Research Article - Der Pharma Chemica ( 2018) Volume 10, Issue 2
A Facile Synthesis of N'-Arylidene-2-((7-bromo-2-methylpyrido[2,3-b]pyrazin-3 yl)oxy)acetohydrazides
Hemalatha Kotakommula1, Shashikala Kethireddy2, Laxminarayana Eppakayala3 and Thirumala Chary Maringanti1*
1Jawaharlal Nehru Technological University, Kukatpally, Hyderabad-500 085, Telangana, India
2Geethanjali College of Engineering and Technology, Keesara, Rangareddy-501301, Telangana, India
3Sreenidhi Institutes of Science and Technology, Ghatkesar, Hyderabad, 501301, Telangana, India
- *Corresponding Author:
- Thirumala Chary Maringanti
Jawaharlal Nehru Technological University
Kukatpally, Hyderabad-500 085, Telangana, India
Abstract
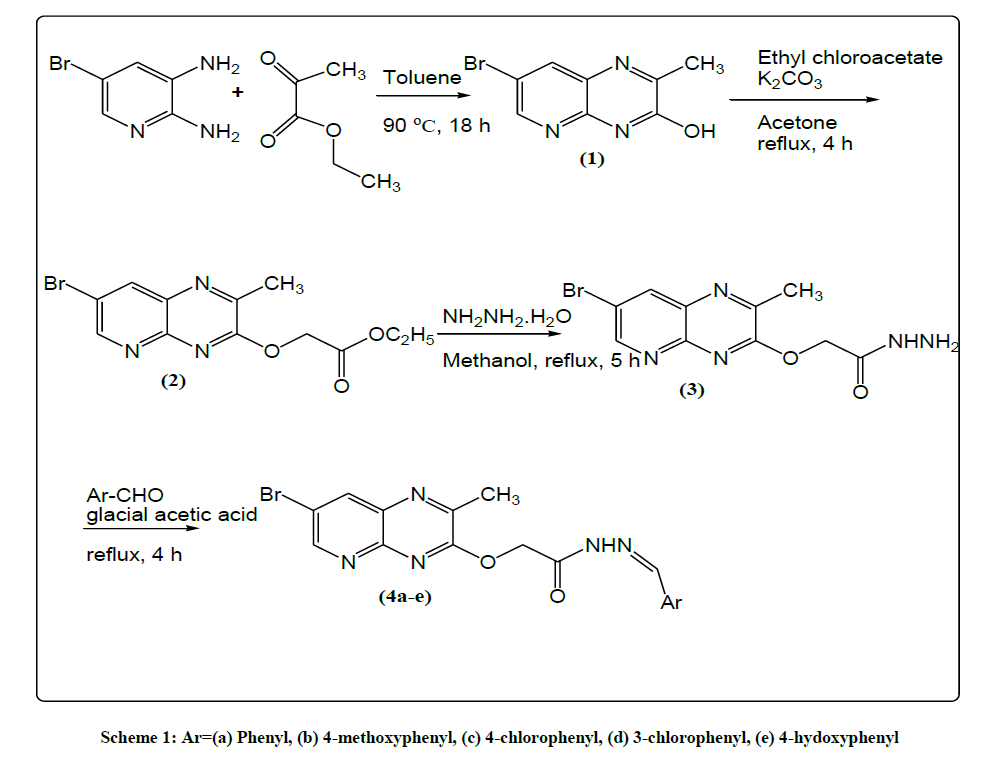
5-bromo 2,3-diamino pyridine and ethyl pyruvate react each other to form 7-bromo-2-methylpyrido[2,3-b]pyrazin-3-ol (1) which further reacts with ethyl chloroacetate and form ethyl 2-((7-bromo-2-methylpyrido[2,3-b]pyrazin-3-yl)oxy)acetate (2). Compound 2 on reaction with hydrazine hydrate gives 2-((7-bromo-2-methyl pyrido[2,3-b]pyrazin-3-yl)oxy)acetohydrazide (3), which on condensation with different aldehydes produce N'-Arylidene-2-((7-bromo-2-methyl pyrido[2,3-b]pyrazin-3-yl)oxy) acetohydrazides (4a-e).
