Research Article - Der Pharma Chemica ( 2018) Volume 10, Issue 1
Triterpenes from Styrax benzoin
1Chemistry of Natural Compounds Department, National Research Center, Pharmaceutical Industries Research Division, El-Buhouth St., Dokki, Cairo 12311, Cairo, Egypt
2Department of Chemistry, College of Science & Arts, Najran Universuty, Najran 1988, Kingdom of Saudi Arabia
- Corresponding Author:
- Mohamed H Abd El-Razek
Chemistry of Natural Compounds Department, National Research Center
Pharmaceutical Industries Research Division, El-Buhouth St.
Dokki, Cairo 12311, Cairo, Egypt
Abstract
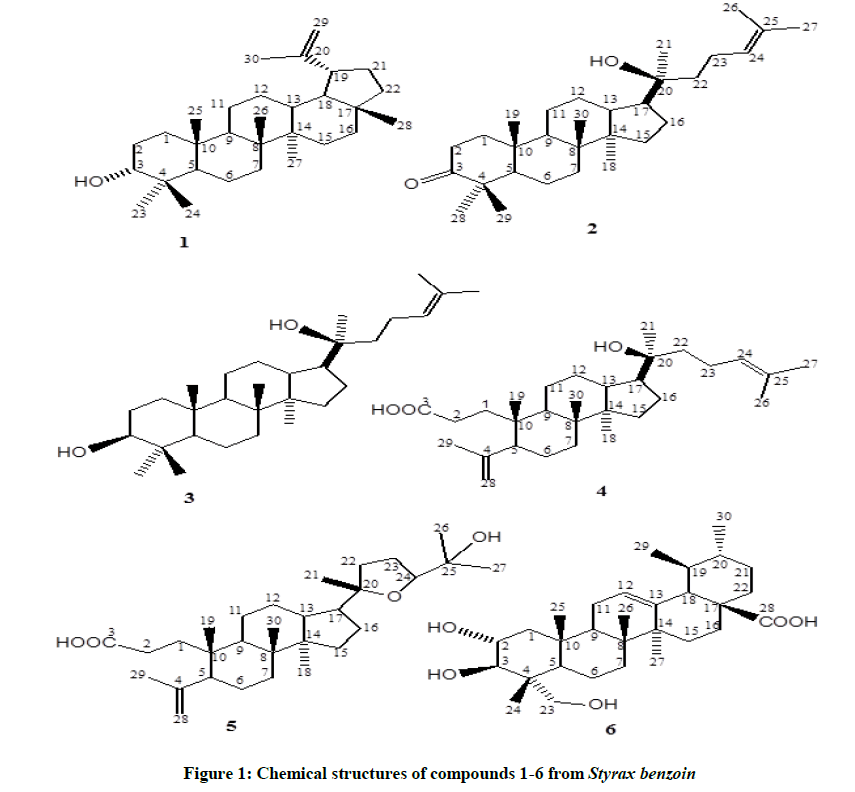
One lupeol, epilupeol (1), four dammaranes, dipterocarpol (2), dammarandiol-II (3), dammarenolic acid (4), and eichlerianic acid (5) and one oleanane, asiatic acid (6), were isolated from the chloroform extract of the gum resin of Styrax benzoin (Styracaceae). The data of Proton Nuclear Magnetic Resonance (1H-NMR) and Carbon Nuclear Magnetic Resonance (13C-NMR) chemical shifts for dammarenolic acid (4) [20Shydroxy- 3,4-seco-4(28),24-dammaradien-3-oic acid] were already reported before, however, the complete assignments have ever been revealed. All known compounds were identified by spectroscopic methods and isolated from the family Styracaceae for the first time. Among them 4, 5 and 6 show significant inhibitory effects on superoxide anion generation by human neutrophils in response to fMLP/CB with IC50 0.52 ± 0.04, 2.44 ± 0.61 and 4.76 ± 0.85 µg/ml, respectively. On the other hand, these compounds have minor inhibition of nitric oxide formation in lipopolysaccharide-induced RAW264.7 macrophages. This is the first study showing anti-inflammatory activity of these compounds. The data of 1H and 13C-NMR chemical shifts for dammarenolic acid (4) [20S-hydroxy-3,4-seco-4(28), 24-dammaradien-3-oic acid] were already reported before, however, the complete assignments have ever been revealed. All known compounds were identified by spectroscopic methods and isolated from the family Styracaceae for the first time.
