Review Article - Der Pharma Chemica ( 2021) Volume 13, Issue 11
A Review on Features, Structure and Possible Treatment of a Pandemic 2019 Novel Coronavirus (COVID-19)
Gurmeet Kaur1* and Jagdeep Kaur22Department of chemistry(UIS), Chandigarh University, Punjab,140413, India
Gurmeet Kaur, Department of Chemistry (UIS), Chandigarh University, Punjab,140413, India, Email: gurmeet.uis@cumail.in
Abstract
In December 2019, an outbreak of a previously known animal virus, currently known as 2019 Novel Coronavirus (COVID-19) or SARS-CoV-2 has been altered to become zoonotic in humans. It is assumed to be started from a popular wholesale Seafood market in Wuhan, Hubei, a city in China, and spread all over the world in just two to three months and hence was announced as a Global Pandemic on 11th March 2020 by the WHO. The virus predominantly affects humans' respiratory systems, and it spreads through sneezing or coughing droplets. WHO considers the incubation period, or the time between infection and onset of symptoms, to be 2-14 days, however, it can vary from person to person and in certain cases can be as long as 29 days [1]. The present review is an attempt to collect information regarding features, structure, possible treatments, and vaccines against this deadly Novel Coronavirus 2019.
Keywords
2019 Novel Coronavirus; COVID 19; SARS-CoV-2; Zoonotic; Seafood; WHO; Pandemic
Introduction
Coronavirus is a type of virus known in animals like a bat, camel, cat, snake, etc., and was known to cause infection mainly in the respiratory system as well as in the gastrointestinal tract of animals. This virus remains mostly confined in the animals only until and unless it comes in direct contact with humans very often. It can then jump to humans; the process is known as Zoonosis, a virus known as Zoonotic virus, and the disease known as a Zoonotic disease (plural zoonoses, or zoonotic diseases) by some sort of mutation or some other unknown processes. In history, several bacteria, viruses, parasites, etc. are known to spread infectious diseases from animals i.e. non-humans (usually vertebrates) to humans [2-4]. Zoonoses include modern-day diseases such as Ebola and salmonellosis. HIV was first transmitted to humans as a zoonotic disease in the early twentieth century, but it subsequently transformed into a separate human-only illness. Although many forms of avian flu and swine flu are zoonoses, the majority of influenza types that infect people are human diseases. When these infections recombine with human flu virus strains, they can generate pandemics, such as the 2009 swine flu. In endemic areas, Taenia solium infection is one of the most overlooked tropical diseases, posing a threat to human health and veterinary concerns [5]. A range of disease pathogens, including as viruses, bacteria, fungi, and parasites, can cause zoonoses. 61 percent of the 1,415 diseases identified as infecting humans were zoonotic [6]. Although most human diseases have their origins in animals, diseases like rabies, which can be transmitted from non-human to human, are usually considered instantaneous zoonosis [7,8]. Zoonoses can be transmitted in a variety of ways. In direct zoonosis, a disease is transmitted directly from an animal to a human through a medium such as air (for example, influenza) or bites and saliva (for example, rabies) [9]. On the other hand, transmission can occur when an intermediary species, also known as a "vector," spreads the disease pathogen without becoming affected. Reverse zoonosis, also known as anthroponosis, occurs when humans infect other animals [10].
Classification of different types of Viruses and their structures
A virus is considered a borderline case between living and non-living organisms. The word “Virus” is derived from the Latin language which means “poison” and can practically infect any living form like plants, animals, humans, etc. It is too tiny to be seen by an ordinary optical microscope and it can only be seen through a scanning microscope. A virus becomes living when it comes in contact with either a host (in which it can cause infection) or a vector (the virus has some lifecycle in the vector but it doesn’t infect or do any harm to the vector) within some specified time. Vector can act as a carrier of the virus and take it to the host where the virus can cause infection. Any typical virus is made of three main parts –the outer fatty envelope (not present in all viruses) containing glycoprotein protruding for attachment to the host, outer protein coat of its body or capsule or capsid which secures the inner genetic material which can be either RNA or DNA or both RNA and DNA depending upon the type of the virus. It can have different genetic material, shapes (usually icosahedral, helical, or complex), sizes, can have an envelope or naked capsid, sense (+) or antisense (-), Strandedness (ds, double-stranded or ss, single Strandedness) [11].
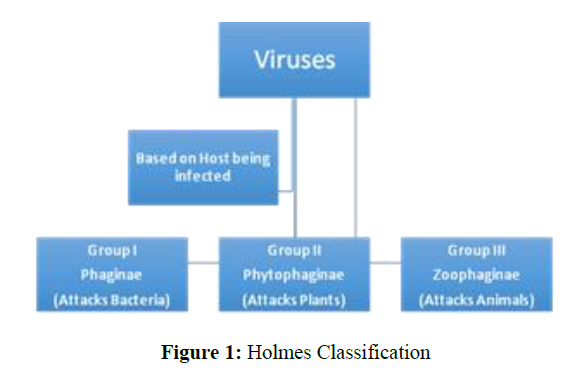
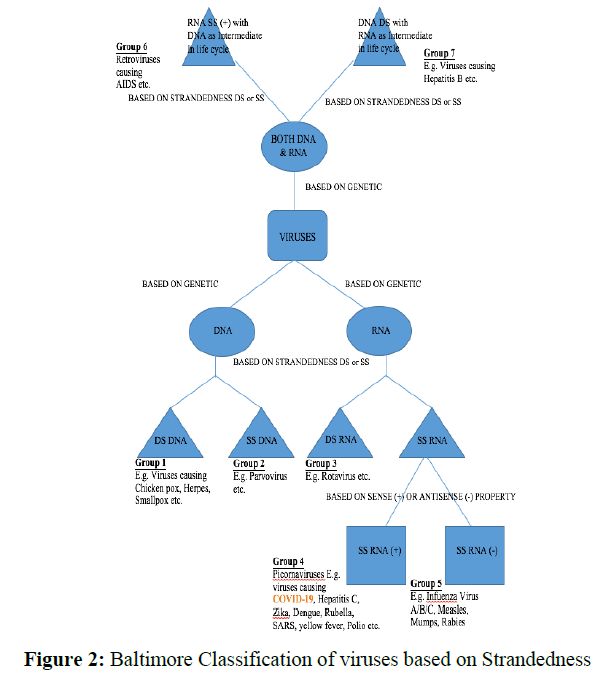
There are many classifications of viruses known in literature but Holmes and Baltimore’s classification seems a bit easy and quite informative. According to Holmes, the classification can be done based on the host on which the virus is attacking (Figure 1). According to Baltimore, any known virus should belong to one of the seven Groups which are classified on basis of genetic material, Strandedness (DS for double-stranded and SS for single-stranded) and sense (means +) or antisense (means -) (Figure 2) [12].
