Abstract
Development and validation of a new simple and stability indicating RP-HPLC method for the determination of vemurafenib in presence of degradant products
Author(s): Pawanjeet. J. Chhabda,M. Balaji, Srinivasarao V. and K. M. Ch. Appa RaoA novel, simple, precise and stability indicating reverse phase high performance liquid chromatography method was developed and validated for the quantitative analysis of Vemurafenib in bulk drug and dosage form using C8 column(150x 4.6, 3.5 μm) with mobile phase consisting of buffer-acetonitrile (50:50 v/v) with a flow rate of 1.0ml/min (UV at 254nm). Linearity was observed over the concentration range of 20-200 μg/ml with r2= 0.9999. The percentage relative standard deviation in accuracy and precision studies was found to be less than 2%. Vemurafenib was subjected to stress conditions including acidic, alkaline, oxidation, photolysis and thermal degradation. Vemurafenib is more sensitive towards acidic and alkaline degradation. The method was validated as per ICH guidelines.
Select your language of interest to view the total content in your interested language
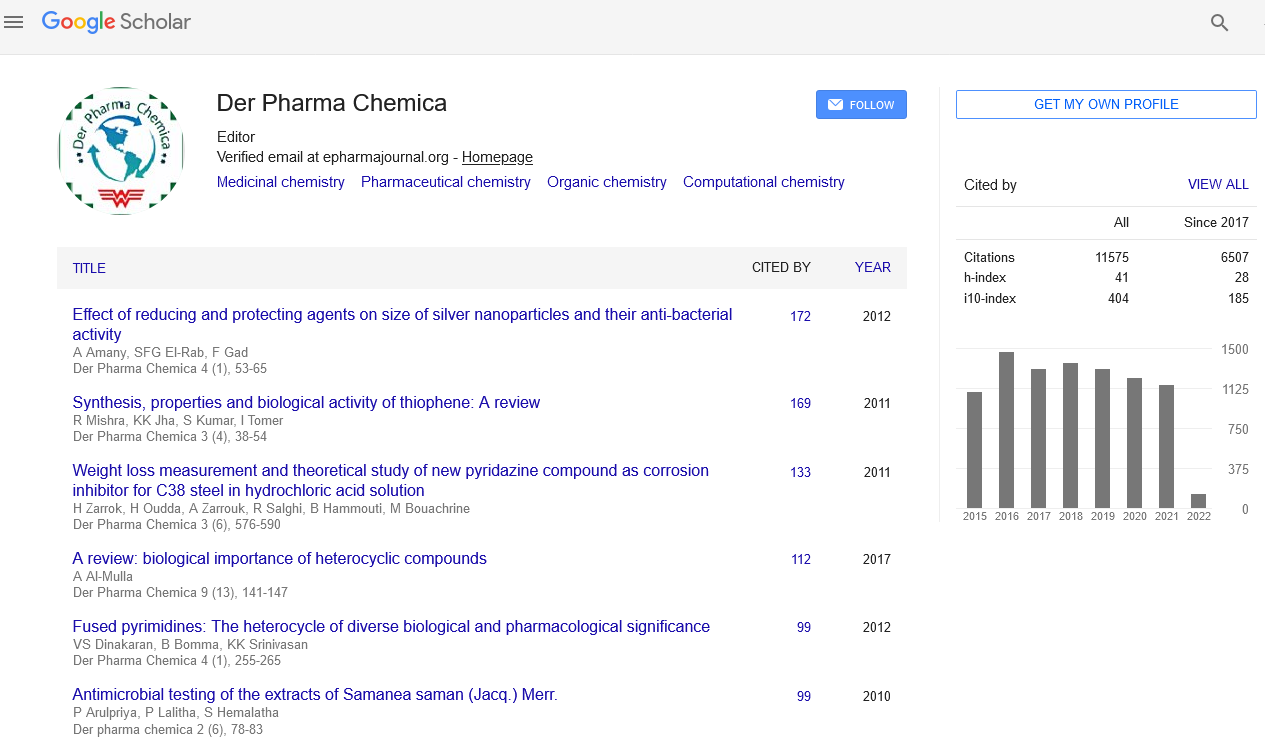
Google Scholar citation report
Citations : 25868
Der Pharma Chemica received 25868 citations as per Google Scholar report
Der Pharma Chemica peer review process verified at publons
DOWNLOADS