Research Article - Der Pharma Chemica ( 2020) Volume 12, Issue 5
A Research Article: Comparison on Phytochemical Analysis and Antioxidant profile of two species of Ocimum (Ocimum basilicum and Ocimum sanctum)
Manisha Bhatti*, Damandeep Kaur and Mohit ThakurManisha Bhatti, University Institute of Pharma Sciences, Chandigarh University, Gharuan, Mohali, Punjab, India, Email: manishabhatti13.mb@gmail.com
Received: 06-Apr-2020 Accepted Date: Aug 18, 2020 ; Published: 31-Aug-2020
Abstract
Ocimum is a genus of aromatic annual and perennial herbs and shrubs of family Lamiaceae. There are number of species of this genus that are used to treat different types of disorders and diseases from ancient times. Out of those the two species i.e Ocimum sanctum and Ocimum basilicum were studied in the present article for their phytochemical testing and antioxidant activity and found that different extracts of these plants possessed various phytoconstituents such as alkaloids, glycosides, saponins, tannins, phenol, flavonoids and essential oils. And the methanol plant extracts of both plants exhibited antioxidant activity. The radical scavenging activity of plants was determined by DPPH scavenging method using Ascorbic acid as standard and it was found that % Scavenging activity of Ocimum basilicum was 53.6 ± 0.021 at 100 mcg/ml and 48.9 ± 0.034 at 100 mcg/ml in Ocimum sanctum respectively
Keywords
Ocimum sanctum, Ocimum basilicum, Phytochemical Constituents, DPPH Scavenging method, Antioxidant Activity
Introduction
The growing advancements, modern technology, stress, environmental pollution and poor diet has led to degradation in the health. Most of the people are suffering from ailments such as hyperthyroidism, diabetes, hyperlipidaemia, cancer etc. Conventional allopathic medicines are used to treat such diseases but they lead to various side effects. Thus, the Ayurveda and Naturopathic plants are used that have minimum side effects and treat various diseases. Ayurveda is unique healthcare discipline as it lay importance on the cause of the disease and suffering. Herbal medicines are beneficial than allopathic ones. Herbal medicines have almost no or less side effects than the commercial medicines. They are easily available and are cheaper.
Phytochemicals are naturally occurring compounds that are found in various parts of plants such as leaves, root, stem, bark etc. They are biologically active and provide health benefits to humans. They prevent the onset of various diseases or decelerate the progress of diseases such as cancer, diabetes, oxidative damage, high blood pressure. Bioactives present in medicinal plants are terpenoids, alkaloids, phenols, flavonoids, carotenoids, tocopherols volatile oils etc [1].
Ocimum, commonly known as basil is one of the medicinal herbs used to treat various ailments. It is known as miracle herb, wonder herb and herb for all reasons due to its various pharmacological properties. Ocimum species possess powerful antioxidant activity due to the essential oil present in its leaves. It contains eugenol as its main chemical constituent. Basil leaves act as anti-stress agent, help in blood purification and also lowers the cholesterol [2]. Cell damage which is caused by free radicals seems to be a major contributor to aging and to degenerative diseases of aging namely cancer, cardiovascular disease, cataracts and immune system decline and brain dysfunction. Antioxidants stabilize or deactivate free radicals before they attack cells. It possesses radical scavenging property and protects from cellular damage. Antioxidant compounds may function as free radical scavengers, complexing agents for pro-oxidant metals, as reducing agents and quenchers of singlet oxygen formation [3]. The antioxidant activity can be determined using various methods such as DPPH radical scavenging assay, ABTS radical scavenging assay, Nitric oxide scavenging activity, Hydrogen peroxide scavenging activity etc [4].
Ocimum sanctum
Ocimum sanctum, also known as Queen of Herbs is one of the holiest and highly respectable for most therapeutic and restorative herbs distributed mainly in the all regions of India. Ocimum sanctum Linn. commonly known as holy basil in English or sacred basil, in Hindi and in Sanskrit it is known as Vishnu priya, divya belongs to the family Lamiaceae (labiatae). As its Latin name implies it considered a very sacred plant. It is high in sattva thereby imparting the quality of lightness and spiritual clarity [5,6].
Ocimum sanctum possess specific aromatic odour due to the presence of essential or volatile oil, mainly concentrated in the leaf. This aromatic volatile oil contains phenols, terpenes and aldehydes. The oil extracted from seeds is called fixed oil and is mainly composed of fatty acids. Besides oil, the plant also has alkaloids, glycosides, saponins and tannins. Ascorbic acid and Carotene is also present in the leaves [7,8]. Vitamin A and Vitamin C found in this herb stimulates antibody production up to 20% so to provide protection against diseases [9].