In Figure 2 the classification of a Virus is presented and COVID-19 belongs to Group 4. This Group seems to include many other Zoonotic viruses. Even Group 5 and 6 seem to be quite dangerous. The size of the virus is generally too small to see through a normal optical microscope.
COVID-19 comes under Group 4, in which genetic material is RNA which is single-stranded (SS) and has (+) sense. The capsid of the virus is enveloped and its shape is helical and its size is about 140 nm, which is much more than dust particles hanging in the air. SARS-CoV-2 belongs to the beta-Coronavirus family among four different families’ alpha, beta, delta, and gamma [13].
Structure of SARS-CoV-2
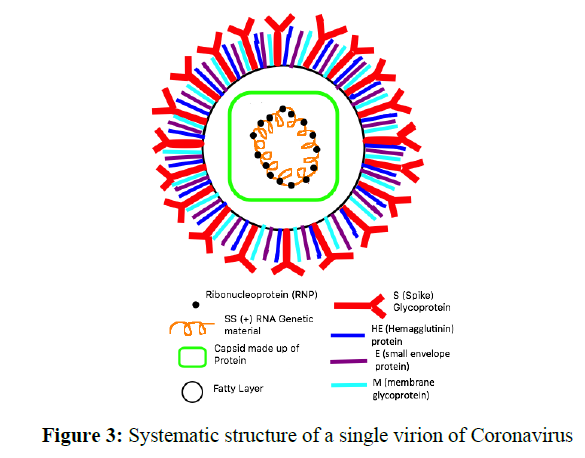
The structure of a single virion of coronavirus is circular in a structure having crown-like structures outside (word “corona” means crown). It is made of an outer fatty layer that encloses the capsid made up of protein and protects the genetic material which is positive single-stranded RNA made up of ribo-nucleoprotein (RNP) of about 28 to 32 Kb which is longer among the viruses and is arranged in a helical shape. There are various spikes over the fatty outer envelope made up of a different type of glycoproteins like bigger Spike (S) glycoprotein, Hemagglutinin (HE) glycoprotein, small Envelope (E) protein, and small membrane (M) glycoprotein for attaching to host and to fuse with the host cell and enter inside host cell for multiplication [14] (Figure 3).
Transmission process of 2019 Novel Coronavirus
The infection of humans starts with droplets of another infected human which have come out from the cough or sneeze of an infected person. The droplet has many virions which can enter through the mucous membrane of the nose or mouth and conjunctiva of eyes and will try to enter finally to the lungs. Droplet infection is different than airborne infection. The droplet infection can occur only when the healthy person is within 1 m distance of either an infected person or comes in direct contact with the droplet on the surface on which the virus is there from an infected person. Airborne infection takes place via the smaller size of the virus which can remain in the air for a longer time and can travel in air as being lighter. Coronavirus infection is a droplet infection and not an airborne infection. It can stay on surfaces from few hours to several hours depending upon the nature of the surface. On the surface of plastic, it can stay for the longest hours till 8 hours while on a metal surface like the copper metal surface it can stay only for 2-3 hours [15].
Lifecycle of SARS-CoV-2 inside Human and possible Drug Targets for 2019 Novel Coronavirus
Attachment to Human cell
SARS-CoV-2 reaches the lungs and attaches itself to the cell of the lung through one of the glycoproteins, mainly through the largest Spike (S) glycoprotein, and attaches itself to the receptor Angiotensin-converting enzyme-2 on the lung cell [16]. If a drug is made which can inhibit ACE-2that will not allow the covid-19 to attach to the alveoli cell of the lungs and hence lifecycle of the Coronavirus can’t start inside the human. ACE-2 inhibitors like Azilsartan (Edarbi), Candesartan (Atacand), Eprosartan, Irbesartan (Avapro), Losartan (Cozaar), Olmesartan (Benicar), Telmisartan (Micardis), Valsartan (Diovan) should be effective against 2019 Novel Coronavirus if the virus has only one mechanism of attachment to the human lung cell [17].
Entry Inside the human cell
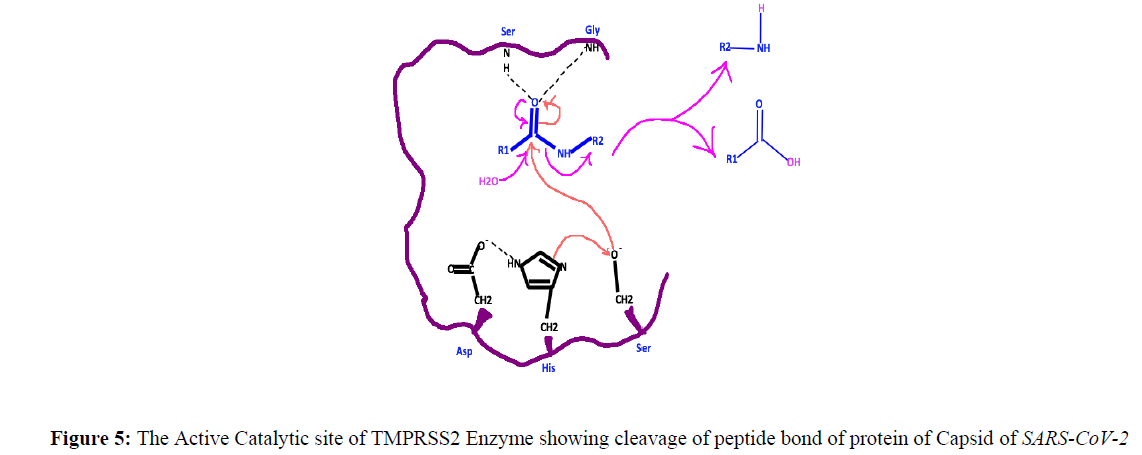
After attachment, the membrane and capsid dissolve so that the genetic material can be transferred inside the cell of the alveoli. This is done by the protease enzyme of the host. So the viral envelope either fuses with the plasma membrane of the host cell or a process called endocytosis occurs by which the genome reaches inside the host cell and is ready for the next step which is its replication. This protease is identified as TMPRSS2 by studies done earlier on SARS which is quite similar to SARS-CoV-2 (Figure 4).
