Abstract
A Validated Stability Indicating HPLC method for the Determination of Impurities in Pioglitazone Hydrochloride
Author(s): N. Rashmitha, Hiriyanna S.G, CH. Sreenivasa Rao, K. Chandra Sekhar Reddy, M. Hari Kiran, Hemant Kumar Sharma, K. MukkantibThe present paper describes the development of a reversed-phase high performance liquid chromatographic (RP-HPLC) method for Pioglitazone hydrochloride in the presence of its impurities and degradation products, generated from forced degradation studies. The drug substance was subjected to stress conditions of hydrolysis, oxidation, photolysis and thermal degradation. The degradation of Pioglitazone hydrochloride was observed under base and oxidative stress conditions. The drug was found to be stable in other stress conditions studied. Successful separation of the drug from the process related impurities and degradation products formed under stress conditions were achieved on an Inertsil ODS-3V (150 x 4.6 mm), 5 μm column. The gradient LC method employs solution A and solution B as mobile phase. The solution A contains phosphate buffer pH 3.1 and acetonitrile as Solution B. The developed RPLC method was validated with respect to specificity, linearity, accuracy, precision and high sensitivity with detection limits and quantification limits ranging from 0.033 mg/ml to 0.049 mg/ml and 0.100 mg/ml to 0.150 mg/ml respectively. To the best of our knowledge, a rapid LC method, which separates all the impurities, disclosed in this investigation was not published elsewhere.
Select your language of interest to view the total content in your interested language
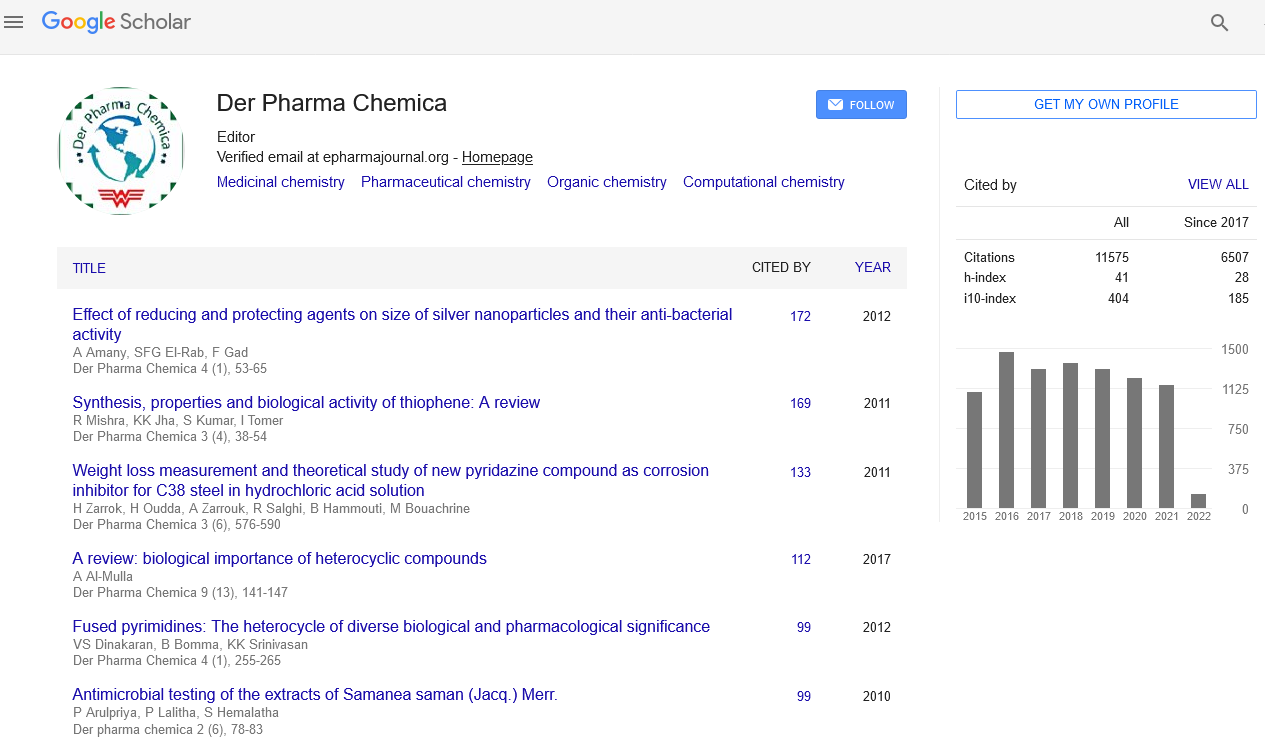
Google Scholar citation report
Citations : 25868
Der Pharma Chemica received 25868 citations as per Google Scholar report
Der Pharma Chemica peer review process verified at publons
DOWNLOADS