Abstract
Comparison and Evaluation of Freely Supplied Government and Ethically Marketed Antihypertensive Drug (Atenolol)
Author(s): Manimala M, Veerashekar J, Hariharasharan S, Praveenkumar MThe main aim of present research work is to evaluate and compare the standards regarding quality of generic and two branded antihypertensive drug (atenolol). The drugs are evaluated and results showed that branded and generic meet the pharmacopoeial specifications. Further all tablets passed weight variation, hardness, thickness, friability, disintegration, dissolution, assay parameters as per pharmacopoeia. Hence we can say that branded and generic drugs of antihypertensive are equal. Hence health care professionals are suggested to prescribe generic drugs so that every man can reach the coast of drugs and maintain health.
Select your language of interest to view the total content in your interested language
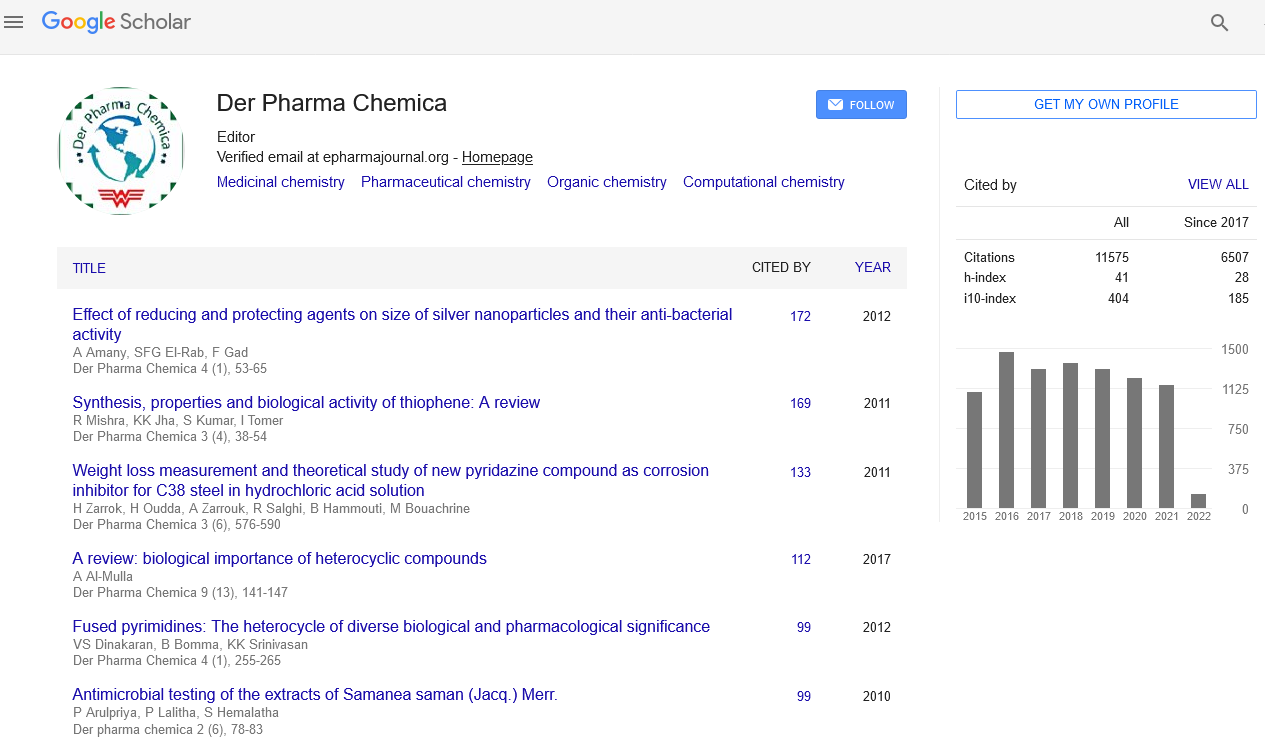
Google Scholar citation report
Citations : 25868
Der Pharma Chemica received 25868 citations as per Google Scholar report
Der Pharma Chemica peer review process verified at publons
DOWNLOADS