Research Article - Der Pharma Chemica ( 2017) Volume 9, Issue 3
A Novel One Pot Facile Synthesis of 1,2,4-Triazolo-1,3,4-Thiadiazepino Fused Coumarins and Their Antimicrobial and Antituberculosis Activity Studies
Dharati S Patel*, Nilesh J Patel, Parin V Shaikh and DI BrahmbhattDharati S Patel, Department of Chemistry, Sardar Patel University, Vallabh Vidyanagar, Gujarat, India,
Abstract
A novel series of [1,2,4]triazolo[3’,4’:2,3][1,3,4]thiadiazepino[7,6-b]coumarins (3a-l) was synthesized by the reaction of appropriate 4-chloro 3-formyl coumarins (1a-c) with various 4-amino-5-substituted-3-mercapto-1,2,4-triazoles (2a-d) in ethanol in the presence of a catalytic amount of K2CO3 under refluxing conditions. The structures of the synthesized compounds were established by elemental analysis and spectral data like IR, 1H-NMR, 13C-APT and mass analysis. The synthesized compounds were screened for their antimicrobial and antituberculosis activity. Among all the synthesized compounds, the compounds 3a, 3e, 3j and 3k were found to be more active against tested pathogens.
Keywords
3-mercapto-1,2,4-triazole, 1,3,4-thiadiazepines, Coumarins, Antituberculosis, Antifungal activity
Introduction
1,2,4-Triazoles, a five member heterocyclic compounds with three nitrogen atom in the ring are best known class of triazoles and these compounds have drawn a special attention due to their promising biological activities such as antimicrobial [1], antiinflammatory [2], antiviral [3], analgesic [4] and anticancer [5]. In addition to these important biological applications, 4-amino-1,2,4- triazole-3-thiols are also of greater utility in the preparation of organic compounds by heterocyclization. The amino and mercapto groups are ready-made nucleophilic centers for the synthesis of condensed heterocyclic rings such as triazolothiadiazoles, triazolothiadiazines, triazolotetrazines and triazolothiadiazepines [6,7].
From the literature survey, it is revealed that 1,3,4-thiadiazepines are better therapeutic agents due to the presence of the -N=C-S group. Various groups of researcher have reported their outstanding biological activities such as, antiviral [8], antimicrobial [9], antitumor [10], antidepressant [11], anticonvulsant [12], anti HIV [13], anti-inflammatory [14] and antifungal [15]. These compounds are not only known for their potent biological activity but they are also known for their excellent charge generating property [16].
During past few decades, considerable reports are documented in literature for the efficiency of triazolo thiadiazepines as good therapeutic agents. They are found to possess good to excellent in vitro antibacterial [17], antifungal [18] and antitubercular activity [19]. Based on these compounds many potential drugs have been documented, particularly in cancer and virus research [20,21].
During our literature survey on the further fusion of triazolo thiadiazepines with other heterocycle we noticed that researcher have reported various furano fused triazolo thiadiazepines [22] benzopyrano fused triazolo thiadiazepines [23], quinolino fused triazolo thiadiazepines [24], hydrazono triazolo thiadiazepines [25], pyrazolo fused triazolo thiadiazepines [26]. All these heterocyclic fused compounds are also reported to have excellent biological activities. The survey also revealed that so far no chemists have made efforts to synthesize triazolo thiadiazepino fused coumarins and therefore in the present work it was thought worthwhile to synthesize triazolo thiadiazepino fused coumarins via simple condensation reaction and therefore herein we report synthesis of various [1,2,4]triazolo-[3’,4’:2,3][1,3,4]-thiadiazepino[7,6-b]coumarins.