Keywords
Heterocycles, Aldehydes, Antitumor agents, Hydrogen bond, Corrosion, Hormones
Introduction
Pyrido[2,3-b]pyrazine (5-azaquinoxaline) derivatives are very important nitrogen-containing heterocycles, that are extensively used for their pharmacological and therapeutic properties [1]. Pteridine and quinoxaline are structural analogues of them. Studies have shown that such compounds are widely involved in several fields, as they exhibit antimalarial, anti-cancer [2], antibacterial and anti-allergic activities [3]. They also exhibit antimitotic behavior [4]. Pyrido[2,3-b]pyrazine derivatives are well-known for their strong inhibitory activities of phosphodiesterase IV (PDE IV), the production of Tumor Necrosis Factor (TNF), Platelet derived growth receptor, gonadotropin releasing harmone, IgE production [5]. Pyrido pyrazine derivatives are broadly used as corrosion inhibitors for metals in acid environments, since they own the nitrogen and oxygen atoms which can easily be protonated to exhibit good inhibitory action on the corrosion of metals [6].
Mutations affecting Epidermal Growth Factor Receptor (EGFR) activity could result in cancers such as squamous-cell carcinoma of the lung, anal cancers, glioblastoma and epithelian tumors of the head and neck. The identification of EGFR as an oncogene (a gene that has the potential to cause cancer) has led to the development of anticancer therapeutics against EGFR, called "EGFR” inhibitors. Among them, using small molecule inhibitors to inhibit the EGFR tyrosine kinase is the most appropriate method, which acts on the cytoplasmic side of the receptor. Without kinase activity, EGFR is unable to activate itself, which is a prerequisite for binding of downstream adaptor proteins [7-9].
The EGFR inhibitor erlotinib is a good example of small molecule inhibitor, which possesses high anti-tumor effect. In search of small molecule inhibitors, pyrido[2,3-b]pyrazine compounds were proved to be effective inhibitors of resistant cells. A series of novel pyrido[2,3-b]pyrazines were synthesized as potential antitumor agents for erlotinib-resistant tumors. Studies revealed that, there is a promising effect of the substituent at position 7 and position 2 of the pyrido pyrazine core. Pyrido[2,3,-b]pyrazine core has hydrogen-bond acceptor site and hydrophobic center roles [9].
Materials and Methods
All the chemicals and solvents used were purchased either from Fluka or Merck. Reagents used were of analytical grade. Thin Layer Chromatography (TLC) was performed on E Merck AL silica gel 60 F254 plates and visualized under UV light. IR spectra were recorded as KBr pellet with a PerkinElmer spectrum gx Fourier Transform Infra-Red (FTIR) instrument and only intense peaks are reported. Proton Nuclear Magnetic Resonance (1H-NMR) spectra were recorded in Deuterated Dimethyl Sulfoxide (DMSO-d6) with a Varian Mercury plus 400 MHz instrument. Tetramethylsilane (TMS) is used as an internal standard. All the chemical shifts were reported in δ (ppm). The 1H-NMR chemical shifts and coupling constants were determined assuming first-order behavior. Multiplicity is indicated by one or more of the following: s (singlet), d (doublet), t (triplet), q (quartet), m (multiplet), br (broad); the list of coupling constants (J) corresponds to the order of multiplicity assignment. Mass spectra were recorded with a PE Sciex model API 3000 instrument. All the reactions were carried out under argon atmosphere.
Results and Discussion
Different pyrazine derivatives are synthesized from 5-bromo-2,3-diamino pyridine. First step involves the cyclisation with ethyl pyruvate using toluene as a solvent under heating condition to get cyclized compound (1) which further reacts with ethyl chloroacetate to give compound (2). It is followed by the condensation with hydrazine hydrate to give acetohydrazide (3) and finally leads to title compounds with different substituted aldehydes (4a-e). All the molecules synthesized in the above scheme 1 shown are characterized by spectrum of 1H-NMR and Mass.
Synthesis of 7-bromo-2-methylpyrido [2,3-b]pyrazin-3-ol (1): To a suspension of 5-bromo 2,3-diamino pyridine (1.0 g, 0.005 mol) in toluene (10 ml) was added ethyl pyruvate (0.6 ml, 0.005 mol) drop wise with vigorous stirring under N2 atmosphere. Then the resulting reaction mixture was stirred at 90oC for 18 h. Progress of the reaction was monitored by TLC. After complete consumption of SM cooled the mixture to room temperature. Then the resulting solid was collected by filtration, dried and recrystallized from methanol to give desired product (500 mg, 39%) as dark yellow solid.