Ocimum basilicum
O. basilicum is being known by different names in different languages around the world. In Hindi and Bengali, it is known as babui ocimum. In English, it is known as Basil, Common Basil or Sweet Basil [10]. In Arabic the plant is known as Badrooj, Hebak or Rihan; as Nasabo or Sabje in gujrati and as Jangli Ocimum in urdu. Tohrakhurasani and Okimon are the names of the plant in persian and unani languages respectively [11]. Linalool, geraniol, eugenol and 1, 8-cineole is the major constituents which were present up to 92.9% [12,13].
Material and Methods
Authentication, Collection and Extraction of Ocimum sanctum & Ocimum basilicum
The leaves of two plant species of Ocimum were precured from plant nursery (Jalandhar Road, Hoshiarpur, Pb), authenticated from NISCAIR Delhi and a herbarium specimen (UIPS/HB/DK/02) of these plants is preserved in the UIPS department, Chandigarh University, Gharuan, Mohali (Pb) for the future reference.
The leaves were cut into pieces and were shade dried for 10 to 15 days. The dried leaves were powdered and crude drug was extracted using Soxhlet apparatus with two solvents that are petroleum ether and methanol. The Soxhlet assembly was run for about 4 hours and the crude extract obtained was filtered and concentrated [14,15].
All the chemicals used were of Analytical grade.
Phytochemical Evaluation
Extraction was carried used using Soxhlet method. The petroleum ether and methanol extracts of the two species of Ocimum were subjected to various phytochemical tests.
Test for alkaloids
To the extract dilute hydrochloric acid was added and was then filtered. The filtrate was treated with various alkaloidal reagents
Mayer’s test:
The filtrate was treated with Mayer’s reagent and appearance of cream colour indicates the presence of alkaloids.
Dragendorff test: The filtrate was treated with dragendorff reagent and appearance of reddish-brown precipitate indicates the presence of alkaloids.
Hager’s test: The filtrate when treated with Hager’s reagent lead to appearance of yellow colour precipitate which indicates the presence of alkaloids [15].
Test for carbohydrates and reducing sugar
The small quantities of the filtrate was dissolved in 4ml of distilled water and filtered. The filtrate will be subjected to following tests:
Fehling’s test: The extract was treated with Fehling’s reagent A and B. Reddish brown colour precipitate indicates the presence of reducing sugar.
Barfoed’s test: The extract was treated with barfoed’s reagent (copper acetate solution) and heated. Reddish orange colour precipitate indicates the presence of non-reducing sugars.
Test for steroids
Libermann burchard’s test: The extract was treated with 3ml of acetic anhydride, few drops of glacial acetic acid followed by a drop of concentrated sulphuric acid. Bluish green colour indicates the presence of steroids [16].
Test for proteins
Biuret test: The extract was treated with copper sulphate solution, followed by addition of sodium hydroxide solution; appearance of violet colour indicates the presence of proteins.
Test for tannins: The extract was treated with 10% lead acetate solution; appearance of white precipitate indicates the presence of tannins.
Test for phenolic compounds: The extract was treated with neutral ferric chloride solution; appearance of violet colour indicates the presence of phenolic compounds. The extract was treated with 10% sodium chloride solution; appearance of cream colour indicates the presence of phenolic compounds [15,17].
Test for flavonoids: 5 ml of extract was hydrolyzed with 10%sulphuric acid and cooled. Then, it was extracted with diethyl ether and divided in to three portions in three separate test tubes. 1ml of diluted sodium carbonate, 1ml of 0.1N sodium hydroxide, and 1ml of strong ammonia solution was added to the first, second and third test tubes respectively. In each test tube, development of yellow colour demonstrated the presence of flavonoids.
Test for gums and mucilage
The extract was treated with 25 ml of absolute alcohol, and filtered. The filtrate will examine for its swelling properties [15,18].
Test for glycosides
When a pinch the extract was treated with glacial acetic acid and few drops of ferric chloride solution, followed by the addition of conc. Sulphuric acid, formation of ring at the junction of two liquids indicates the presence of glycosides.
Test for saponins
Foam test: About 1 ml of the extract was diluted to 20 ml of with distilled water and shaken well in a test tube. The formation of foam in the upper part of test tube indicates the presence of saponins [17,19].