Experimental
p>All the melting points are uncorrected. All reactions were performed with commercially available reagents and they were used without further purification. Organic solvents were purified by standard methods and stored over molecular sieves. All the IR spectra (KBr disc) were recorded on Shimadzu FT-IR 8400-S spectrometer. 1H-NMR and 13C APT spectra were recorded on Bruker Advance 400 spectrometer operating at 400 MHz for 1H-NMR and 100 MHz for 13C-APT. The chemical shift (δ) is reported in ppm using chloroform-d as a solvent and calibrated standard solvent signal. Mass spectra were recorded on Shimadzu QP 2010 spectrometer. Elemental analysis was carried out on Perkin-Elmer 2400 C-H-N-S-O Analyzer Series-II. Column chromatography was performed with silica gel 60-120 mesh (Merck, Mumbai, India.). All the compounds were routinely checked for completion of the reaction on silica gel 60 F254 TLC plates and their spots were visualized by exposure to a UV lamp, iodine vapour or KMnO4 reagents.General method for the synthesis of [1,2,4]triazolo[3’,4’:2,3][1,3,4]thiadiazepino[7,6-b]coumarins(3a-l)
A solution of appropriate 4-amino-5-substituted-3-mercapto-1,2,4-triazoles (0.025 mol) (2a-d) in ethanol (5 ml) was taken in 100 ml round bottom flask. To this catalytical amount of K2CO3 (0.03 mol) was added and stirred for 30 min at room temperature. The after an appropriate 4-chloro-3-formyl coumarin (1a-c) (0.025 mol) in ethanol (15 mL) was added dropwise followed by addition of 2-3 drops of acetic acid to above well stirred solution during 10 min at room temperature. The reaction mixture was then refluxed for 10 min and further stirred at room temperature for two hrs. The solid obtained was filtered out and washed with hexane and dried. The compounds were obtained as yellowish colored solid, which were recrystallized from chloroform-hexane.
The structure of all the synthesized compounds (3a-l) were confirmed by their analytical and spectral data like IR, 1H-NMR,13C-APT, elemental analysis and representative mass spectral data given below.
3-Methyl-[1,2,4]triazolo[3’,4’:2,3][1,3,4]thiadiazepino[7,6-b]coumarins(3a)
Yellow solid, Yield= 85%; M.P; 198-201 Anal. Calcd. For C13H8N4O2S: C, 54.92; H, 2.84; N, 19.71, %. Found: C, 54.90; H, 2.80; N, 19.69 %. IR (KBr, νmax, cm-1); 756(C-S-C Stretching), 1714 (C=O stretching of -lactone of coumarin), 1586 (aromatic C=C stretching), 1476 (aromatic C=N stretching), 2852(aliphatic C-H Stretching), 3043 (aromatic C-H stretching). 1H NMR (400MHz, CDCl3, δ): 2.47 (3H, s, CH3), 7.20-8.39 (5H, m, aromatic protons). 13C APT (100MHz, CDCl3, δ): 20.88 (CH3), 112.80 (C), 113.76(C), 115.83(C), 117.54 (C), 125.24(CH), 128.77(CH), 130.48(CH), 134.98(CH), 136.63(C), 138.50(CH), 155.90(C), 160.43(CO of coumarin). The mass spectrum of compound showed M+ peak at 284 (18%) (m/z%) along with some other fragments peaks at 257(23%), 77(12%), 57(11%), 44(100%) etc. The appearance of molecular ion peak at 284 mass unit supports the structure of compound 3a.
3,9-Dimethyl-[1,2,4]triazolo[3’,4’:2,3][1,3,4]thiadiazepino[7,6-b]coumarins (3b)
Yellow solid; yield=75%; mp 193-196°C; Anal. Calcd. For C14H10N4O2S: C, 56.34; H, 3.36; N, 18.78%. Found: C, 56.37; H, 3.38; N, 18.75%. IR (KBr, νmax, cm-1); 750(C-S-C Stretching), 1714 (C=O stretching of -lactone of coumarin), 1586 (aromatic C=C stretching), 1476 (aromatic C=N stretching), 2852(aliphatic C-H Stretching), 3043 (aromatic C-H stretching). 1H NMR (400 MHz, CDCl3, δ): 2.64 (6H, s, 2 × CH3), 7.33-8.42 (4H,m, Ar-H). 13C APT (100MHz, CDCl3, δ): 20.85 (CH3), 23.14 (CH3), 115.14(C), 117.85(CH), 119.40(C), 123.15(C) 124.55(CH), 128.05(C), 129.45 (CH), 131.51 (CH), 135.95(C), 138.43(C), 152.80(C), 162.61 (CO of coumarin).