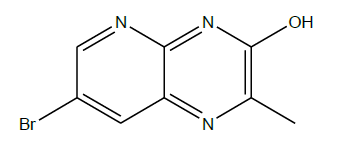
C8H6BrN3O, Exact Mass: 238.97, Mol. Wt.: 240.06, m/e: 238.97 (100.0%), 240.97 (97.6%), 239.97 (9.8%), 241.97 (8.5%), 241.96 (1.1%), C, 40.03; H, 2.52; Br, 33.29; N, 17.50; O, 6.66; 1H-NMR (400 MHZ, DMSO-d6): (ppm) 2.42 (s, 3H), 2.43 (s, 1H), 8.38 (d, J=2 HZ, 1H), 8.57 (d, J=2 Hz, 1H). MS (ESI) 239.7 m/z (M+H) +.
Synthesis of ethyl 2-((7-bromo-2-methylpyrido[2,3-b]pyrazin-3-yl)oxy)acetate (2): To a suspension of 7-bromo-2-methylpyrido[2,3- b]pyrazine-3-ol (500 mg, 0.002 mol) in dry acetone (5 ml) was added ethyl chloroacetate (0.22 ml, 0.002 mol) followed by the addition of dry K2CO3 (138 mg, 0.001 mol). The reaction mixture was stirred at reflux temperature (50-60°C) for 24 h. After completion of the reaction checked by TLC, the mixture was allowed to cool to room temperature and removed the solvent under reduced pressure. The obtained residue was dissolved in ethyl acetate and washed with water. The organic layer was dried over Na2SO4, filtered and concentrated and the residue was recrystallized from methanol to give desired product (2) as brown solid (500 mg, 73%).
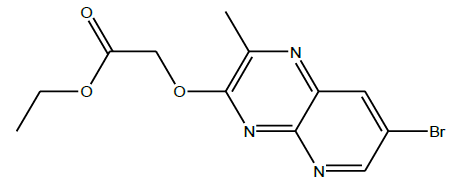
C12H12BrN3O3; Exact mass: 325.01, Mol. Wt.: 326.15, m/e: 325.01 (100.0%), 327.00 (97.3%), 326.01 (13.2%), 328.01 (13.0%), 327.01 (1.6%), 329.01, (1.4%), 326.00 (1.1%), 328.00 (1.1%), C, 44.19; H, 3.71; Br, 24.50; N, 12.88; O, 14.72; 1H-NMR (400 MHZ, DMSO-d6): (ppm) 2.42 (s, 3H), 2.43 (s, 1H), 8.38 (d, J=2 HZ, 1H), 8.57 (d, J=2 Hz, 1H). MS (ESI) 239.7 m/z (M+H) +.
2-((7-bromo-2-methylpyrido[2,3-b]pyrazin-3-yl)oxy)acetohydrazide (3): To a solution of ethyl 2-((7-bromo-2-methylpyrido[2,3-b]pyrazin-3- yl)oxy)acetate (500 mg, 0.001 mol) in methanol (10 ml) was added hydrazine hydrate (80%, 20 ml) and heated to reflux temperature (70°C) for 5 h. Progress of the reaction was monitored by TLC. The mixture was cooled to room temperature and poured in to crushed ice with stirring. Then the resulting solid was collected by filtration and dried in vacuum to give the title compound (3) as a pink solid (100 mg, 21%).
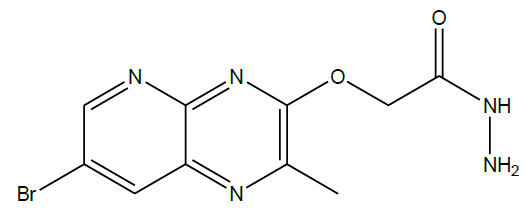
C10H10BrN5O2, Exact mass: 311, Mol. Wt.: 312.12, m/e: 311.00 (100.0%), 313.00 (97.5%), 314.00 (12.4%), 312.01 (11.0%), 312.00 (1.8%) C, 38.48; H, 3.23; Br, 25.60; N, 22.44; O, 10.25; 1H-NMR (400 MHZ, DMSO-d6): (ppm) 2.42 (s, 3H), 4.42 (brs, 2H), 4.78 (s, 2H), 8.52 (s, 1H), 8.69 (s, 1H), 8.80 (brs, 1H). MS (ESI) 312 m/z (M+H) +.
N'-benzylidene-2-((7-bromo-2-methylpyrido[2,3-b]pyrazin-3-yl)oxy)acetohydrazide (4a): A mixture of 2-((7-bromo-2-methylpyrido[2,3- b]pyrazin-3-yl)oxy)acetohydrazide (100 mg, 0.0003 mol) and benzaldehyde (33 mg, 0.0003 mol) were taken in DMF (1 ml) followed by the catalytic amount of glacial acetic acid. Then the reaction mixture was heated to 120oC for 4 h. The completion of the reaction was checked by TLC, the mixture was poured in to crushed ice and the resulting solid was collected by filtration and dried to give title compound (4a) as a brown solid (50 mg, 40%).