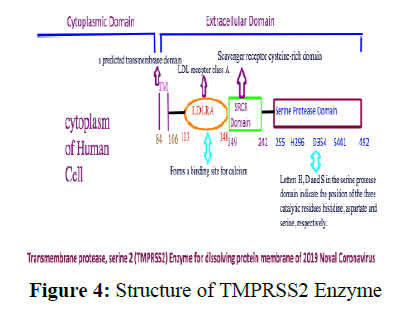
TMPRSS2 is an abbreviation of Transmembrane protease, serine 2 and it is an enzyme that in humans is used to hydrolyze the protein membrane of the viruses having outer protein coat [18]. What triggers TMPRSS2 is not that clear in any of the research papers. That can also become an important drug target. This is present on the plasma membrane of the cell. Its structure has 492 amino acids and gives a single entry to the cell. It has the structure shown in figure 4.
The structure of TMPRSS2, which can be a good target of the 2019 Novel Coronavirus, has three main units as shown in Figure 4. The main catalytic active site is called Serine Protease Domain and has three active amino acids Histidine (H) at position 296, Aspartate (D) at position 354, and Serine (S) at position 441. The mechanism of peptide cleavage of virus protein starts by an attack of the nucleophile –O-of serine which is generated by deprotonation by histidine residue to the carbonyl carbon of the peptide of the virus capsid layer, forming a tetrahedral intermediate linking both virus capsid and the host cell covalently (Figure 5).
“The negative charge is stabilized by NH of both Serine and Glycine present at some other position. When the negative charge on the oxygen of carbonyl group of the amide linkage of the peptide bond of the protein layer of the Coronavirus, comes back to form carbonyl group then amine is cleaved when water adds to the carbonyl carbon forming acid and amine and hence cleaving the amide bond of the peptide bond of the protein moiety of the Coronavirus (Figure 5). So drug compounds could be even small organic compounds that can bind with Serine OH, NH of histidine, or H of COOH of Aspartate and block cleavage of peptide linkage could also be the target for the SARS-CoV-2” [19].
According to an unpublished preprint, one commercial medicine known asTMPRSS2 inhibitor, Camostat mesylate [20], is known to impede the entry of the SARS-CoV-2 virus. JAK inhibitor can be another target for killing COVID-19. JAK kinase is responsible for signal transduction or finally cytokine signaling, a phenomenon through which any cellular response is there. So blocking JAK Kinase will stop cytokine signaling which ultimately going to stop any cellular response like a fusion of Spike /(S) protein of COVID-19 with the host cell. Olumiant (barticinib) is a Janus-associated kinase (JAK) inhibitor that has been approved for the treatment of autoimmune diseases. Rheumatoid arthritis, which is known to block ACE-2 endocytosis, can be used to treat COVID-19. Jakafi (ruxolitinib), another JAK inhibitor, is being tested against COVID-19. The viral spike (S) protein attaches to the host cell and initiates COVID-19 infection. Endocytosis or direct fusing of the envelope with the host cell membrane is conceivable depending on the protease enzyme of the host [20].
When the virus enters the host cell, it releases viral RNA into the cytoplasm, where it can attach to the host ribosome and begin the translation process [21]. So any drug which can start this translation process by inhibiting its binding to the host ribosome can also become a drug but it can harm all other human cells also, so may have a lot of side effects. The initially formed polypeptide of the Virus has its protease enzyme and is cleaved into several unstructured proteins. Because complete protein production can be stopped if this COVID-19 protease enzyme is blocked, it can be a better target. Other drugs which are effective to stop the entry of the virus into the host are umifenovir, chloroquine, hydroxychloroquine, or interferon. Out of these Chloroquine and hydroxychloroquine works as Endosomal acidification fusion inhibitors [22,23].
Replication
A multiprotein that can operate as a Replicate Transcriptase Complex is made up of several non-structured proteins (RTC). The RNA-dependent RNA polymerase (RdRp) is the most important RTC protein, as it is directly responsible for RNA replication and transcription from an RNA strand [24]. First, it will form a negative strand from the main positive COVID-19 genome. Then with the help of other non-structured proteins, mRNA is formed from the negative strand which is again positive and it is passed to the Golgi apparatus for the next translation step.
So in this step also has a drug target is to block RdRp after finding out its structure. There is a lot of similarity between the genome of COVID-19 with a genome of SARS, MERS and so almost the same types of proteins are involved in the replication. The drugs which are known to hinder DNA replication in SARS or MERS can be tried against SARS-CoV-2 also. They are lopinavir/ritonavir, ASC09, or darunavir/cobicistat, which blocks 3C-like protease (3CLpro) and viral RNA synthesis (which is blocked by remdesivir, favipiravir, emtricitabine/tenofovir alafenamide, or ribavirin). Many other protease inhibitor drugs, such as saquinavir, indinavir, lopinavir, and ritonavir, as well as the proteasome inhibitor carfilzomib, two respiratory syncytial virus drugs, a schizophrenia medication, and an immunosuppressant, are undergoing clinical trials following the discovery of the crystal structure of 3CLpro protein [23].
Release outside the Host Cell
This is the penultimate step inside the host cell, and after exocytosis, the host cell releases a large number of virions. Each virion can then infect new fresh cells, and the lifecycle can continue in other host cells until it raptures, releasing a large number of virions. Translation i.e. the protein synthesis from the newly synthesized mRNA of COVID-19 takes place in the Endoplasmic reticulum on the Ribosomes of the host cell. Essential (structural and accessory) proteins like M, S, E, etc. are synthesized which can enter the Golgi Apparatus of the host cell where full virion is formed by binding to its nucleocapsid and is released outside the cell via exocytosis through secretory vesicles [24]. Here also a lot of Drug targets are there like blocking the enzymes involved in the whole process.
Symptoms of Covid-19
symptoms associated with coronavirus infection are well listed on the site of the WHO (world health organization). Sometimes less or most common symptoms disappear without hospitalization; however, in case of severe symptoms, hospitalization is required. Fever, dry cough, and fatigue are the most common symptoms. The less common symptoms include ageusia (loss of taste), anosmia (loss of smell), sore throat, headache, body ache, nasal congestion, skin rash, nausea, vomiting, conjunctivitis, diarrhea, chills, depression, anxiety, seizures, stroke, brain inflammation. High temperature, dyspnea, pressure on the chest, reduction of speech and movement, loss of appetite are severe symptoms.