3-Methyl-9-Chloro-[1,2,4]triazolo[3’,4’:2,3][1,3,4]thiadiazepino[7,6-b]coumarins (3c)
Pale yellow solid; yield=79%; mp 200-202°C; Anal. Calcd. For C13H7N4O2SCl: C, 48.99; H, 2.21; N, 11.12%. Found: C, 48.96; H, 2.18; N, 11.08%. IR (KBr, νmax, cm-1); 752(C-S-C Stretching), 1719 (C=O stretching of -lactone of coumarin), 1584 (aromatic C=C stretching), 1473 (aromatic C=N stretching), 2854 (aliphatic C-H Stretching), 3041 (aromatic C-H stretching). 1H NMR (400MHz, CDCl3, δ): 2.35 (3H, s, CH3), 7.33-8.38 (4H, m, Ar-H). 13C APT (100MHz, CDCl3, δ): 20.95 (CH3), 115.34(C), 117.35(CH), 119.50(C), 123.25(C), 125.55(CH), 128.69(C), 129.99(CH), 131.25(C), 134.95(C),138.43(CH).157.80(C),161.81(CO of coumarin).
3-Phenyl-[1,2,4]triazolo-[3’,4’:2,3][1,3,4]-thiadiazepino[7,6-b]coumarins (3d)
Pale yellow solid; yield=79%; mp 212-214ºC; Anal. Calcd. For C18H10N4O2S: C, 62.42; H, 2.91; N, 16.18 %. Found: C,62.40; H, 2.89; N, 16.13%. IR (KBr, νmax, cm-1); 756(C-S-C Stretching), 1715 (C=O stretching of -lactone of coumarin), 1585 (aromatic C=C stretching), 1476 (aromatic C=N stretching), 3045 (aromatic C-H stretching). 1H NMR (400 MHz, CDCl3, δ):7.21-8.95 (10H,m, Ar-H). 13C APT (100 MHz, CDCl3, δ): 114.55(C), 115.60(C), 115.65(C), 117.12(CH) 124.05(CH), 125.36(CH), 125.99(CH), 128.95(CH), 129.55(C), 130.01(CH), 133.70(CH), 141.94(C), 143.56(C), 150.88(C), 156.66(C), 161.56(CO of coumarin).
3-Phenyl-9-methyl-[1,2,4]triazolo-[3’,4’:2,3][1,3,4-]thiadiazepino[7,6-b]coumarins (3e)
Pale yellow solid; yield=75%; mp 230-232ºC; Anal. Calcd. For C19H12N4O2S: C,63.32; H, 3.36; N, 15.55 %. Found: C,62.32; H, 3.35; N, 15.50%. IR (KBr, νmax, cm-1); 751(C-S-C Stretching), 1719 (C=O stretching of -lactone of coumarin), 1581 (aromatic C=C stretching), 1475 (aromatic C=N stretching), 2956 (aliphatic C-H stretching), 3041 (aromatic C-H stretching). 1H NMR (400MHz, CDCl3, δ):2.34(3H, s, CH3) 7.30-8.52 (9H, m, Ar-H). 13C APT (100MHz, CDCl3, δ): 20.68(CH3), 112.80(C), 113.70(C), 115.63(C), 117.52(CH), 118.46(C), 125.04(CH), 128.70(CH), 130.43(CH), 135.03(C), 136.33(CH), 138.90(C), 143.43(CH), 145.73(CH), 153.48(C), 155.04(C), 163.98 (CO of coumarin).
3-Phenyl 9-Chloro-[1,2,4]triazolo-[3’,4’:2,3][1,3,4]-thiadiazepino[7,6-b]coumarins (3f)
Pale yellow solid; yield=80%; mp 215-217°C; Anal. Calcd. For C18H9N4O2SCl: C, 56.77; H, 2.38; N, 14.71 %. Found: C, 56.72; H, 2.35; N, 14.68 %. IR (KBr, νmax, cm-1); 754(C-S-C Stretching), 1719 (C=O stretching of -lactone of coumarin), 1582 (aromatic C=C stretching), 1476 (aromatic C=N stretching), 3043 (aromatic C-H stretching). 1H NMR (400 MHz, CDCl3, δ): 7.32-8.59 (9 H,m, Ar-H). 13C APT (100 MHz, CDCl3, δ): 111.57(C), 113.80(C), 114.70(C), 115.63(C), 117.52 (CH), 118.90(C), 125.04(CH), 128.70(CH), 130.43(CH), 135.03(CH), 136.33(C),138.98(CH), 143.43(CH), 153.04(C), 156.98(C),162.48(CO of coumarin).
