Research Article - Der Pharma Chemica ( 2018) Volume 10, Issue 1
Chemical Composition and Antimicrobial Activity of Torreya nucifera Essential Oils against Oral Pathogens
Ji-Hye Ko1, Su-Hee Cho1, Ji-Seon Hyun1, Ju-Mi Hyun1, Je-Hwan Jeong2, Duk Soo Kim1, Choon Il Kang3, Nam Ho Lee1 and Chang-Gu Hyun1*
1Department of Chemistry and Cosmetics, Jeju National University, Jeju 63243, Korea
2ISLAND Co., 21 Jungang-ro 14-gil, Jeju 63169, Korea
3JejuIndi Inc., 4150-30, Jungsangandong-ro, Seongsan-eup, Seogwipo-si, Jeju 63635, Korea
- Corresponding Author:
- Chang-Gu Hyun
Department of Chemistry and Cosmetics
Jeju National University, Jeju 63243, Korea
Abstract
Essential oils are natural substances extracted from various organs of multiple plant species, such as buds, flowers, leaves, stems, branches, roots, seeds, fruits, wood, and bark. The oils are used as a raw material for food flavoring, and as food additives and additives to cosmetics and perfumes. In this study, we investigated the composition of essential oils extracted from the branches and leaves of Torreya nucifera and their cytotoxicity and antibacterial activity against oral bacteria. Hydro-distilled T. nucifera essential oils (TNEs) were analyzed using gas chromatography-mass spectrometry (GC-MS). In total, 16 components were identified, with dl-limonene (43.95%), α-pinene (14.76%), δ-3 carene (8.94%), and δ-cadinene (5.42%) representing the main components. Antimicrobial activity tests were carried out using MIC, MBC and time kill assays against the oral bacteria, Streptococcus mutans (KCOM 1054) and Streptococcus sobrinus (KCOM 1157). TNEs showed bactericidal effect at 2X MIC against the oral bacteria. In vitro time kill assays were performed at 0.5, 1, and 2X MICs. Cytotoxicity was confirmed using MTT assay by analyzing the amount of formazan crystals via ELISA. Based on these results, we confirm the feasibility of TNEs as a natural antimicrobial agent for oral microorganisms.
Keywords
Cytotoxicity, Essential oil, GC-MS, Keratinocyte, Oral Pathogen, Torreya nucifera
Introduction
Despite advances in public policy, dental caries remains the most prevalent and costly infectious disease worldwide [1,2]. It represents a global public health issue requiring effective management by the authorities and dental professionals [2,3]. Effective caries-preventive methods have been developed and revised over the last decades [4]. It is well known that chemical control of plaque is an effective way to prevent dental caries. The synthetic compounds, fluorides [5], chlorhexidine [6], triclosan, cetylpyridinum chloride, as well as natural products [4,7] are currently available. Natural products have been proposed as new therapeutic agents for dental caries [8] in order to minimize the side effects of synthetic compounds [9].
Essential oil (EO) is a concentrated hydrophobic solution containing natural volatile aromatic compounds and various unique components such as monoterpenes, sesquiterpenes, or their oxidative derivatives from plants [10]. Monoterpenes are the major compounds found in EOs and have been shown to exhibit potent antibacterial activity against caries-associated microorganisms [11,12].
Despite the progress made so far, there has been little research into the potential application of EOs in the dental field [13]. A number of phytochemical components of EOs have been used in anti-plaque and anti-gingivitis mouthwashes [14-16]. Therefore, additional exploration is required to establish EOs that can be used as adjunctive anti-caries chemotherapy.
In this investigation, we conducted a study on Torreya nucifera essential oils (TNEs). T. nucifera is a warm temperate zone, evergreen conifer, native to some parts of South Korea and Japan. In particular, Jeju island, in Korea, has a large population, and is known as the world’s largest single-species forest.
EOs are extracted from T. nucifera seeds; are used as edible oil, hair oil, and medicine [17]; and have been used commercially as a raw material for cosmetics (Bija essential oil, Innisfree Co., Korea). Analyses of seed EO constituents, and the antibacterial [18], antioxidant effects [19,20], anti-inflammatory effects [19], neuroprotective effects [21] of EOs have been reported.
However, the chemical composition and biological activities of EOs derived from the leaf and branch organs are not well-known. In our previous study, we investigated TNEs and reported their anti-inflammatory effects, cytotoxicity based on lactate dehydrogenase (LDH) assay, and antibacterial effects against skin pathogens [22].