Keywords
Styrax benzoin, Styracaceae, Resin, Triterpenes
Introduction
Styracaceae is a family constituted of small trees and shrubs, mostly native to tropical and subtropical regions [1]. The genus Styrax is different from other genera of this family due to the production of resinous material, usually secreted when the barks and trunks are injured by sharp objects [2]. This resin, in the past considered a miraculous remedy in several parts of Asia and America, has been used in traditional medicine to treat inflammatory disease and was also used by Romans, Egyptians, Phoenicians and Ionians as incense and in therapeutic uses [3]. Styrax species contain egonol, a natural benzofuran, which is known to be an effective pyrethrum synergist [4].
Earlier chemical studies on several Styrax plant species have revealed them to be a rich source of arylpropanoids, lignans, triterpenoids and saponins [5-15]. It was reported that triterpenoids from the resin of Styrax tonkinensis show antiproliferative and differentiation effects in human leukemia HL-60 cells [16]. Also, benzoic acid-(4-hydroxy-3-methoxy-trans-cinnamyl ester) and the benzoate of trans-p-coumaryl alcohol were reported from Styrax benzoin [17]. Our research on this source led to the isolation of six triterpenes, epilupeol 1 [18], dipterocarpol 2 [19], dammarandiol-II 3 [19], dammarenolic acid 4 [20], eichlerianic acid 5 [20] and asiatic acid 6 [21], from the chloroform extract of the gum resin of Styrax benzoin. Among them 4, 5, and 6 showed significant inhibitory effects on superoxide anion generation by human neutrophils in response to fMLP/CB. The IC50 of 4, 5 and 6 were found to be 0.52 ± 0.04, 2.44 ± 0.61, and 4.76 ± 0.85 µg/ml, respectively. On the other hand, these compounds have minor inhibition of nitric oxide formation in lipopolysaccharide-induced RAW264.7 macrophages.
The earliest systematic investigation of dammar resin was reported by Zinke and Unter-Kreuter [22], who described a compound called dammarolic acid 4, a name also given to a different compound isolated by Mladenovic and Barkovic [23] and subsequently shown by Brewis and Halsall [24] to be asiatic acid, Bisset et al. [25], reported an interesting study in which the shoreic and dammarenolic acid content, as well as the neutral terpene content of the resin was used as a means of chemotaxonomic differentiation of the genus Sborea from related subgenera. It was also reported that damarenolic acid had in vitro antiviral activity [26]. A detailed spectroscopic analysis of dammarolic acid 4 was conducted in order to assign unambiguously all hydrogens and carbons in their NMR spectra.
Materials and Methods
General experimental procedure
Melting points were determined using a Yanagimoto micro-melting point apparatus and are uncorrected. IR spectra were measured on a Mattson Genesis II spectrophotometer. The optical rotation was determined on a Perkin-Elmer Model 341 Polarimeter. Low resolution EIMS were collected on a Joel JMS-SX/SX 102A mass spectrometer at 70 eV. NMR spectra were obtained on a Bruker-400 FT NMR spectrometer. Chemical shifts were expressed in δ (ppm) relative to the solvent signal as internal standard and coupling constants (J) were given in hertz. TLC was carried out on precoated Silica gel 60 F254 (Merck, art. 5715). Column chromatography was performed on Silica gel 60 (Merck, 40-63 and 63-200 µm) and Sephadex LH-20 (Sigma, 25-100 µm) HPLC was carried out a JASCO PU-980 apparatus equipped with a JASCO UV-970 detector. Hypersil ODS 5 µm (250 × 4.6 mm i.d.) and preparative ODS 5 µm (250 × 21.2 mm i.d.) columns were used for analytical and preparative purposes.
Sample collection
The gum of Styrax benzoin Dryand., ‘Jurur’, was a chocolate brown colored resin with some lighter areas, the particle size was about 1 cm. The resin was harvested from the outside of the bark and therefore, exposed to light for several days. It was imported from India and obtained from El-Gomhouria Co., for Drugs and Equipments, 13 Mahmoud Basuny St., El-Tahrir Sq., Cairo, Egypt.