3-Pyridyl-[1,2,4]triazolo-[3’,4’:2,3][1,3,4]-thiadiazepino[7,6-b]coumarins (3g)
Pale yellow solid; yield=82%; mp 252-254˚C; Anal. Calcd. For C17H9N5O2S: C,58.78; H, 2.61; N,20.16 %. Found: C,58.72; H, 2.63; N, 20.10 %. IR (KBr, νmax, cm-1); 751(C-S-C Stretching), 1719 (C=O stretching of -lactone of coumarin), 1581 (aromatic C=C stretching), 1475 (aromatic C=N stretching), 3041 (aromatic C-H stretching). 1H NMR (400 MHz, CDCl3, δ): 7.22-8.88 (9 H, m, Ar-H). 13C-APT (100 MHz, CDCl3, δ): 110.21(C), 113.02(C), 115.65(C), 117.76(CH), 118.29(C), 120.23(C), 125.86(CH), 125.97(C), 128.96(CH), 131.81(CH), 135.28(CH), 138.39(CH), 142.98(CH), 152.98(C), 163.81(CO of coumarin).
3-Pyridyl-9-methyl-[1,2,4]triazolo-[3’,4’:2,3][1,3,4]-thiadiazepino[7,6-b]coumarins (3h)
Pale yellow solid; yield=86%; mp 268-270˚C; Anal. Calcd. For C18H11N5O2S: C, 59.82; H,8.07; N,19.38 %. Found: C, 59.75; H, 8.10; N,19.36 %. IR (KBr, νmax, cm-1); 756(C-S-C Stretching), 1712 (C=O stretching of -lactone of coumarin), 1585 (aromatic C=C stretching), 1475 (aromatic C=N stretching), 2854(Aliphatic C-H stretching), 3045 (aromatic C-H stretching). 1H NMR (400 MHz, CDCl3, δ): 2.58(3 H.s, CH3), 7.23-8.89 (8 H, m, Ar-H). 13C-APT (100 MHz, CDCl3, δ):20.43(CH3), 111.31(C), 112.73(C), 113.80(C), 115.57(C), 117.58(CH), 118.37(C), 125.16(C), 126.13(CH), 131.72(CH), 136.03(C), 139.89(CH), 142.37(CH), 145.13(CH), 152.43(C), 163.79(CO of coumarin).
3-Pyridyl-9-Chloro-[1,2,4]triazolo-[3’,4’:2,3][1,3,4]-thiadiazepino[7,6-b]coumarins (3i)
Pale yellow solid; yield=79%; mp 236-237˚C; Anal. Calcd. For C17H8N5O2SCl: C, 53.48; H,2.11; N,18.34 %. Found: C,53.45; H, 2.08; N,18.30 %. IR (KBr, νmax, cm-1); 754(C-S-C Stretching), 1715 (C=O stretching of -lactone of coumarin), 1580 (aromatic C=C stretching), 1472 (aromatic C=N stretching), 3045 (aromatic C-H stretching). 1H NMR (400 MHz, CDCl3, δ): 7.21-8.58 (8 H, m, Ar-H). 13C-APT (100MHz, CDCl3, δ):111.22(C), 112.82(C), 115.65(C), 117.77(CH), 118.29(C), 120.23(C), 125.66(CH), 125.77(C), 129.16(CH), 131.81(CH), 136.18(C), 140.36(CH), 143.82(CH), 153.28(C), 163.41(CO of coumarin).