In this study, we investigate the potential applicability of TNEs from branches and leaves as a new dental formulation. We also report cytotoxicity in the HaCaT cell line, and antibacterial effects on oral pathogens (Streptococcus mutans and Streptococcus sobrinus).
Material and Methods
Plant materials and extraction
T. nucifera leaves and branches used in the experiment were supplied by JejuIndi Inc. EOs were extracted using steam distillation. T. nucifera leaves and branches (200 g) were heated with 4.5 l of distilled water at 110°C for 6 h. The resulting steam was passed through the cooler and separated into distillated and EO, and only the upper layer of the oil was collected. This sample was stored at 4°C until analysis.
Gas chromatography-mass spectrometry
T. nucifera essential oils (TNEs) were analyzed using gas chromatography-mass spectrometry (GC-MS) and quantified according to the analytical methods reported by the Korean Ministry of Food and Drug Safety. The 7890A GC system (Agilent Technologies, Santa Clara, CA, USA) was used, with DB-5ms Ultra Inert (0.15-0.32 mm, 5-60 m, 0.15 1.00 μm; Agilent Technologies) columns and the 5975C mass selective detector (Agilent Technologies). Electron-impact ionization of mass spectrometry was performed at 70 eV. TNEs were dissolved in ethanol and injected into the column. The temperature was first set at 60°C (5 min) and increased to 240°C (5 min) at a rate of 3°C/min. Helium was used as the carrier gas at a flow rate of 1.5 ml/min. The TNE compounds were identified by comparing their retention times and mass spectra with those of standards given in the Wiley 138 library data of the GC-MS system. C7-C30 saturated alkanes (Sigma-Aldrich, St. Louis, MO, USA) were used as standard solutions.
Determination of antibacterial activity
We measured the antibacterial activity of TNE against the gram-positive oral bacteria S. sobrinus (KCOM 1157) and S. mutans (KCOM 1054) obtained from Korean Collection for Oral Microbiology (Gwang-ju, Korea). The bacterial strains were cultured with Brain Heart Infusion medium (BHI, Becton Dickinson GmbH, Heidelberg, Germany) in an AnaeroGen 2.5 l Sachet (Thermo Fisher Scientific, Waltham, MA USA) in a 37°C incubator for 18 h. Minimum inhibitory concentration (MIC) was measured by a modified Broth Microdilution method [23]. TNE was serially diluted 50-fold with ethanol. Following this, 20 ml of BHI medium was added to 200 μl of TNE. The starting microorganism concentration was approximately (30-100) x 106 CFU/ml. The bacteria were inoculated to 1% of the total volume. After incubation for 18 h under anaerobic conditions, the minimum concentration at which the bacteria did not grow was determined by visual inspection. Minimum bactericidal concentration (MBC) was determined based on the MIC required to prevent colony growth on 1.5% agar medium.
Time-kill curve assay
The time-kill curve assay was performed to confirm the rate of bacterial death [24] for samples with concentrations ranging from 7 to 10 log colony-forming units (CFU)/ml. BHI medium (950 μl) and 10 μl of the cultured bacteria were added to the four micro tubes and cultured for 18 h. The TNE was diluted to 0.5, 1, and 2 x MIC and 40 μl added to each tube. Medium (40 μl) was used as a control. The cultures were incubated at 37°C under anaerobic conditions and colony counts were determined at 0, 2, 4, 6, 8, 10, 12, and 24 h.
Cell culture
The HaCaT (human keratinocyte) cell line was cultured with Dulbecco’s Modified Eagle medium (DMEM) containing 10 % fetal bovine serum (FBS) and 1% penicillin at 37°C in a 5% CO2 incubator.
MTT assay for cell viability
Cell viability was determined using the 2-(4, 5-dimethylthiazol-2-yl)-5-diphenyltetrazolium bromide (MTT) reduction assay. HaCaT cells were seeded in a 24-well plate for 24 h, and treated with TNE. After 24 h, cells were treated with MTT solution (2 mg/ml) and the formazan crystals were dissolved in dimethyl sulfoxide (DMSO). Absorbance was measured at 540 nm with an enzyme-linked immunosorbent assay (ELISA) reader. The results were then expressed as percentage of MTT reduction, with the absorbance exhibited by the control cells being arbitrarily set as 100%.