Isolation of the chemical constituents of the gum of S. benzoin dry
The gum resin of S. benzoin (500 g) was exhaustively extracted at room temperature with CHCl3 over 2 days. After filtration the combined extracts (6 L) were evaporated under reduced pressure to give 150 g of a brown oily material. 20 g of this oily residue were chromatographed over silica gel using a n-hexane/ EtOAc and a CHCl3/MeOH gradient to get thirty-six fractions. Fractions were combined based on their TLC pattern to yield subfraction designated as SB1-SB6. Subfraction SB1 (500 mg) was chromatographed over silica gel eluting with n-hexane/ EtOAc (97:3) to get a white crystalline compound 1 (60 mg).
Subfraction SB2 (700 mg) was recrystallized from n-hexane/EtOAc (9:1) followed by column chromatography over silica gel eluting with n-hexane/ CH2Cl2 (6:1) to give compound 2 (31 mg). Subfraction SB3 (550 mg) was applied to a silica gel column chromatography n-hexane/ EtOAc (5:1) and further purified on Sephadex LH-20 column using n-hexane/EtOAc (1:5) to obtain 3 (25 mg). Subfraction SB4 (400 mg) which was chromatographed on silica gel elution with CHCl3 as eluent to obtain four subtraction’s (SB4-1 ~ SB4-4). Subfraction SB4-4 (120 mg) was further purified on a RP-HPLC [Hypersil ODS 5 µm (250 × 4.6 mm i.d.)] with an UV detection at λ=224 nm, elution with MeOH/H2O (4:1) to receive compound 4 (40 mg, Rt=55 min). Subfraction SB5 (250 mg) was repeatedly chromatographed on Sephadex LH-20 column using (EtOAc) to get seven subfraction (SB5-1 ~ SB5-7). Subfraction SB5-6 (88 mg) was subjected to a RP-HPLC [ODS 5 µm (250 × 21.2 mm i.d.)] with an UV detection at λ=224 nm, elution with MeOH/ H2O (3:1) to give compound 6 (22 mg, Rt=33 min) and compound 5 (33 mg, Rt=55).
All isolated compounds were identified with a series of 1-D and 2-D NMR techniques, including 1H, 13C, DEPT, COSY, HMQC, HMBC, as well as by comparison of physical and spectroscopic properties with already published data. A complete assignment of 1H and 13C-NMR data of compound 4 is until now not published.
Results and Discussion
Current study has been designed to estimate the possible antimicrobial potential of six triterpenes were isolated from the chloroform extract of the gum resin of Styrax benzoin (Styracaceae). Considering these results, it is concluded that S. benzoin can be used as a potential source for the antimicrobial agents. The CHCl3 extract of the gum resin S. benzoin was purified by column chromatography using silica gel as well as a combination of chromatography and Sephadex LH-20 as well as reverse phase HPLC to yield six triterpenes 1-6. The structures were elucidated by analysis of the NMR spectra and comparison of the physical and spectral data with those of literature (Table 1 and Figure 1).
| Compounds | fMLP/CB | LPS |
|---|---|---|
| Inh %a or IC50 (mg/ml)b | Inh % | |
| Epilupeol (1) | -3.23 ± 2.69a | 9.74 ± 6.16 |
| Dipterocarpol (2) | 1.47 ± 1.78a | 12.10 ± 4.71 |
| Dammarandiol-II (3) | -3.02 ± 1.04a | 23.80 ± 4.09 |
| Dammarenolic acid (4) | 0.52 ± 0.04b | 5.19 ± 2.10 |
| Eichlerianic acid (5) | 2.44 ± 0.61b | 2.02 ± 3.56 |
| Asiatic acid (6) | 4.76 ± 0.85b | 12.54 ± 6.14 |
Table 1: Inhibitory effects of compounds 1-6 on superoxide anion generation by fMLP/CB-activated human neutrophils and nitric oxide generation by LPS-induced RAW264.7 macrophages
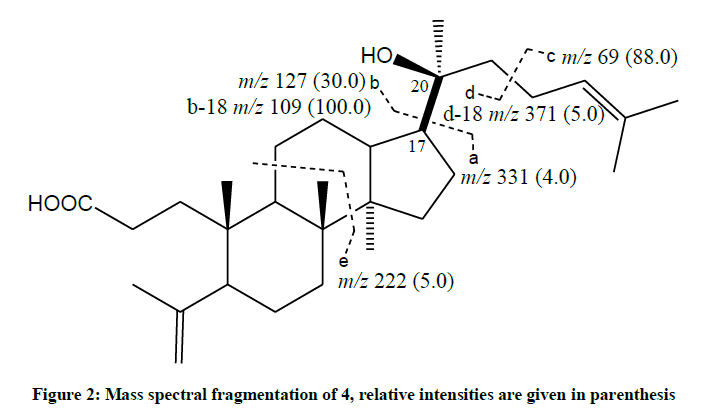