3-Thiophenyl-[1,2,4]triazolo-[3’,4’:2,3][1,3,4]-thiadiazepino[7,6-b]coumarins (3j)
Pale yellow solid; yield=84%; mp 211-214°C; Anal. Calcd. For C16H8N4O2S2: C, 54.53; H,2.29; N,15.90 %. Found: C, 54.50; H, 2.25; N, 15.86 %. IR (KBr, νmax, cm-1); 754(C-S-C Stretching), 1712 (C=O stretching of -lactone of coumarin), 1584 (aromatic C=C stretching), 1475 (aromatic C=N stretching), 3045 (aromatic C-H stretching). 1H NMR (400 MHz, CDCl3, δ): 7.06-8.34 (8 H, m, Ar-H). 13C-APT (100 MHz, CDCl3, δ): 101.43(CH), 109.89(CH), 112.72(C), 113.80(C), 115.54(C), 117.50(CH), 118.37(C), 125.06(CH), 126.03(C), 129.11(CH), 131.73(CH), 136.52(C), 139.08(CH), 153.49(C), 163.99(CO of coumarin).
3-Thiophenyl-9-methyl-[1,2,4]triazolo-[3’,4’:2,3][1,3,4]-thiadiazepino[7,6-b]coumarins (3k)
Yellow solid; yield=75%; mp 225-227ºC; Anal. Calcd. For C17H10N4O2S2: C, 55.72; H,2.75; N,15.29 %. Found: C, 55.70; H, 2.74; N, 15.27 %. IR (KBr, νmax, cm-1); 754(C-S-C Stretching), 1715 (C=O stretching of -lactone of coumarin), 1580 (aromatic C=C stretching), 1472 (aromatic C=N stretching), 2854 (aliphatic C-H stretching) 3045 (aromatic C-H stretching). 1H NMR (400 MHz, CDCl3, δ): 2.34(3H, s, CH3), 7.06-8.33 (7 H, m, Ar-H). 13C-APT (100 MHz, CDCl3, δ):23.32(CH3), 101.32(CH), 109.92(C), 112.74(C),115.28(C), 115.57(C), 118.39(C), 119.23(CH), 124.89(CH), 129.10(CH), 131.72(CH), 132.05(C), 137.58(CH), 138.42(CH), 142.38(C), 153.48(C), 163.31(CO of coumarin).
3-Thiophenyl-9-Chloro-[1,2,4]triazolo-[3’,4’:2,3][1,3,4]-thiadiazepino[7,6-b]coumarins (3l)
Yellow solid; yield=90%; mp 226-229ºC; Anal. Calcd. For C16H7N4O2S2Cl: C,49.68; H,1.82; N,14.40 %. Found: C, 49.65; H, 1.84; N,14.36 %. IR (KBr, νmax, cm-1); 756(C-S-C Stretching), 1718 (C=O stretching of -lactone of coumarin), 1585 (aromatic C=C stretching), 1475 (aromatic C=N stretching), 3041 (aromatic C-H stretching). 1H NMR (400 MHz, CDCl3, δ): 7.08-8.36 (7 H, m, Ar-H). 13C-APT (100MHz, CDCl3, δ):101.43(CH), 109.89(CH), 112.72(C), 113.80(C), 115.54(C), 117.50(CH), 118.37(C), 125.06(CH), 126.03(C), 129.11(CH), 131.73(CH), 136.52(C), 139.08(CH), 143.37(C), 155.52(C), 163.49(CO of coumarin).