Results and Discussion
GC-MS analysis
The TNE yield was 0.022 %. Table 1 shows the general chemical profile, percent content, and retention time of the constituents of TNE. In total, 16 compounds were identified on the basis of their mass spectra, which were compared to those in the Wiley 138 library data of GC-MS system. The main constituents of TNE were dl-limonene (43.95%), α-pinene (14.76%), δ-3-carene (8.94%), and δ-cadinene (5.42%).
| RT (min) | %Area | Hit Name | Quality |
|---|---|---|---|
| 8.746 | 14.764 | α-Pinene | 97 |
| 9.33 | 1.382 | Camphene | 96 |
| 10.955 | 1.51 | β-Pinene | 97 |
| 12.317 | 3.066 | β-Myrcene | 96 |
| 13.209 | 8.942 | δ-3-Carene | 98 |
| 14.754 | 43.946 | dl-Limonene | 99 |
| 18.348 | 2.958 | α-terpinolene | 98 |
| 43.822 | 1.359 | β-Farnesene | 98 |
| 44.377 | 1.729 | α-Amorphene | 98 |
| 46.466 | 5.42 | δ-Cadinene | 99 |
| 49.853 | 2.662 | Naphthalene | 96 |
| 50.248 | 1.691 | 2-Isopropyl-5-methyl-9-methylene | 96 |
| 50.442 | 4.703 | δ-Cadinol | 98 |
| 50.643 | 1.664 | 2-Isopropyl-5-methyl-9-methylene | 96 |
| 51.524 | 1.967 | α-Bisabolol | 90 |
| 67.402 | 2.235 | Triacontane | 99 |
Table 1: Chemical composition of the Torreya nucifera essential oils
The primary constituent of TNE, dl-limonene, is a terpene hydrocarbon. It is mainly found in orange, lemon, mandarin, and pine tree EOs. dl- Limonene is used as a raw material for cosmetics to provide fragrance and aid absorption. Together with α-pinene, also detected in our GC-MS analysis, dl-limonene has been shown to have stress-reducing, anti-inflammatory, and anxiolytic effects.
Components were characterized based on library and literature searches and only those components showing matches exceeding 90% were selected. RT, retention time
MIC and MBC assay for antibacterial activity
We evaluated the antibacterial activity of TNE against the oral bacteria S. mutans (KCOM 1054) and S. sobrinus (KCOM 1157) (Table 2). S. mutans and S. sobrinus are well-established contributors to dental caries in humans [25]. S. mutans is a facultative anaerobic gram-positive, round bacterium commonly found in the human oral cavity and is known as a contributor to tooth decay [26]. S. sobrinus is a gram-positive, catalase-negative, non-motile, anaerobic bacterium and has been found with S. mutans on decaying teeth of humans and certain species of rats [27,28].
| Bacterial strain | TNE (μL / mL) | |
|---|---|---|
| MIC | MBC | |
| KCOM 1054 (S. mutans) | 0.1 | 0.1 |
| KCOM 1157 (S. sobrinus) | 0.1 | 0.1 |
Table 2: In vitro activity of TNE on oral bacteria KCOM 1054 and KCOM 1157
To establish MIC of TNE, concentrations of TNE ranging between 0.009766 μl/ml and 10 μl/ml were applied to the bacterial strains S. mutans (KCOM 1054) and S. sobrinus (KCOM 1157).