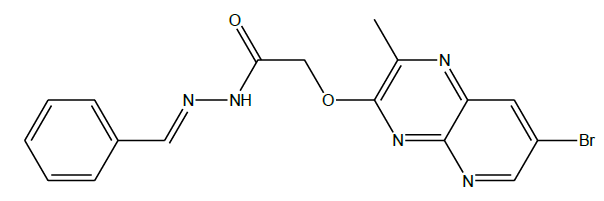
C17H14BrN5O2, Exact Mass: 399.03, Mol. Wt.: 400.23, m/e: 399.03 (100.0%), 401.03 (97.6%), 402.03 (19.7%), 400.04 (18.6%), 401.04 (2.0%), 403.04 (2.0%), 400.03 (1.8%), C, 51.02; H, 3.53; Br, 19.96; N, 17.50; O, 8.00; 1H-NMR (400 MHZ, DMSO-d6): (ppm) 2.89 (s, 3H), 4.78 (s, 2H), 7.52 (s, 4H), 7.88 (s, 2H), 7.95 (s, 1H), 8.72 (s, 1H). MS (ESI) 400 m/z (M+H) +.
N'-(4-methoxybenzylidene)-2-((7-bromo-2-methylpyrido[2,3-b]pyrazin-3 yl)oxy)acetohydrazide (4b):
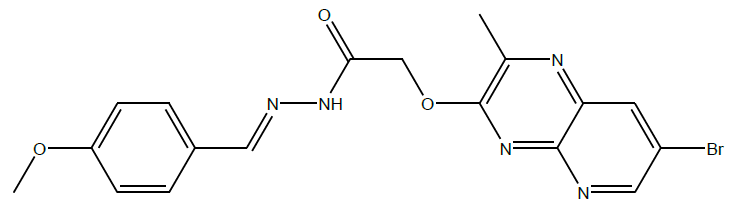
C17H14BrN5O2, Exact mass: 399.03, Mol. Wt.: 400.23, m/e: 399.03 (100.0%), 401.03 (97.6%), 402.03 (19.7%), 400.04 (18.6%), 401.04 (2.0%), 403.04 (2.0%), 400.03 (1.8%), C, 51.02; H, 3.53; Br, 19.96; N, 17.50; O, 8.00; 1H-NMR (400 MHZ, DMSO-d6): (ppm) 2.90 (s, 3H), 3.81 (s, 3H), 4.76 (s, 2H), 7.50 (s, 4H), 7.87 (s, 1H), 7.96 (s, 1H), 8.71 (s, 1H). MS (ESI) 431 m/z (M+H) +.
N'-(4-chlorobenzylidene)-2-((7-bromo-2-methylpyrido[2,3-b]pyrazin-3-yl)oxy)acetohydrazide (4c):
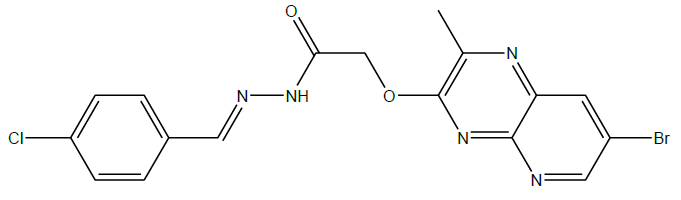
C17H13BrClN5O2, Exact mass: 432.99, Mol. Wt.: 434.67, m/e: 434.99 (100.0%), 432.99 (77.2%), 436.99 (24.3%), 434.00 (14.4%), 436.00 (14.2%), 435.99 (6.4%), 437.99 (4.9%), 437.00 (2.0%), 435.00 (1.6%), 433.99 (1.4%), C, 46.97; H, 3.01; Br, 18.38; Cl, 8.16; N, 16.11; O, 7.36; 1H-NMR (400 MHZ, DMSO-d6): (ppm) 2.90 (s, 3H), 4.74 (s, 2H), 7.54 (s, 4H), 7.86 (s, 1H), 7.96 (s, 1H), 8.74 (s, 1H). MS (ESI) 435 m/z (M+H) +.