Biological Results
Antimicrobial activity
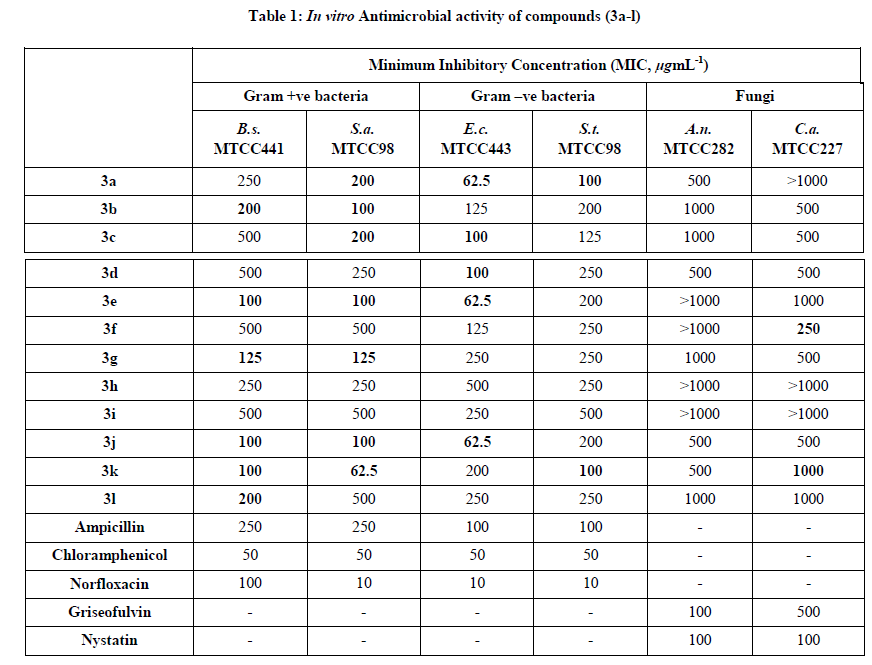
The newly synthesized target compounds (3a-l) were evaluated for their in vitro antibacterial activity against two Gram positive bacteria Staphylococcus aureus (MTCC 96) and Bacillus subtilis (MTCC 441) and two Gram negative bacteria Escherichia coli (MTCC 443) and Salmonella typhi (MTCC 98). They were also evaluated for their in vitro antifungal activity against Candida albicans (MTCC 227) and Aspergillus niger (MTCC 282) as fungal strains. Broth dilution method was used for the determination of the antibacterial and antifungal activity as recommended by NCCLS [32]. Ampicillin, Chloramphenicol and Norfloxacin were used as standard antibacterial drugs, whereas Griseofulvin and Nystatin were used as standard antifungal drugs. The synthesized compounds (3a-l) were screened for their antibacterial and antifungal activity at the concentration of 1000, 500 and 250 μg/mL for the primary screening. The synthesized compounds showing activity against microbes in the primary screening were further screened in a second set of dilution at concentrations of 200, 100, 62.5, 50 and 25 μg/mL. The suspensions of 10 μL from each well were further incubated and growth was noted at 37ºC after 24 h for bacteria and 48 h for fungi. The lowest concentration which showed no visible growth (turbidity) after spot subculture was considered as the Minimum Inhibitory Concentration (MIC) for each compound. The investigation of the data summarized in Table 1 reveals that many compounds were found to be active against Gram-positive bacteria while some of the compounds were found to be active against Gram-negative bacterial and fungal species as compared to that of the standard antimicrobial drugs.
Antimicrobial evaluation
The compounds (3a-l) were screened for their in vitro antibacterial and antifungal evaluation against various bacterial and fungal pathogens by broth dilution method. Ampicillin, Chloramphenicol, Norfloxacin, Griseofulvin and Nystatin were used as standard drugs. The values of MIC are summarized in Table 1.
Review of the antimicrobial activities of synthesized compounds (3a-l) in Table 1 indicated that compounds 3e, 3j and 3k (MIC=100 μg/mL) exhibited excellent activity toward Gram-positive bacteria Bacillus subtilis as compared to Ampicillin (MIC=250 μg/mL) and showed equipotent activity to Norfloxacin (MIC=100 μg/mL). Against Gram-positive bacteria Bacillus subtilis, compound 3g (MIC=125 μg/mL) showed activity higher than that of Ampicillin (MIC=250 μg/mL). Compounds 3b and 3l (MIC=200 μg/mL) displayed better activity than Ampicillin (MIC=250, μg/mL) toward Gram-positive bacteria Bacillus subtilis. Compounds 3a and 3h (MIC=250 μg/mL) showed results equivalent to that of Ampicillin (MIC=250 μg/mL) toward Gram-positive bacteria Bacillus subtilis. Compounds 3b, 3e and 3j (MIC=100 μg/mL) were found to be more effective against Gram-positive bacteria Staphylococcus aureus than Ampicillin (MIC=250 μg/mL). Against Gram-positive bacteria Staphylococcus aureus, compounds 3g (MIC=125 μg/mL) showed activity higher than that of Ampicillin (MIC=250 μg/mL). Compounds 3a and 3c (MIC=200 μg/mL) showed good activity against Gram-positive bacteria Staphylococcus aureus as compared to Ampicillin (MIC=250 μg/mL). Against Gram-positive bacteria Staphylococcus aureus, compounds 3d and 3h (MIC=250 μg/mL) showed equipotent activity to that of Ampicillin (MIC=250 μg/mL).