Compound 4 was isolated as a white powder, m.p 146-148°C, [α] D25: +131.0 (c, 1.0). Its EIMS and FABMS did not show the molecular ion peak, instead, both exhibited an intense peak at m/z=440, due to the elimination of H2O from the [M]+. An analysis of the EIMS spectrum implied the tetracyclic nature of 4. Peaks at m/z=249, 207 and 205 indicate the A, B and C rings of the tetracyclic skeleton [26], limiting the choice to the dammarane ring system and excluding the euphane or the lanostane type skeleton [27]. Peaks at m/z=331 and 127 are due to the rupture of the bond between C-17 and C-20 [28]. The peak at m/z=127 corresponds to the side chain ([C8H15O]+) while m/z=109 corresponds to the side chain less water see Figure 2.
The IR spectrum indicates that a carbonyl group of a carboxylic acid (1708 cm-1) and a double bond (1640 cm-1) are present. The 13C-NMR spectrum of 4 (in CDCl3), shows 30 carbons, DEPT experiments proofed the presence of seven methyls, eleven methylenes (one of them was assigned to be vinyl exo-methylene), five methines (One of them attributed to olefinic double bond), and seven quaternary carbons (including one carbon signal appears at δC=179.3, suggesting a carboxylic acid residue, one tertiary alcoholic carbon centered at δC=75.6, and two trisubstituted double bonds) which is indicating the molecular formula of C30H50O3. The 1H-NMR spectrum of 4 displays signals for seven tertiary methyl groups, two of which were vinylic in nature at δH=1.65 and 1.59, one was angular methyl at δH=1.70, one tertiary alcoholic at δH=1.11 and the other three methyls located at δH=0.97, 0.86 and 0.82. The spectrum also showed triplet signal for the trisubstituted vinylic proton at δH=5.08, in addition to two oxo-olefinic protons at δH=4.81 and 4.63.
The configuration at C-20 was determined to be S-isomer, according to Asakawa et al. [29]. The chemical shifts differences of the carbons around the C-20 epimers such as C-21 and C-22 used for determination of the C-20 configuration. The resonance of the (20S)-epimer for C-21 is more deshielded while C-22 is more shielded than that of the (20R)-epimer. All one bond connections between 13C and 1H-NMR were elucidated by HMQC experiment. In order to obtain further assignments of NMR signals, the 1H-1H COSY spectrum was measured. Owing to the two signals at δH=2.34 and 2.15 showed geminal coupling and strong vicinal coupling with the multiplet signal at δH=1.56, this supported the proponic acid side chain. Also, there was a coupling between angular methyl signal at δH=1.70 with the exo-methylene protons caused by allylic long range coupling. The covalent connectivity and planar structure of 4 was determined from HMBC analysis. From the chemical shift of the carbonyl carbon of the group C-3 (δC=179.3) showed HMBC connectivity with the methylene protons of H-2 at δH=2.34, 2.15 and H2-1 (δH=1.56, m). H2-1 protons showed HMBC correlations with C-2 (δC=28.3), C-3 (δC=179.3), C-5 (δC=50.7), C-10 (δC=39.0) and C-19 (δC=41.0). Long-range correlation between hydrogen atoms attached C-19 (δH=20.1) and C-5 (δC=50.7) indicated that C-19 is a β=oriented angular methyl group in position 19.
The correlations between the protons at δH=4.63, 4.81 of C-28 (δC=113.4) and C-4 (δC=147.4) can be seen in the HMBC spectrum. This, coupled with the correlation between Ha, b (C-28) and C-29 (δC=23.2), suggests that there is a 1-methylvinyl group in the side chain. By the correlations between the methine carbon C-5 (δC=50.7) and the hydrogen atoms attached to C-28 and C-29, they can be deduced that the 1-methylvinyl group is attached to C-5. The primary carbon atom C-30 had a chemical shift at δH=0.97 showed HMBC correlations with the methylene carbon C-7 (δC=33.8), the methine carbon C-9 (δC=41.0) and quaternary carbon C-14 (δC=50.6), indicated that the chemical shift at δC=40.0, a quaternary carbon atom, should belong to C-8.
