Abstract
Kinetics and thermodynamics study of biosorption of Hg2+ by sulphonated biomass of Cicer arientinum- batch studies
Author(s): A. A. Kale, A.S.Burungle N. R. Deshpande, R. V. KashalkarPresent work deals with fundamental investigation on the removal of heavy metal from aqueous solutions by chemically treated biomass of Cicer arientinum is conducted in batch conditions. Adsorption kinetics and isotherm data are determined, and effect of different parameters such as contact time, sorbent dose, pH and temperature has been studied. Modeling of kinetics results shows that sorption process is best explained by pseudo – second order model with determination coefficients 0.997 for S-III under all experimental conditions. Weber and Morris intraparticle diffusion model is used to determine the mechanism. Thermodynamic parameter via KD, ΔG has also been calculated to determine the spontaneity of the process.
Select your language of interest to view the total content in your interested language
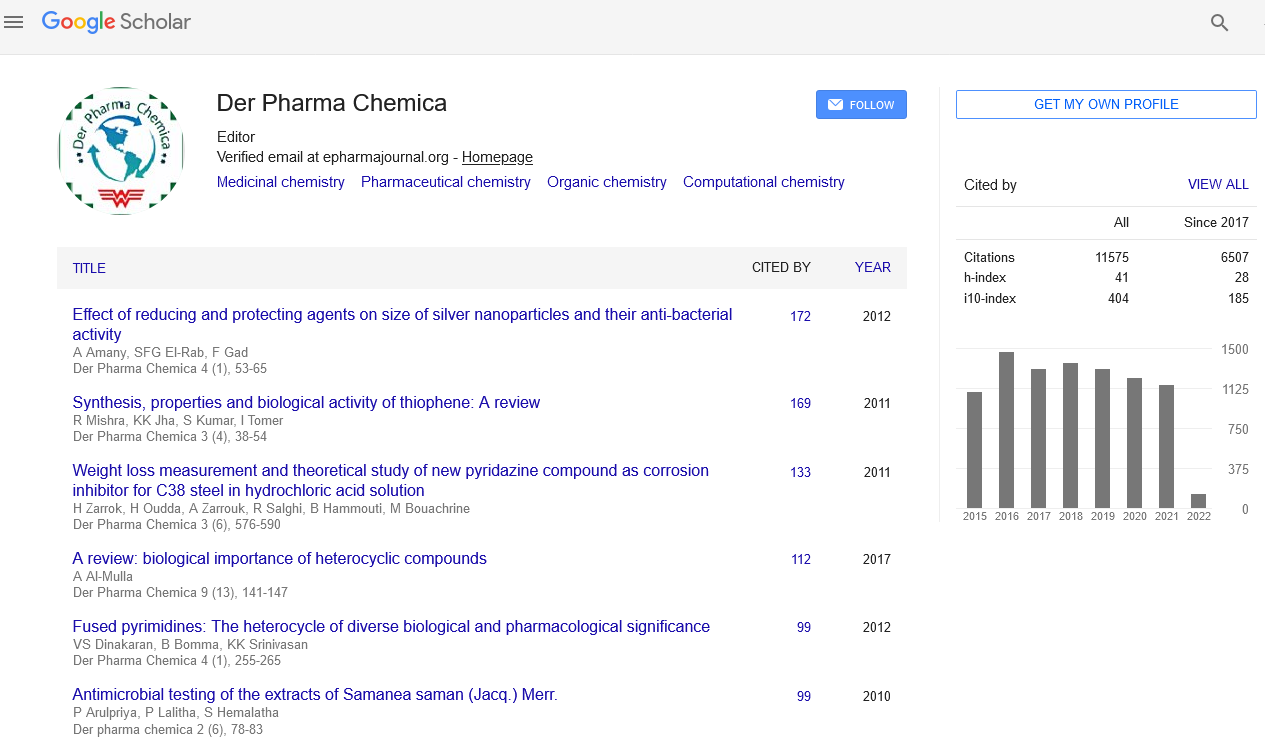
Google Scholar citation report
Citations : 25868
Der Pharma Chemica received 25868 citations as per Google Scholar report
Der Pharma Chemica peer review process verified at publons
DOWNLOADS