Possible Treatments and Therapies for Pandemic
Treatments under Allopath
“In the allopathic approach, therapeutic drugs are used against the virus-based and host-based targets. The table below enlisted both types of targets along with their potential drugs that are used to combat the virus. The virus-based targets include structural proteins and non-structural proteins. The structural proteins like spike protein, envelope protein, membrane protein, and nucleocapsid protein whereas; non-structural proteins are the main protease (3CLpro), the papain-like protease (PLpro), the RNA dependent RNA polymerase (RdRp), and helicase. The host-based targets are ACE2 (Angiotensin-converting enzyme 2), TMPRSS2 (Transmembrane Serine Protease 2), Cathepsin L, AAK1 (Adaptor-Associated Kinase 1) and GAK (Cyclin G-Associated Kinase), PIKfyve (phosphatidylinositol 3- phosphate 5 kinase, Two pore channel (TPC2), DPP4 (dipeptidyl peptidase 4)” [25-28]. (Table 1)
| Category | Potential Drugs |
|---|---|
| S protein inhibitors | Arbidol (Umifenovir) Chloroquine/Hydroxychloroquine Lipopeptide inhibitor (IBP02) |
| 3CLpro inhibitors | Disulfiram (NCT04485130) Carmofur Lopinavir/ritonavir (NCT04455958) |
| RdRp inhibitors | Remdesivir (NCT04292730) Favipiravir (NCT04359615) Triazavirin (NCT04581915) |
| Helicase inhibitors | Bananin Idobananin, Vanillinabin Eubananin |
| ACE2 inhibitor | Telmisartan soluble ACE2/APN01 |
| TMPRSS2 inhibitors | Camostat (NCT04608266) Nafamostat (NCT04418128) Bromhexine (NCT04355026) Genistein Estradiol Enzalutamide(NCT04475601) |
| Cathepsin L inhibitors | SID 26681509 SSAA09E1 |
| AAK1 (Adaptor-Associated Kinase 1) and GAK (Cyclin G-Associated Kinase | Baricitinib(NCT04421027) 3,5-disubstitured pyrrolol [2,3-b] pyridines (LKB1) Sunitinib Eriotinib Nintedanib(BIBF 1120) |
| PIKfyve (phosphatidylinositol 3- phosphate 5 kinase) inhibitors | Aplimod (NCT04446377) YM201636 |
| Two pore channel (TPC2) | Fluphenazine and Pimozide (dopamine antagonists) Raloxifene, Clomifene and Tamoxifen (selective estrogen receptor modulators) |
| DPP4 (dipeptidyl peptidase 4) inhibitors | Gliptins |
Though there is no new medicine developed for the virus. The FDA only approved the remdesivir (Anti-viral) drug [29], which is usually given by an injection into a vein of the hospitalized adults and pediatrics.
Targeting transcriptional regulation of Covid-19 entry points (ACE2, TMPRSS2)
The entry of coronavirus into a cell particularly of lungs, kidney, heart, intestines through ACE2 (Angiotensin-converting enzyme 2) receptors and a surface protein TMPRSS2 (transmembrane protease serine 2) is the pivotal step for initiation of the life cycle of coronavirus. Angiotensin-converting enzyme 2 is an important enzyme for the changing of Angiotensin II to Angiotensin in RAAS (Renin-angiotensin system) [30]. RAAS is the regulatory system in the human body that maintains blood pressure and fluid balance. An endothelial cell surface serine protease TMPRSS 2 is produced from TMPRSS 2 gene whose expression is regulated by androgens in some of the lung cells. TMPRSS 2 is extensively studied in the case of prostate cancer as its expression linked with the level of androgens. More the protease serine 2 effective will be the entry of virus in the cells. Since males have more androgens in comparison to females, it has been studied that the severity of the disease is more pronounced in males (high mortality rates) than females [31]. Both ACE 2 and TMPRSS 2 are regulated by androgens. Hence, targeting the transcriptional regulation of covid-19 entry points could be an effective approach to get rid of it. The transcription of TMPRSS 2 and ACE 2 is linked with androgen receptor (AR) activity. It is thought to block AR activity by AR antagonists that are approved by the FDA like enzalutamide, apalutamide, darolutamide [32], and AR degraders like ARD-61 that lead to the reduction in expression of TMPRSS 2 and ACE 2 which is helpful in attenuation of positive single-stranded RNA coronavirus [33]. It is very important to mention that not all lung epithelial cells have androgen-regulated TMPRSS 2, ACE 2, and AR signaling expressions. That’s why in the case of cells that are not mediated by androgens, for inhibition of entry factors (TMPRSS 2 and ACE 2) expression BET protein inhibitors are used but showed different efficacy than AR inhibitors. BET protein inhibitors work by reducing the amount of surplus cytokine production during SARS-CoV 2 infection. Generally, BET inhibitors belong to a class of drugs that binds to bromodomain and extra terminal motif proteins and interferes with the interaction among BET proteins and transcription factors in case of cancer and inflammation. BET proteins play an important role as co-activator in regulating AR activity [34]. It is observed that the utilization of BET inhibitors can reduce the TMPRSS 2 and ACE 2 genes expression. More research and clinical trials are required to prove it to greater efficiency. In conclusion, BET protein inhibitors block the expression of TMPRSS 2 and ACE 2 hence, making sites unavailable for the attachment of the virus. Recently, it is suggested that α1-Antitrypsin (a protein produced by the immune system to combat infections) blocks TMPRSS 2 expression and Coronavirus infection [35].
Sequential doxycycline and colchicines combination therapy
When coronavirus take up by the human body it comes in contact with ACE 2 and dipeptidyl peptidase 4(DPP 4) receptors present mainly on lung cells which trigger the macrophages as a part of immune response that further facilitates the liberation of pro-inflammatory cytokines like interleukins and tumor necrosis factor (TMF). This response mainly depends upon the concentration of virus in blood also known as viral load and response is called the innate immune response. Coronavirus induces autoimmunity [36], an exaggeration of the immune system of the body due to the cytokine storm in the second phase of the infection which is responsible for pneumonia, lung injury, and acute respiratory distress syndrome. It has been observed in five patients admitted to the hospital with pneumonia and acute lung injury that sequential combination therapy of doxycycline and colchicine reduces the symptoms of Covid-19 and patients were discharged well from the hospital [37]. Colchicine is known to inhibit the spindle (microtubule) formation of cells with a high division rate like neutrophils (a kind of WBC’s that helps in fighting infection). Moreover, it has an anti-inflammatory property that works by inhibiting neutrophils function or by suppressing cytokine formation [38]. Due to its anti-inflammatory nature, it is recommended for the control of covid-19 infection. Doxycycline is a tetracycline-based antibiotic as well as anti-inflammatory medicine [39]. It helps in attenuation of the virus by blocking DPP 4 expression and inferring the replication process of the virus by breaking the non-structural proteins of the virus or by inhibition of an enzyme called RNA-dependent polymerase. Approximately, 80 % of covid-19 infections are not severe because of normal immune response with regards to low viral load, and the percent of the population that requires mechanical ventilation is only 5 %. Since it is a small case study and needs a large-scale study of this combination therapy to evaluate the importance of this combination therapy.