Determination of Antioxidant property
The antioxidant activity was determined by using 2, 2-Diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging assay. DPPH scavenging activity or the hydrogen donating capacity was quantified in presence of stable DPPH radical on the basis of Blois method [20]. The antioxidant property was determined using methanol extract of plants. Briefly, to a methanol solution of DPPH (100 mM, 2.95 ml), 0.05 ml of test compounds dissolved in methanol was added at different concentration (20-100 μg/ml). Reaction mixture was shaken and absorbance was measured at 517 nm using ascorbic acid as standard. The degree of discoloration indicates the scavenging efficacy of the extracts. The capability to scavenge the DPPH radical was calculated using the following equation: [21].
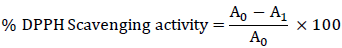
Where A0 was the absorbance of the control and A1 was the absorbance in the presence of the sample of extract and standard [22].
All the experimental measurements were carried out in triplicate and are expressed as average of three analysis ± standard deviation (SD) (n=3).
Results and Discussions
The preliminary phytochemical analysis of the two species using pet. ether and methanol extracts was performed. Phenols and flavonoids were found present in petroleum ether extract of Ocimum sanctum and Carbohydrates, proteins, phenols and flavanoids were detected in pet. ether extract of Ocimum basilicum. Whereas, alkaloids, tannins, phenols, flavonoids, glycosides, saponins were found positive in Ocimum sanctum as well as in Ocimum basilicum. The results of phytochemical analysis of Ocimum sanctum and Ocimum basilicum in two different extracts of pet. ether and methanol are presented in Table 1.
Table 1: Results for Phytochemical Analysis of Pet. Ether and Methanol Extracts of Ocimum sanctum and Ocimum basilicum
| S.no. | Chemical Test | Ocimum sanctum (Pet ether Extract) | Ocimum basilicum (Pet ether Extract) | Ocimum sanctum (Methanol Extract) | Ocimum basilicum (Methanol Extract) |
|---|---|---|---|---|---|
| 1 | Alkaloids | - | - | + | + |
| 2 | Carbohydrates | - | + | - | + |
| 3 | Steroids | - | - | - | - |
| 4 | Proteins | - | + | - | - |
| 5 | Tannins | - | - | + | + |
| 6 | Phenols | + | + | + | + |
| 7 | Flavanoids | + | + | + | + |
| 8 | Gums and Mucilage | - | - | - | - |
| 9 | Glycosides | - | - | + | + |
| 10 | Saponins | - | - | + | + |
+ means Positive result (present) and – means negative result (absent)
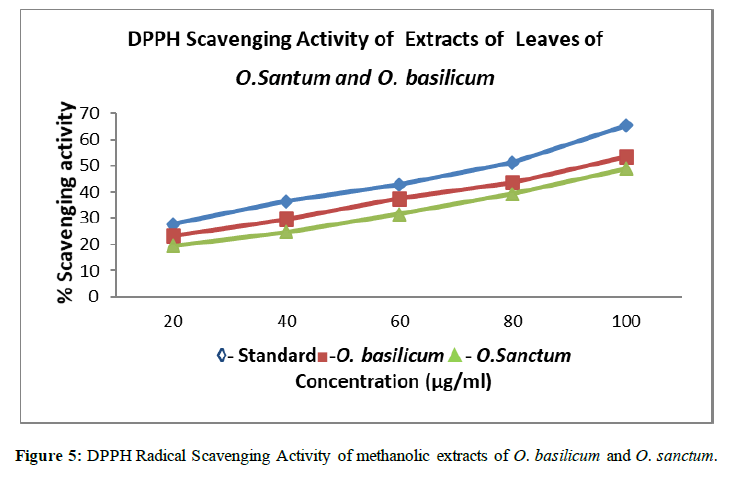
Table 2: Results for %DPPH Scavenging Activity
| S.no. | Concentration (mcg/ml) | % Scavenging activity of Standard(Ascorbic Acid) | % Scavenging activity of Sample A Ocimum basilicum | % Scavenging activity of Sample B Ocimum sanctum |
|---|---|---|---|---|
| 1 | 20 | 27.7 ± 0.040 | 23.4 ± 0.010 | 19.5 ± 0.031 |
| 2 | 40 | 36.6 ± 0.035 | 29.6 ± 0.015 | 24.7 ± 0.024 |
| 3 | 60 | 42.8 ± 0.020 | 37.5 ± 0.034 | 31.6 ± 0.028 |
| 4 | 80 | 51.3 ± 0.025 | 43.7 ± 0.029 | 39.4 ± 0.036 |
| 5 | 100 | 65.4 ± 0.030 | 53.6 ± 0.021 | 48.9 ± 0.034 |
The DPPH scavenging activity was determined for methanol extracts of Ocimum basilicum and Ocimum sanctum. The results were expressed in % Scavenging activity of free radical. The methanol extracts of Ocimum basilicum and Ocimum sanctum showed good radical scavenging property. The % scavenging activity of ascorbic acid (standard) was 65.4 ± 0.030 at 100 mcg/ml while at the same concentration i.e 100 mcg/ml the % scavenging activity of Ocimum basilicum and Ocimum sanctum was found to be 53.6 ± 0.021and 48.9 ± 0.034 respectively.