In our experiment, a methyl group (C-18) with chemical shifts δC=16.3 and δH=0.86 showed a single peak in the 1D 1H-NMR spectrum. This suggests that the methyl group is attached to C-14, confirmed by the correlation between the hydrogen of this methyl with two quaternary carbon atoms C-8 (δC=40.0), C-14 (δC=50.6), the methine carbon C-13 (δC=42.3) and the methylene carbon C-15 (δC=31.1). From another correlation between C-13 (δC=42.3) and the hydrogen atoms attached to the methylene (δC=24.7), the chemical shift of position 16 was determined. The methyl signal at (δC=25.2, δH=1.11), which fits the characteristic of methyl group attached to tertiary alcoholic carbon atom at δC=75.6, which in turn have correlations with the methine carbon at δC=49.6 (C-17) and the methylene carbon at δC=40.6 (C-22) in the HMBC spectrum. The later carbon provides us the beginning for assignment the isoproplene side chain. As the HMBC cross peaks from the signals included a protonated olefinic carbon at δC=124.6 (C-24) and a non-protonated olefinic carbon at δC=131.5. Me-26 (δH=1.59) and Me-27 (δH=1.65) protons correlated with C-24 (δC=124.6) and C-25 (δC=131.5).
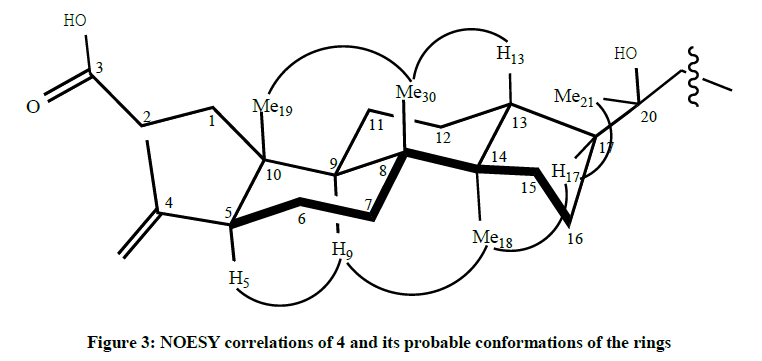
The H-24 olefinic proton (t, δH=5.08) showed correlations with C-25 (δC=131.5), C-26 (δC=17.7), C-27 (δC=25.7), and C-22 (δC=40.6). The assignments of δC and δH of the dammarenolic acid have been completed. NOESY spectra can give the stereo character of chemical compounds. The relative stereochemistry of rings system was clarified by NOESY spectrum as shown in Figure 3. It is known that the angular methyl group at site 19 is on the β-face. The fact that there is no correlation between the hydrogen atoms attached to C-19 and the hydrogen atom attached to C-5 suggests that this hydrogen atom is on the α-face. The correlation between Me-19 and Me-30 suggests the conformation is β. The configuration of C-17 was assigned to be S-isomer according to the above data, also the correlations between H-17 and the methyls signals of H- 18 and H-21 were observed. Furthermore, strong interactions between H-9 and H-30 and H-5 were detected.