Why did hydroxychloroquine fail to act as a potent antiviral drug against COVID-19?
Chloroquine and hydroxychloroquine both in combination found very effective in preventing viral infections in vitro conditions however, this therapy shows no significant improvement of coronavirus infection during clinical trials on humans. There are two ways by which a virus can enter a cell: one way which depends on cathepsin L (an endosomal protease) and the second way rely on TMPRSS 2. Cathepsin L (present mainly in cellular compartments) mediated entry affected by the pH of endosomes whereas, TMPRSS 2 protease (present on the cell membrane) mediated entry is independent of pH. Hydroxychloroquine works by raising the pH of intracellular vacuoles which affects the proteolytic activity of cathepsin L. Hence, an increase in pH has no profound impact on TMPRSS 2 activity which is essential for the entry of SARS-CoV 2. From various experiments, it is found that due to the presence of TMPRSS 2 the inhibitors of endosomal acidification do not affect managing infection caused by SARS-CoV 2. That’s why alone hydroxychloroquine is not effective in managing infection. However, a combination of hydroxychloroquine and TMPRSS 2 inhibitors can be effective in controlling the infection [40].
Treatment under Homeopathy
Homeopathy is a system of medicine used to treat several diseases which are based upon the principle of using naturally occurring chemicals in less concentration that becomes symptomatic when present in high concentrations. An outbreak around 1918-1919 of Spanish flu is the clear evidence to support homeopathy as a therapeutic medicine where 21 million patients died and mortality of those who treated with homeopathic medicines is very low or negligible than treated with allopathic medicines. There are so many historical pieces of evidence that favor homeopathy for the treatment of epidemics like cholera, chikunguniya, malaria, hepatitis, etc [25]. Some examples of homeopathic medicines that are effective in alleviating pain, ache, fever associated with viral infections are Belladona 3 C, Eupatorium perfoliatum Q, Grindelia, Calcarea carb, phosphorous, Camphora, Thymulin, Gelsemium, etc. A medicine included in health advisory by Ministry of AYUSH is Arsenicum album is considered as the best prophylactic homeopathic medicine for covid -19 infections because Arsenic toxicity, inflammation due to SRAS-CoV-2, an infection caused by HIV are similar. It is prepared by heating arsenic in distilled water for about 2-3 days continuously. Arsenic present in the medicine acts on different macrophages and tumor cells [41]. It reduces the NF-κβ which is the transcription factor that regulates gene expression of response linked with innate and adaptive immunity and it also decreases the TNF-α or tumor necrosis factor. When infection is detected by the macrophages they release TNF-alpha to alert other cells (cell signaling) for immune response and inflammation-causing cells. According to the guidelines given by the Ministry of Ayush the list of homeopathic medicines for treating the symptoms of coronavirus infection are Aconite napellus, Arsenicum album, Bryonia alba, Gelsemium sempervirens, Rhus tox. Eupatorium perfoliatum, Ipecacaucunha, Belladona, Camphora, phosphorus, Chelidonium, Veratrum Viride, Iodum, Cinchona officinalis, Lycopodium, Stannum met, Carbo veg, Antimonium arsenicum [42].
Treatment under Ayurveda
Ayurveda is practicing for more than 3000 years in India in healing diseases. In this approach body, mind and spirit are balanced with the use of proper diet, herbal medicines, meditation, exercise, and other methods. The ayurvedic medicines are thought to be worked by inhibiting endocytosis of virus particles and by blocking signaling of ACE 2 receptors in the case of coronavirus. The exact mechanism is not known how they help in reducing the severity of covid-19. Pre-existing ayurvedic medicines are used for covid infection which gives positive results in recovering infection. The two case studies are published where only ayurvedic medicines are used for SARS-CoV-2 infection. First, a 26 yr old woman was admitted to the hospital in Mumbai with difficult breathing, high fever, cough, chills, etc and she consciously shifted her allopathic treatment to ayurvedic treatment [43]. From the Ayurved practitioners, she took Sadangapaniyam with Guduci (alleviate fever and boost immunity), Saddharanacurna (helps to overcome digestive disorders, indigestion), Suksmatriphala (to treat viral infection), Kanakasaram (acts as a bronchodilator and anti-inflammatory and also increase airflow to the lungs), Indukantam kasayam (to treat loss of appetite). On the 19th day, she completely recovered and RT-PCR tests came negative. This combination of drugs is proved effective in treating the deadly virus of that lady. There are so many valuable herbs are present which need to be studied for the eradication of diseases. Second, a 43 yr old male in New York, USA presented with covid symptoms and he was administrated with Sudarsana churna (boost immunity against bacteria and viruses), Talisadi churna (for cough, lung infection, and respiratory disorders), Dhanwantara Gutika (Antimicrobial, Antioxidant, Antipyretic, Anti-stress and treat breathlessness), Vidaryadi Ghritam (helps in strengthening of lungs, improves muscle fatigue and increase the immunity of body). After a month of regular intake of prescribed medicines by the doctor test report also came negative [44]. All the remedies in Ayurved work better when taken with proper diet and exercise. This field is rich with abundant therapeutic drugs which need to be explored.