Moreover, Against Gram-negative bacteria Escherichia coli, compounds 3c and 3d (MIC=100 μg/mL) showed activity comparable to Ampicillin (MIC=100 μg/mL). Against Gram-negative bacteria Salmonella typhi, compounds 3a, 3e and 3j (MIC=62.5 μg/mL) showed excellent activity as compared to Ampicillin (MIC=100 μg/mL). Whereas compounds 3a and 3k (MIC=100 μg/mL) showed equipotent to Ampicillin (MIC=100 μg/mL) toward Gram-negative bacteria Salmonella typhi.
Furthermore, against Candida albicans fungal pathogen, however compound 3f (MIC=250 μg/mL) showed better inhibition action as compare to the standard drug Griseofulvin (MIC=500 μg/mL). Whereas compounds 3b, 3c, 3d, 3g and 3j (MIC=500 μg/mL) showed activity comparable to Griseofulvin (MIC=500 μg/mL) against fungal pathogen Candida albicans.
Anti-tuberculosis activity
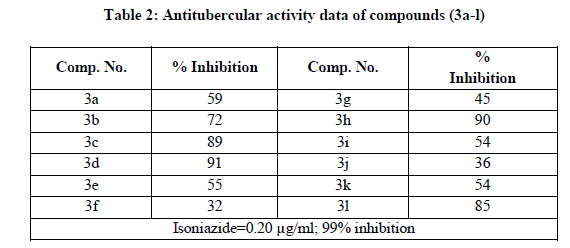
The in vitro antitubercular activity of all the synthesized compounds were determined by using Lowenstein-jensen medium (Conventional method against Mycobacterium tuberculosis H37Rv strain as described by Rattan [33]. The results of the activity data are presented in Table 2 in the form of % inhibition, relative to that of standard drugs isoniazide and rifampicin. Upon study of the activity data it was observed that compound 3d and 3h showed good activity in comparison with isoniazid.
Conclusion
Present study described successful hybridization strategy of three bioactive moieties, coumarin, triazole and thiadiazepines in a single scaffold. The target compounds were synthesized in good yield by adopting simple condensation reaction. Majority of the compounds were found to be active against Gram positive and gram negative bacteria. Antimicrobial screening results revealed that compounds 3a, 3e, 3j and 3k were found to be the most proficient members of the series and antitubercular activity data revealed that compound 3d and 3h showed good activity in comparision with isoniazide.
Acknowledgment
The authors are thankful to the Head, Department of Chemistry, Sardar Patel University for providing research facilities. Financial assistance to DSP, NJP and PVS from the UGC, New Delhi, India, is highly acknowledged.
References
[1] T. Karabasanagouda, A.V. Adhikari, N.S. Shetty, Eur. J. Med. Chem., 2007, 42, 521-529.
[2] I. Küçükgüzel, S.G. Küçükgüzel, S. Rollas, M. Kiraz, Bioorg. Med. Chem. Lett., 2001, 11, 1703-1707.
[3] A. El-Essaway, W.A. El-Sayed, S.A. El-Kafrawy, A.S. Moorshedy, A.H. Abdel Rahman, Naturforsch., 2008, 63, 667-674.
[4] B.S. Holla, B.S. Rao, B.K. Sarojini, P.M. Akberali, N.S. Kumari, Eur. J. Med. Chem., 2006 41, 657-663.
[5] S.H. Fang, V. Padmavathi, Y.K. Rao, D.R.C.V. Subbaiah, P. Thriveni, M. Geethangili, A. Padmaja, Y.M. Tzeng, Int. Immunopharm., 2006, 6, 1699-1705.