Time-kill assay
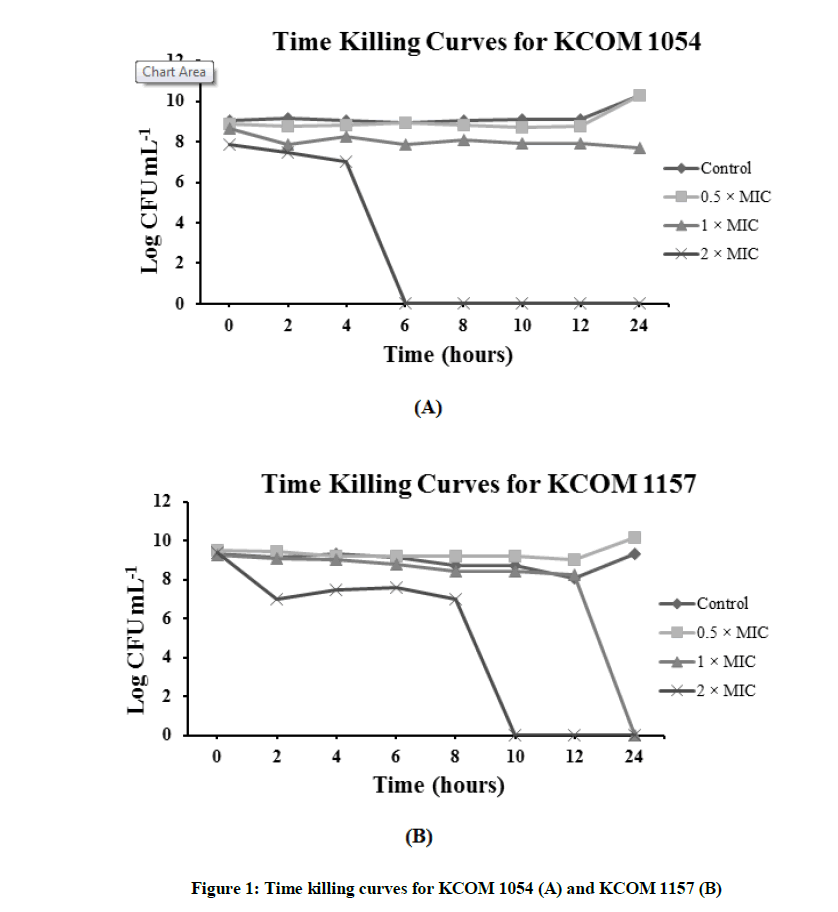
The time-kill assay was performed over a period of 24 h. The bacteria were exposed to 0.5, 1, and 2X MIC of TEOs. Figure 1 shows the result of the time kill curve for KCOM 1054. In control and at 0.5X MIC of TEO, the number of bacterial colonies increased after 12 h. When considering the curve for 1X MIC, the number of bacterial colonies appears to decrease until the initial stage (6 h), after which, the number of colonies remained the same. At 2X MIC, no colonies remained by 6 h. In the previous experiments, the MIC value was visually confirmed to be the same as the MBC value. However, the MBC value was found to be twice as high as the MIC when the time kill curve was drawn (Figure 1A).
Figure 1B represents the time kill curve for KCOM 1157. Similar to KCOM 1054, in the control and at 0.5X MIC, the number of bacterial colonies increased over time. However, at 1X and 2X MIC, the number of colonies began to sharply decrease after 12 h and 8 h, and it was confirmed that the number of bacterial colonies decreased to zero after 24 h and 10 h, respectively.
The time-kill assay was performed as shown in material and methods. The oral pathogens were exposed to 0.5, 1, and 2X MIC of TNEs.
Cytotoxicity test
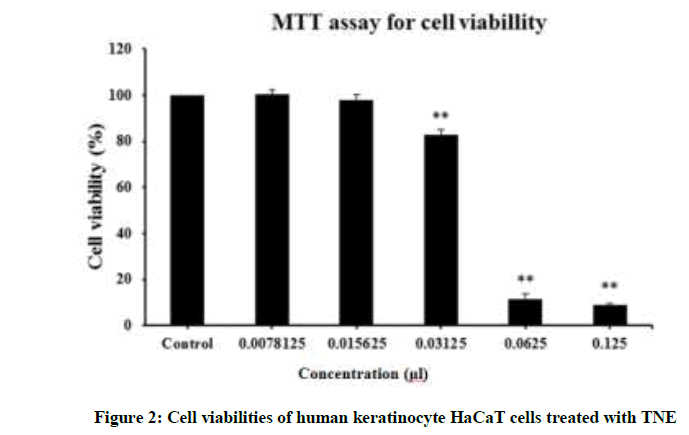
Cytotoxicity was determined by measuring cell viability (Figure 2). Exposure of HaCaT cells to a high dosage (≥ 62.5 μl/ml) of TNE resulted in a significant decrease in cell viability. However, the cells showed more than 80% viability at 31.25 μl/ml of TNE. In particular, at 15 μl/ml, more than 3X MIC, cell viability was close to 100% and therefore TNE had negligible cytotoxicity for HaCaT cells even at a concentration that induced bactericidal effects.
TNE exhibited relatively low cytotoxicity (cell viability almost 100% at 15 μl/ml). MTT assays were performed after incubation of human keratinocyte HaCaT cells with various concentrations of TNE (0.78 ~ 125 μl/ml) for 24 h at 37°C in 5% CO2 atmosphere.
Conclusion
The time-kill assay confirmed that TNE had a bactericidal effect on the oral bacteria S. mutans and S. sobrinus, which is corroborated by the results of the MIC and MBC assays. The MTT assay revealed that TEO showed almost no cytotoxicity against human skin cells even at the concentration showing a bactericidal effect. From these results, we found that TNE could feasibly be used as a natural antibiotic against oral bacteria. The GC-MS data revealed that TNE has not only antibacterial components but also anti-inflammatory components such as dl-limonene and α-pinene. Therefore, we want to elucidate the possible anti-inflammatory applications of TNEs through further study.