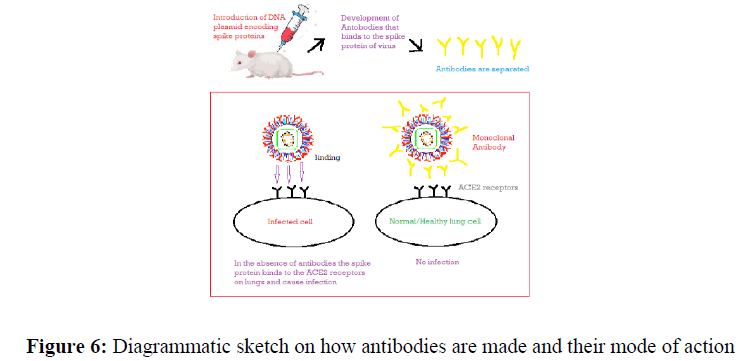
Monoclonal antibody therapy
It is very important to understand what kind of antibodies are produced by B-type lymphocyte cells in our body in response to antigens (coronavirus) so that these can be extracted from the blood of an infected patient and can use in the treatment of other covid 19 patient. Convalescent plasma therapy can be a potential practice to alleviate the infection. It was used in the 1930s and based upon a simple principle that antigen triggers the production of antibodies specific to that virus in the body [45]. Usually, blood is collected after the recovery of the patient from a viral infection which particularly contains antibodies, and serum containing antibodies are extracted from blood and injected into a newly infected patient that helps to do battle against the infection. After reviewing the literature it is thought to employ convalescent plasma therapy to treat this infection. Shen et al. used this therapy on 5 critically ill patients out of which 3 are recovered well and 2 recovered after some time. Later Casadevall and Pirofski explained the risks associated with this therapy are: danger of getting other infections by transfusion process and risk of ADE (antibody-dependent enhancement) which can exacerbate Covid-19 [46]. That’s why monoclonal antibody therapy is required that targets specifically the spike proteins of the virus and prevents its entry into our cells (Figure 6) [47].
A spike protein of coronavirus binds to the ACE 2 receptors and 77.5 % of the amino acid sequence is similar in both [48]. The receptor-binding domain (RBD) of spike protein is highly variable [49]. SARS-CoV 1 and SARS-CoV 2 have distinct RBDs and Tian et al. found that monoclonal antibodies that target RBD like m396, CR3014 used for SARS-CoV 1 is ineffective for SARS-CoV-2. Further, their research stated that CR3022 can nullify both SARS-CoV 1 and SARS-CoV 2 effectively. Tian advised CR3022 as a potential therapeutic for covid 19 infections [50]. Another monoclonal antibody 47 D 11 binds to the spike’s RBD of the virus which is reported by Chunyan Wang et al. and neutralizes the virus [51]. The use of monoclonal antibodies may also reduce the spread of the virus. US-FDA approved the only emergency use of Casirivimab (REGN10933) and Imidevimab (REGN10987) together to close the entry of virus into the human cells. These monoclonal antibodies developed by Regeneron Pharmaceuticals against spike glycoprotein on SARS-CoV-2 that are sold in the market under the brand name REGEN-COV [52]. This therapy is given to adults and pediatric patients and not administrated to the hospitalized patients. In February 2021 CHMP (The Committee for Medicinal Products for Human Use) declared that REGWN-COV2 can be used as a remedy to treat covid 19 especially those patients who do not require mechanical ventilation. Another monoclonal antibody developed by Eli Lilly is called bamlanivimab (LY-CoV555) [53] also approved by FDA for emergency use. A combination of bamlanivimab and etesevimab seems very effective in reducing viral infection.
Recent Development in which treatment can be beyond the lifecycle of the SARS-CoV-2
Recently it was found by some Chinese Scientists that covid 19 impairs the RBC cells inside the lungs. According to them some of the SARS-CoV-2 nonstructural proteins attacks the 1--a strings of the hemoglobin preferably deoxyhaemoglobin in such a way that hemoglobin breaks into a porphyrin ring and free iron by some process which is not very clear, which can be toxic for the body. According to them the patient dies due to a shortage of oxygen as the hemoglobin in RBC keeps breaking and hence the body is neither getting oxygen nor the carbon dioxide is removed from the body, so treatment can include blood transfusion having fresh RBC rather than a ventilator (until some other complication is there) although still, it needs to be confirmed with more studies [54].
Scientists have found that few animals like crocodiles and sharks etc. are almost free of any bacterial or viral infection. So few scientists studied these animals and isolated the compound especially from sharks known to provide antiviral properties. The compound is like a steroid molecule and is known as Squalamine. The Squalamine has a positively charged chain and it attaches to the inner layer of the cell and in-process displaces the positively charged proteins inside the cell which are attached to the inner membrane of the cell, which is found to be essential for any virus multiplication inside the cell [55]. So such types of compounds can also become a treatment for the SARS-CoV-2 treatment.
Role of micronutrients in alleviating Covid-19 symptoms
Vitamins and minerals play a crucial role in the maintenance as well as the proper functioning of our body. They also help our immune system to work. From the literature studies, it has been found that there is a correlation between covid-19 symptoms and micronutrient deficiency symptoms. They are not the complete cure but can help us to recover fast and can minimize the severity of SARS-CoV-2 infection. Supplements of vitamins like B, C, D, E, and other micronutrients like zinc, selenium, magnesium are used along with other medications to treat symptoms of coronavirus infection. Omega-3 fatty acids also assist in alleviating symptoms of the virus. Detailed functions and their deficiencies are well listed in table 2. It is not necessary to take supplements; a properly balanced diet enriched with these micronutrients could also prevent us from infection. Zinc, Vitamin C and Vitamin D help to adhere to epithelial cells (biological barrier), deficiency of which results in weak interactions between cells and makes easy penetration of a pathogen [56-59].