NMR correlation data of compound 4
Dammarenolic acid (4) (20S-hydroxy-3,4-seco-4 (28), 24-dammaradien-3-oic acid): White crystal from n-hexane; m.p. 146-148°C; [α] 25D : +131.0° (c, 1.0); EI-MS m/z (rel.int.%): 440 (9), 371 (5), 331 (4), 222 (5), 127 (30), 109 (100), 69 (88); UV (MeOH), λmax (log ε)=218 (3.27); IR (KBr), υmax=3408 broad (OH), 2931 broad (CH), 1708 (C=O), 1640 (C=C), 1667 (di-Me-C=C) cm-1; 1H-NMR (CDCl3, 400 MHz), δ=5.08 (1H, t, J=8Hz, H-24), 4.81 (1H, brs, H-28a), 4.63 (1H, brs, H-28b), 2.34 (1H, ddd, J=16, 12, 8 Hz, H-2a), 2.15 (1H, ddd, J=16, 12, 8 Hz, H-2b), 1.95 (1H, dd, J=12, 3 Hz, H-5), 1.84 (1H, m, H-6α), 1.77 (1H, m, H-12β), 1.74 (2H, m, H-17), 1.70 (3H, s, H-29), 1.65 (1H, s, H-27), 1.62 (1H, m, H- 13), 1.59 (3H, s, H-26), 1.56 (2H, m, H-1), 1.47 (3H, m, H-9, H-7α, H-22), 1.40 (1H, m, H-15), 1.37 (1H, m, H-16), 1.36 (1H, m, H-11), 1.35 (1H, m, H-6), 1.34 (1H, m, H-12), 1.22 (1H, m, H-11), 1.17 (1H, m, H-7), 1.11 (3H, s, H-21), 1.04 (1H, m, H-15), 0.97 (3H, s, H-30), 0.86 (3H, s, H-18), 0.82 (3H, s, H-19); 13C-NMR (CDCl3, 100 MHz), δ=179.3 (C-3), 147.4 (C-4), 131.5 (C-25), 124.6 (C-24), 113.4 (C-28), 75.6 (C-20), 50.7 (C-5), 50.6 (C-14), 49.6 (C-17), 42.3 (C-13), 41.0 (C-9), 40.6 (C-22), 40.0 (C-8), 39.0 (C-10), 34.2 (C-1), 33.8 (C-7), 31.1 (C-15), 28.3 (C- 2), 27.4 (C-12), 25.7 (C-27), 25.2 (C-21), 24.7 (C-16), 24.6 (C-6), 23.2 (C-29), 22.5 (C-23), 22.0 (C-11), 20.1 (C-19), 17.7 (C-26), 16.3 (C-18), 15.3 (C-30).
Conclusion
Compounds 4, 5, and 6 showed significant inhibitory effects on superoxide anion generation by human neutrophils in response to fMLP/CB with IC50 of 0.52 ± 0.04, 2.44 ± 0.61, and 4.76 ± 0.85 µg/ml, respectively. On the other hand, these compounds have minor inhibition of nitric oxide formation in lipopolysaccharide-induced RAW264.7 macrophages. Previously, it has been reported that dammarenolic acid (4) possessed antifungal activity against Colletotrichum musae [30]. In addition, compounds 4 and 5 show cytotoxicity against Human Telomerase Reverse Transcriptase-Retinal Pigment Cells (hTERT-RPE1) [31]. Asiatic acid 6 shows antiproliferative activity against human breast cancer MCF-7 cells [32] and human fibroblast ATCC-CRL-2450 cells [33], besides of anti-inflammatory activity against ICR mouse [34]. According to the above results, S. benzoin is a useful material and a good source for the isolated triterpenes. It is a meaningful. The material can be developed a good resource of these isolated compounds, and potential to be a candidate for anti-inflammatory natural products development.
References
- M. Pio Correa, Dictionary of Useful Medicinal Plants of Brazil and Cultivated Exotic, Ministry of Agriculture, Rio de Janeiro, Brazil, 1931, 2, 363.
- A.F. Costa: Farmacognosia, 2nd Edi., Lisboa. Calouste Gulbenkia Foundation., 1968, 1, 737.
- V. Vardar, S. Oflas. Qual. Plant Mater. Veg., 1973, 22, 145-151.
- P.M. Pauletti, A.R. Ara´ujo, M.C.M. Young, A.M. Giesbrecht, V.S. Bolzani, Phytochemistry., 2000, 55, 597-601.
- H.I. Moon, M.R. Kim, E.R. Woo, J.H. Chung, Bio.Pharm. Bull., 2003, 28, 2003-2006.
- Q.L. Li, B.G. Li, H.Y Qi, X.P. Gao, G.L. Zhang, Planta Medica., 2005, 71, 847-851.
- T. Kinoshita, Y. Haga, S. Narimatsu, Heterocycles., 2005, 65, 1471-1480.
- H.L. Teles, J.P Hemerly, P.M. Pauletti, J.R.C Pandolfi, A.R Araujo, S.R Valentini, M.C.M. Young, V.D.S Bolzani, D.H.S. Silva, Nat. Prod. Res., 2005, 19, 319-323.