Conclusion
The Phytochemical Analysis of O. basilicum and O. sanctum revealed that different chemical constituents were present in two different extracts (pet. ether and methanol) of both species. Such as Phenols and flavonoids were found present in petroleum ether extract of Ocimum sanctum and carbohydrates, proteins, phenols and flavonoids were detected in pet. ether extract of Ocimum basilicum. Whereas, alkaloids, tannins, phenols, flavonoids, glycosides, saponins were found positive in Ocimum sanctum as well as in Ocimum basilicum. Moreover, from the experimental study it was also concluded that both species possessed good antioxidant activity and the % scavenging activity was found to be 53.6 ± 0.021 at 100 mcg/ml in Ocimum basilicum and 48.9 ± 0.034 at same concentration in Ocimum sanctum. Which proved that Ocimum basilicum possessed more % scavenging activity that other species i.e Ocimum sanctum. Due to its powerful antioxidant properties, the plants can be used as anti-cancer and in prevention of various disorders that occurs due to generation of free radicals such as heart diseases, alzheimer etc.
References
[1] PSS. Harichandan, AK Sahu, S. Gautam et al., GSC Biological and Pharmaceutical Sciences., 2019. 8(2): p. 22-33.
[2] Marc Cohen and Ocimum - Ocimum sanctum: A herb for all reasons. J. Ayurveda Integr. Med., 2014. 5(4).
[3] A. D. Sathisha, H. B. lingaraju and K. Sham Prasad. E-Journal of Chemistry., 2011. 8(2): p. 882-886.
[4] S. Sahoo, G. Ghosh, D Das et al., Asian Pac J Trop Biomed., 2013. 3(11): p. 871-876.
[5] N. Bano, A. Ahmed, M. Tanveer et al., Pharmacological Evaluation of Ocimum sanctum, J Bioequiv Availab., 2017. 9(3): p. 387-392.
[6] SI. Vidhani, VG. Vyas, HJ. Parmar et al., Am J Food Sci Technol., 2016. 4: p. 52-57.
[7] DS. Sharma. Matchless healing properties of Ocimum sanctum (Review). Indian J Appl Res., 2016. p. 5.
[8] Andrea Muráriková and Anton Tažký. Characterization of Essential Oil Composition in Different Basil Species and Pot Cultures by a GC-MS Method, Molecules. 2017. 22: p. 1221.
[9] P. Kumar, SG. Krishna and RT. Bhavani. Am J Ethnomed., 2014. 1: p. 89-95.
[10] CP.Khare., Indian medicinal plant- an illustrated dictionary. Springer publication. 2007. P. 445-449.
[11] Fakhroo Ameena and Sreerama Lakshmaiah. Int. j. App Pharm Bio R. 2016. 1(4): p. 1-7
[12] D. Beatovic, D. Krstic-Miloševic. S. Trifunovi´c et al., Rec. Nat. Prod. 2015. 9: p. 62-75
[13] Al. Abbasy, DW. Pathare, N. Al-Sabahi et al., Asian Pac. J. Trop. Dis., 2015. 5: p. 645-649.
[14] U. Zlotek, M. Michalak-Majewska and U. Szymanowska. Food Chem., 2016. 213: p. 1-7.
[15] Ghani Sobia and Sathish Pai. Biochemistry, 2015. 5(2).
[16] Kumar Joshi, Rakesh, Setzer N William et al., Am. J. Essent. Oil., 2017. 5(1): p. 18-21
[17] A. Kumar, K. Agarwal, AK. Maurya et al., Pharmacogn Mag., 2015. 11: p. 217.
[18] GE. Trease and WC. Evans. Pharmacognosy, BailliereTindall, London, 13th edition. P. 176-180.
[19] AK. Panday, P. Singh and NN. Tripathi. Asian Pac. J. Trop., 2014. 4: p. 682- 694.
[20] Inampudi Sailaja. Asian Journal of Bio Science., 2010. 5(2): p. 195-199
[21] Deo Bandita, Nath Monalisha, Nayak K Preetam et al., Int. J Plan. Env Sci., 2013. 3(2): p. 150
[22] Behera Saiprasanna, Babu S Manohar, Ramani Y Roja et al., J. Drug Del Ther., 2012. 2(4): p. 122-128