Acknowledgement
This research was supported by the 2017 Scientific Promotion Program funded by Jeju National University.
References
- N.C.D. Panel, J. Am. Dent. Assoc., 2001, 132, 1009.
- A. Robert, F.G.G. Bagramian, R.V. Anthony, Am. J. Dent., 2009, 21, 3.
- M. Bönecker, L.M.A. Tenuta, G.A.P. Junior, P.B. Costa, N. Pitts, Braz Oral. Res., 2013, 27, 5.
- J.C. Gunsolley, J. Dentist., 2010, 38, S6.
- A. Maguire, Evid. Based Dent., 2014, 15, 38.
- I. Yévenes, S.R. Alvarez, M.N. Jara, P.M. Wolfenson, L.P. Smith, Rev. Odonto Ciênc., 2009, 24, 345.
- J.T.G. Noah Samuels, J.S. Aron, D.W. Isaiah, C.W. Ray, Compend. Contin. Educ. Dent., 2012, 33, 1.
- J.G. Jeon, P.L. Rosalen, M.L. Falsetta, H. Koo, Caries Res., 2011, 45, 243.
- P. Magee, Side Eff. Drugs Annu., 2007, 29, 241.
- F. Bakkali, S. Averbeck, D. Averbeck, M. Idaomar, Food. Chem. Toxicol., 2008, 46, 446.
- L.C. Galvao, V.F. Furletti, S.M. Bersan, M.G. da Cunha, A.L. Ruiz, J.E. de Carvalho, A. Sartoratto, V.L. Rehder, G.M. Figueira, M.C. Teixeira Duarte, M. Ikegaki, S.M. de Alencar, P.L. Rosalen, Evid. Based Complement. Alternat. Med., 2012, 2012, 1.
- A. Wagner. R.L. Bernardes, G.T. Marcos, G. Luzio, F. Bocalon, G.M.S. Maria, C.C.T. Isabel, L. Márcio, C.H.G.M. Andrade e Silva, A. Ademar, F. da Silva, R.C. Wilson. Z. Naturforsch., 2010, 65c, 588.
- T.M. Sreevidhya, V.R. Geetha, Int. J. Drug Dev. Res., 2014, 6, 65.
- H.R. Preus, O.C. Koldsland, A.M. Aass, L. Sandvik, B.F. Hansen, Acta Odontol. Scand., 2013, 71, 1.
- R. A. d. S. Marco Antonio Botelho, G.M. Jose, O.C. Cintia, C.P. Mabel, A. Cláudio, S.R. Ronaldo, B.Q. Dinalva, S.R. Wagner, M. Gloria, I.R. Francisca, J. Med. Plants Res., 2008, 2, 341.
- M.P. Van Leeuwen, D.E. Slot, G.A. Van der Weijden, Int. J. Dental Hygiene., 2014, 12, 160.
- Y. Endo, Y. Osada, F. Kimura, K. Fujimoto, Nutrition., 2006, 22, 553.
- H.J. Oh, H.M. Ahn, K.H. So, S.S. Kim, P.Y. Yun, G.L. Jeon, K.Z. Riu, Appl. Biol. Chem., 2007, 50, 164.
- M.K. Cho, Y.P. Jang, Y.C. Kim, S.G. Kim, Int. Immunopharmacol., 2004, 4, 1419.
- H.S. Jeon, Y.S. Lee, N.W. Kim, J. Kor. Soc. Food Sci. Nutr., 2009, 38, 1.
- Y.P. Jang, S.R. Kim, Y.C. Kim, Planta Med., 2001, 67, 470.
- W.J. Yoon, S.S. Kim, T.H. Oh, N.H. Lee, C.G. Hyun, Int. J. Pharmacol., 2009, 5, 37.
- J.M. Andrews, J. Antimicrob. Chemother., 2001, 48, 5.
- G. Rukholm, C. Mugabe, A.O. Azghani, A. Omri, Int. J. Antimicrob. Agents, 2006, 27, 247.
- J.J. De Soet, W.P. Holbrook, M.O. Magnusdóttir, J.D. Graaff, Microb. Ecol. Health Dis., 1993, 6, 237.
- W.J. Loesche, Microbiol. Rev. 1986, 50, 353.
- J.J. de Soet, T.J. van Steenbergen, J. de Graaff, Alpe Adria Microbiol. J., 1992, 3, 127.
- T. Oho, Y. Yamashita, Y. Shimazaki, M. Kushiyama, T. Koga, Oral Microbiol. Immunol., 2000, 15, 258.