| Micronutrient | Functions | Deficiency |
|---|---|---|
| Vitamin B [63] | Innate and adaptive immune response activation, Helps in reducing levels of pro-inflammatory cytokines, Play role in endothelial integrity, Checks hyper-coagulability, Reduces breathing difficulty. |
|
| Vitamin B1 (Thiamine) | Reduces risk of Cardiovascular diseases, Kidney diseases, Neurodegenerative disorders, Cancer, Type-2 diabetes. It acts as a carbonic anhydrase isoenzyme Inhibitor. |
Neuroinflammation, Poor antibody response (especially T-cells, which involves in the Eradication of coronavirus), Severe symptoms of SARS-CoV-2. |
| Vitamin B 2 (Riboflavin) | The presence of ultraviolet light causes damage to the DNA and RNA, Inhibits the replication of pathogens, An essential component of FMN (flavin mononucleotide) and FAD (flavin adenine dinucleotide), Maintains a level of homocysteine. |
Anemia, Cataract, Endocrine abnormalities, Affects the metabolism of nutrients like other B vitamins. |
| VitaminB3 (Niacin) [NCT04809974] |
Form NAD and NADP (both involved in chronic inflammation) help in immunomodulation, Reduces the level of IL-β, IL-6, and TNF-α (tumor necrosis factor), Anti-inflammatory, Strengthens our immune system, Prevents lung tissue from damage, Anti-aging. |
Pellagra, Reduced immunity. |
| Vitamin B5 (Pantothenic acid) | Reduce inflammation, Maintains good mental health, Helps in lowering cholesterol, Quick healing of the wound. |
Numbness, Burning of hands and feet, Headache, Fatigue, Restlessness, Irritability, Improper sleep. |
| Vitamin B6 (pyridoxine, PLP: pyridoxal 5’-phosphate, an active form) | Cofactor in several inflammatory pathways. | Immune dysregulation, Risk of Coagulopathy, Lower the PLP, the higher the risk of covid-19 infection. |
| Vitamin B9 (folic acid) | DNA and protein synthesis, Adaptive immune response, Prevents binding of spike proteins, cell entry by inhibiting furin, Prevents pregnant women from infection. | Anemia, Colo-rectal cancer. |
| Vitamin B12 (cyanocobalamin) | RBC synthesis, Myelin sheath synthesis, Enhances the rate of DNA synthesis (cellular growth), Modulates gut microbiota, Reduction in SARS-CoV-2 related organ destruction and severity of symptoms. |
Increases the amount of homocysteine, methylmalonic acid which contributes to inflammation and elevates oxidative stress, Hyperhomocysteinemia leads to coagulation and platelet activation, megaloblastic anemia, damaged myelin sheath, and reduces the response of the immune system; Creates problems in respiratory, central nervous, and gastrointestinal systems, |
| Vitamin C (Ascorbic acid) [NCT04323514] |
Anti-oxidant, Anti-histamine, Controls cytokine storm, Prevention of biomolecules destruction like DNA, RNA, lipids, proteins, carbohydrates) from toxins and pollutants, Co-factor of various enzymes, Biosynthesis of hormones like vasopressin, etc. Reduces inflammation, Boosts immune system, Prohibits pathogen entry, Remove neutrophils from damaged tissues, Reduces sepsis in pulmonary dysfunction, In adults and children, it shortens the duration of a common cold., Reduces pneumonia. |
Increases the risk of respiratory failure by escalating the level of IL-6 (pro-inflammatory cytokine interleukin). |
| Vitamin E (α-tocopherol) [64] |
Anti-oxidant, Modifies host immune response. (Limited role in SARS-CoV-2 prevention) |
Impairs humoral and cellular immunity. |
| Zinc [NCT04621461] |
Antiviral, Immunomodulatory, Inhibits the ACE 2 enzyme from binding with spike proteins of the virus, Prevents pathogen entry as Zn plays role in intercellular protein structure between cells, Reduces the risk of diarrhea, Regulates the activity and development of T-cells, helpful in reducing cytokine storm, Increase the interferon cytokine signaling, Decreases the replication of RNA viruses. |
Natural killer cells and cytolytic T cells are both affected (both are essential in destroying viruses, bacteria, and tumor cells), Immunosenescence (dysregulation of the immune response), Affects intercellular junctions and attenuates the barrier for virus entry, Higher susceptibility to infectious diseases. |
| Selenium [NCT04869579] |
Antioxidant, Cofactor (in glutathione peroxidize), Prevents entry of virus in the cytoplasm, Strengthens the immune system, Increases the production of natural killer cells and T-lymphocytes, Anti-coagulant reduces the formation of blood clots in covid-19 patients. |
The weak immune system, Higher mutation rates in viruses, Keshan disease. |
| Magnesium [65] | Anti-inflammatory (by inhibiting L-type calcium channels), Bronchial smooth muscle relaxation, Anti-oxidant, Anti-spasm, Vasodilator, Regulates respiratory systems, Protection of the nervous system. |
Hypocalcemia, Hypokalemia, Electrolyte imbalance. |
| Omega-3 fatty acids (eicosapentaenoic and docosahexaenoic fatty acids) [NCT04836052] |
Anti-viral, Improves oxygenation, Synthesis of inflammatory mediators like, prostaglandins, leukotrienes, thromboxanes, protectins, and resolvins, Modulate membrane fluidity, Activation of macrophages, Promoting phagocytosis, Downregulation of NF-κβ, Inhibits viral replication, It Checks viral entry. |
Change in composition of the cell membrane, Susceptible to viral infection. |
| [NCT: The National Clinical Trial Number] | ||
The numbers of clinical trials of the above-listed micronutrients (in the form of supplements) are registered on the clinicaltrials.gov site. Results revealed the great importance of these micronutrients in the quick recovery of patients with respiratory viral infections. Some of the supplements are still under clinical trials. The use of these micronutrients as a treatment is cost-effective, safe, and widely available that’s why more clinical studies are required not to evaluate them thoroughly but to find other therapeutic micronutrients against a worldwide pandemic.
Vaccines
After getting infection from coronavirus formed antibodies generally attacks the spike proteins to neutralize the virus and inhibit its entry into the host cell. The basis for the development of a vaccine is to target the spike proteins (antigen) of coronavirus. During the first encounter of the coronavirus, our innate immunity becomes active by secreting interferons and cytokines also called the phase1 of infection where interferons try to hinder the replication process of the virus. Symptoms include fever, body aches, etc. After 6-8 days, phase 1 of infection enters phase 2 characterized by the adaptive immune response which involves white blood cells particularly T-cells and B-cells. The cell-mediated immunity due to T-cells in which no antibody formation takes place however activates phagocytic cells, release cytokines and antigen-specific cytotoxic lymphocytes. B-cells are responsible for humoral immunity that allows the production of antibodies specific to antigen and neutralizes the virus. CD8+ cytotoxic T cells and CD4+ helper T cells are the two types of T cells involved in adaptive response (cellular) that prevent the multiplication of the virus and activates B cells to make antibodies. B cells secrete antibodies like IgG (protects lower respiratory tract) and IgA (protects upper respiratory tract) and our body produces the first IgM to combat viral infection [60]. On 16/05/2021 statistical data (worldometer) showed a total of 163 million cases worldwide of coronavirus, 141 million cases recovered and 3 million are death cases. These figures keep on rising day by day that’s why a vaccine with greater efficacy is required. The normal period for the development of a vaccine is 15 years or more whereas; the development of the COVID-19 vaccine takes 10 months to 1.5 years. “According to WHO (4, October 2020), There are 42 covid candidate vaccines out of which 10 are in phase 3 of a clinical trial: Sinovac, Wuhan Institute of Biological Products/Sinopharm, Beijing institute of Biological Products/Sinopharm, University of Oxford/AstraZeneca, CanSino Biological Inc. / Beijing institute of biotechnology, Gamaleya Research institute, Janssen Pharmaceutical Companies, Novavax, Moderna/NIAID, BioNTech/Fosun pharma/Pfizer. The number of the candidate vaccine in pre-clinical evaluation is 151. All of the top candidate vaccines are intramuscular injections and based on a two-dose schedule except AstraZeneca and CanSino Biological Inc”[61]. 58 vaccines candidates are under clinical trials phase 2/3 based on Nanoparticle vaccine, plant-based adjuvant vaccine, DNA vaccine, multitope peptide-based vaccine, CVLP based vaccine, Recombinant vesicular stomatitis virus vaccine, SF9 cell vaccine, self-replication vaccine, dendritic cell vaccine, Intranasal vaccine, modified vaccinia virus Ankara vector vaccine, etc all are well listed on Covid-19 vaccine tracker/RAPS site [62]. The list is given ahead in this review of vaccines with their efficacy and most of them have completed their phase 3 clinical trials (Table 3).