N'-(3-chlorobenzylidene)-2-((7-bromo-2-methylpyrido[2,3-b]pyrazin-3-yl)oxy)acetohydrazide (4d):
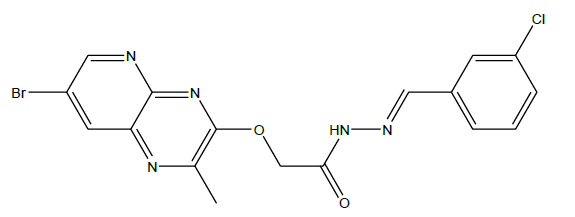
C17H13BrClN5O2, Exact mass: 432.99, Mol. Wt.: 434.67, m/e: 434.99 (100.0%), 432.99 (77.2%), 436.99 (24.3%), 434.00 (14.4%), 436.00 14.2%), 435.99 (6.4%), 437.99 (4.9%), 437.00 (2.0%), 435.00 (1.6%), 433.99 (1.4%), C, 46.97; H, 3.01; Br, 18.38; Cl, 8.16; N, 16.11; O, 7.36; 1H-NMR (400 MHZ, DMSO-d6): (ppm) 2.91 (s, 3H), 4.76 (s, 2H), 7.53 (s, 4H), 7.85 (s, 1H), 7.95 (s, 1H), 8.76 (s, 1H). MS (ESI) 435 m/z (M+H) +.
N'-(4-hydoxybenzylidene)-2-((7-bromo-2-methylpyrido[2,3-b]pyrazin-3-yl)oxy)acetohydrazide (4e):
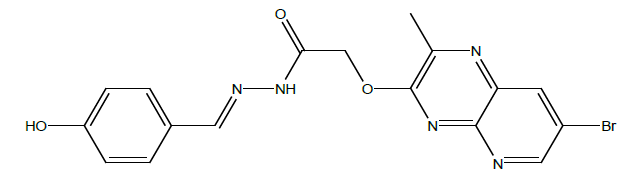
C17H14BrN5O3, Exact mass: 415.03, Mol. Wt.: 416.23, m/e: 415.03 (100.0%), 417.03 (99.8%), 416.03 (20.5%), 418.03 (18.2%), 419.03 (2.5%), 418.02 (1.8%) C, 49.06; H, 3.39; Br, 19.20; N, 16.83; O, 11.53; 1H-NMR (400 MHZ, DMSO-d6): (ppm) 2.92 (s, 3H), 4.76 (s, 2H), 7.52 (s, 4H), 7.85 (s, 1H), 7.94 (s, 1H), 8.76 (s, 1H), 11.23 (brs, 1H). MS (ESI) 417 m/z (M+H) +.
Conclusion
The present paper describes the synthesis of N'-benzylidene-2-((7-bromo-2-methylpyrido [2,3-b]pyrazin-3-yl)oxy)acetohydrazide from commercially available 5-bromo 2,3-diamino pyridine and ethyl pyruvate as starting materials in three steps.
References
- T.J. Haley, A.M. Flesher, R. Vcomelt, Proc. Soc. Biol. Med., 1957, 96, 579-584.
- B. Mathew, S. Srivastava, Bioorg. Med. Chem., 2011, 19, 7120-7128.
- F. Wiseloge, W.L.F. Armarego, Quinazolines, Advances in heterocyclic chemistry, New York, Academic Press, 1963, 1, 304.
- J.D Blythin, N. Caldwell, S. Ho-Jane, P. Brook, Aryl substituted naphthyridine and pyridopyrazine derivatives, useful as anti-allergic agents, U.S Patent Documents, 1988, 4760073.
- Shimazaki, Norihiko, Nagakuni, Tsuchiura-shi, Ibaraki, Sawada, Akihiko, Watanabe, Shinya, Pyrido(2,3-b)pyrazine derivatives, 1997; PCT/JP 96/03666, patent WO97/24355.
- E.E. Ebenso, N.O. Eddy, A.O. Odiongenyi, Portugaliae Electrochimica Acta., 2009, 27(1), 13.
- I. Cherif Alaoui, Y. Kandri Rodi, E.M. El Hadrami, A. Haoudi, M. Skalli, B. Garrigues, E.M. Essassi, J. Mar. Chim. Heterocyc., 2007, 6(1), 32-37.
- L. Kékesi, A. Sipos, G. Németh, A. Dancsó, E. Illyés, S. Boros, N. Breza, Z. Nemes, B. Hegymegi-Barakonyi, J. Pató, Z. Greff, G. Kéri, L. Őrfi, Acta Pharm Hung., 2014, 84(3), 91-104.
- L. Kékesi, A. Sipos, G. Németh, J. Pató, N. Breza, F. Baska, L. Őrfi, G. Kéri, Bioorg. Med. Chem. Lett., 2013, 23(22), 6152-6155.