[6] O.V. Dyablo, A.F. Pozharskii, Chem. Heterocycl. Comp., 1997, 33, 1003-1027.
[7] P. Vainilavicius, R. Smicius, V. Jakubkiene, S. Tumkevicius, Monatsh. Chem. Chem. Mon., 2001, 132, 825-831.
[8] A.R. Farghalya, E. De Clercq, H. El-Kashefa, Arkivoc., 2006, 137-151.
[9] J.K. Sahu, S. Ganguly, A. Kaushik, J. Adv. Pharm. Technol. Res., 2014, 5, 90-95.
[10] G. Marfe, C. Di Stefano, Recent. Pat. Anti-Canc., 2010, 5, 58-68.
[11] D. Giannotti, G. Viti G, P. Sbraci, V. Pestellini, G. Volterra, F. Borsini, A. Lecci, A. Meli A, P. Dapporto, P. Paoli, J. Med. Chem., 1991, 34, 1356-1362.
[12] A. Chimirri, R. Gitto, S. Grasso, M. Zappala, A. Desarro, G.B. Desarro, Farmaco., 1994, 4, 193-196.
[13] R. Silvestri, M. Artico, E. Pag Nozzi, Syn. Farmaco., 1996, 51, 425-430.
[14] P. Karegoudar, D.J. Prasad, M. Ashok, M. Mahalinga, B. Poojary, B.S. Holla, Eur. J. Med. Chem., 2008, 43, 808-815.
[15] Z.A. Kaplancikli, M.D. Altinto, Zitouni GT, Ozdemir A, Demirel R, Mohsen UA. Hussein W, Cukurova Med J, 2013, 38: 103-107.
[16] M. Kuroda, M. Amano, F. Noboru, Ger. Often. DF., 1991, 4, 184.
[17] A. Subageetha, R. Vijayraj, T. Rajkumar, R.S. Anand, Int. J. Res. Pharm. Biomed. Sci., 2011, 2, 155-159.
[18] E. Banfi, G. Scialino, C.M. Bragadin, J. Antimicrobial. Chemother., 2003, 52, 796.
[19] U.V. Laddi, M.B. Talwar, S.R. Desai, R.S. Bennur, S.C. Bennur, Ind. J. Chem., 2001, 40B, 828.
[20] A. Brucato, A. Coppala, S. Gianguzza, P. Provenzano, Bull. Soc. Ital. Bio. Sper., 1978, 54, 1051-1057.
[21] M.A. Raslan, M.A. Khalil, J. Het. Atom. Chem., 2003, 14, 114-120.
[22] D. Anshu, S. Ruby, K. Sarita, Biorg. Med. Chem., 2006, 14, 1303.
[23] M.R. Vang, R.R. Kundurun, Chem. Pharm. Bull., 2010, 58, 1081.
[24] B. Kalluraya, J. Nayak, H.M. Vagdevi, Ind. J.Het. Chem., 2005, 14, 257-258.
[25] S.S. Rajput, Int. J. Pharm. Pharmaceut. Sci., 2013, 5, 717-718.
[26] G. Monica, P. Satya, G. Rajive, Ind. J. Het. Chem., 2009, 48B, 460-466.
[27] S.R. Moorthy, V. Sundaramurthy, N.V. Subba Rao, Ind. J. Chem, 1973, 11, 854.
[28] M. Quaraishi, A. Dandia, S. Gupta, L. Sudheer, J. Mater. Envi. Sci., 2012, 3, 993-1000.
[29] P.K. Sahoo, R. Sharma, P. Pattanayaka, Med. Chem. Res., 2010, 19, 127-135.
[30] R.K. Mali, R.R. Somani, M.P. Toraskar, K.K. Mali, P.P. Naik, P.Y. Shirodkar, Int. J. Chem. Tech. Res., 2009, 2, 168-173. [31] M.A. Al Omar, Molecules., 2010, 15, 502-514.
[32] National Committee for Clinical Laboratory Standards (NCCLS), 2002, 1-56238-454-6, M100-S12 (M7).
[33] A. Rattan, A. Kalia, N. Ahmad, Emerg. Infect. Dis., 1998, 4, 195-209.