- H.I. Moon, D.W. Seo, K.H. Kim, K.H. Cho, H.C. Eun, J.H. Chung, J. Ethnopharmacol.,2005, 97, 567-571.
- M.R. Kim, H.I. Moon, J.H. Chung, Y.H. Moon, K.S. Hahm, E.R. Woo, Chem. Pharm. Bull., 2004, 52, 1466-1469.
- B.S. Min, S.R. Oh, K.S. Ahn, J.H. Kim, J. Lee, D.Y. Kim, E.H. Kim, H.K. Lee, Planta Medica., 2004, 70, 1210-1215.
- B.S. Min, M.K. Na, S.R. Oh, K.S. Ahn, G.S. Jeong, G. Li, S.K. Lee, H. Joung, H.K. Lee, J. Nat. Prod., 2004, 67, 1980-1984.
- M.R. Kim, H.H. Lee, K.S. Hahm, Y.H. Moon, E.R. Woo, Arch. Pharm. Res., 2004, 27, 283-286.
- Y.Y. Akgul, H. Anil, Fitoterapia.,2003, 74, 743-745.
- Y. Yayla, O. Alankus-Caliskan, H. Anil, R.B. Bates, C.C. Stessman, V.V. Kane, Fitoterapia., 2002, 73, 320-326.
- F. Wang, H. Hua, Y. Pei, D. Chen, Y. Jing. J. Nat. Prod.,2006, 69, 807-810.
- S.A. Popravko, I.V. Sokolov, I.V. Torgovm, Khim. Prir. Soedin., 1984, 2, 152-160.
- L. Carvalho, J. Seita, Fitoterapia.,1995, 66, 273.
- M. Tori, R. Matsuda, M. Sono, Y. Asakawa, Magn. Reson. Chem., 1988, 26, 581-590.
- D. Roux, M.T. Martin, M.T. Adeline, T. Sevenet, A.H.A. Hadi, M. Pais, Phytochemistry.,1998, 49, 1745-1748.
- Y. Sashida, K. Ogawa, N. Mori, T. Yamanouchi, Phytochemistry., 1992, 31, 2801-2804.
- A. Zinke, E. Unter-Kreuter, J. Chem. Soc., 1918, 116, 166.
- M. Mladenovic, D. Barkovic, Monatshefte fǘer Chemie., 1940, 73, 206-213.
- S. Brewis, T.G. Halsall, J. Chem. Soc., 1961, 646-650.
- N.G. Bisset, V. Chavanel, P.J. Lantz, R.E. Wolff, Phytochemistry., 1971, 10, 2451-2463.
- B.I. Poehland, B.K. Carté, T.A. Fracis, L.Y. Hyland, H.S. Allaudeen, N. Troupe, J. Nat. Prod.,1987, 50, 706-713.
- F.J.Q. Monte, J.P. Kintzinger, R. Braz-Filho, Magn. Reson. Chem., 1998, 36, 381-384.
- A.M. Duffield, Appleton Century-Crofts, New York, 1969, 3, 107.
- J. Asakawa, R. Kasai, K. Yamasaki, O. Tanaka,Tetrahedron., 1977, 33, 1935-1939.
- E. Lahlou, N. Hirai, T. Kamo, M. Tsuda, H. Ohigashi. Biosci. Biotechnol. Biochem.,2001, 65, 480-483.
- J.F. Rivero-Cruz, H.B. Chai, L.B.S. Kardono, F.M. Setyowati, J.J. Afriatini, S. Riswan, N.R. Farnsworth, G.A. Cordell, J.M. Pezzuto, S.M. Swanson, A.D. Kinghorn, J. Nat. Prod., 2004, 67, 343-347.
- Y.L. Hsu, P.L. Kuo, L.T. Lin, C.C. Lin, J. Pharmacol. Exp. Ther., 2005, 313, 333-344.
- C.D. Coldren, P. Hashim, J.M. Ali, S.K. Oh, A.J. Sinskey, C.K. Rha, Planta Med.,2003,69, 725-731.
- Y.M. Fan, L.Z. Xu, J. Gao, Y. Wang, X.H. Tang, X.N. Zhao, Z.X. Zhang, Fitoterapia., 2004, 75, 253-260.