| Vaccine Name | Origin | Type of vaccine | Manufacturing Company | Dose schedule/Efficacy based on clinical 3 trial |
|---|---|---|---|---|
| Comirnaty (BNT162b2) |
Multinational | mRNA based vaccine | Pfizer, BioNTech, Fosun Pharma | 2 doses; 21 days apart. 95% efficacy in 16 years of age or older [66]. |
| Moderna Covid-19 vaccine(m-RNA -1273 | The United States | mRNA based vaccine | Moderna, BARDA (biomedical advanced research and developmental authority), NIAID (national institute of allergy and infectious diseases). | 2 doses; 28 days apart. 94.1% efficacy [67] |
| AstraZeneca(AZD1222) also called Covishield | The United Kingdom | Adenovirus vaccine | BARDA, OWS | 2 doses; 4-12 weeks apart. 76% efficiency [68] |
| Sputnik V | Russia | Recombinant adenovirus vaccine(rAd26 and rAd5) | Gamaleya Research institute Acellena Contract Drug Research and Development | 2 doses; 21 days apart. 91.6 % Efficacy [69] |
| Janssen(JNJ-78436735; Ad26.COV2.S) | The Netherlands, US | Non-replicating viral vector | Janssen Vaccines (Johnson and Johnson) | Single-dose; 66 % effective [70] |
| CoronaVac | China | Inactivated vaccine(formalin with alum adjuvant) | Sinovac | 2 doses; 14-28 days apart. 50.65% effective in preventing infection (Trials in Brazil reported in the Wall Street Journal) |
| BBIBP-CorV | China | Inactivated Vaccine | Beijing Institute of Biological Products; China National Pharmaceutical Group, Sinopharm | 2 doses; 28 days. 86 % effective [71]. |
| EpiVacCorona | Russia | Peptide Vaccine | Federal budgetary Research Institute State Research Center of Virology and Biotechnology | phase 3 trial will begin in November/December |
| Convidicea (Ad5-nCoV) |
China | Recombinant vaccine (adenovirus type 5 vaccine) | CanSino Biologics | Single dose vaccine 65.7 % effective [72] |
| Covaxin | India | Inactivated Vaccine | Bharat Biotech, ICMR | 2 dose; 4 weeks apart. 81 % effective [73]. |
| WIBP-CorV | China | Inactivated Vaccine | Wuhan Institute of Biological Products; China National Pharmaceutical Group, Sinopharm | Phase 3 trials are underway, from phase1/2 72.5% efficacy is recorded [74] |
| CoviVac | Russia | Inactivated Vaccine | Chumakov Federal Scientific Center for Research and Development of Immune and Biological Products | Approved for use in Russia, trials are not yet begun |
| ZF2001 | China, Uzbekistan | Recombinant vaccine | Anhui Zhifei Longcom Biopharmaceutical, Institute of Microbiology of the Chinese Academy of Sciences | Approved for emergency use in China [75]. |
| QazVac (QazCovid in) |
Kazakhstan | Inactivated Vaccine | Research Institute for Biological Safety Problems, Kazakhstan | 2 doses; 21 days apart; 96% effective [76]. |
Viruses can change their genetic material via mutations. SARS-CoV-2 variants due to mutations or unknown factors are spreading globally which is the major challenge in the development of a vaccine. It leads to quick transmission of the virus, increases mortality rates, develops resistance to Anti-SARSCoV-2 drugs or vaccines [77]. B.1.1.7 is the variant first detected in the United States in December 2020 then identified in the UK. This variant mutates very fast than other variants which also the reason for the large number of deaths in these countries. The preprint bioRxiv delineated that the Comirnaty vaccine is effective in opposition to the B.1.1.7 variant of SARS-CoV-2 [78]. Vaccine BBIBP-CorV is found to be effective against the South African variant [79]. The whole DNA sequence of the covid 19 is recorded on 11, January 2020 in GenBank [80]. Recently, a case of the double mutant (third variant formed from a combination of two variants) was found in Maharashtra, India that is formed by E484Q and L452R mutations. In other words Indian variant B.1.617 is formed by two mutations [81]. The E484Q mutations are caused by the exchange of glutamic acid by lysine at position number 484 whereas; L452R is S protein RBD (receptor binding domain). In April 2021 another additional mutation V382L (in the spike) was found in B.1.617 hence named it triple mutant in India which makes it more dangerous. The World Health Organization considered this variant as a variant of concern worldwide. Though it is thought to be the reason for the surge in covid cases of India, investigation of these variants is still required.
Conclusion
So Scientists around the world are exploring to find out some fast cure for the COVID-19. So the best is to look into all the inhibitors of the enzymes, targets involved in the life span of the SARS-CoV-2 as its shape and processes have close resemblance with already known viruses like SARS, MERS, etc. So FDA-approved drugs can be seen against the identified enzymes or targets and clinical trials can be done against those. Almost 66 human proteins in host pathways are identified and are targeted by 69 existing drugs approved by the FDA or compounds and they are currently under investigation [82]. Another approach is to do molecular docking studies and by using data from chem informatics databank which will contain the exact structure of enzymes and drug targets and find out which exact structure should be the best fit and find out the probable structure of the drug which can be most effective against COVID-19. It is found that people vaccinated against BCG vaccination for the disease Tuberculosis are either not having the SARS-CoV-2 infection or the intensity of the infection is not in the fatal direction [83]. Since BCG is not required in countries like the USA, Europe, etc. which are developed countries and have a comparatively cleaner environment as compared to India where it’s part of the health regime of the infants and almost everyone gets this vaccine in their childhood, comparatively fewer deaths and less severity of the disease is seen in India as compared to the USA or European countries. The best approach to treat this virus is vaccination. Presently, vaccination drives are going on to vaccinate almost all the population of the world. Some of the vaccines are very effective like Pfizer on the other hand a few are under controversies because of their side effects that have been reported in some cases or no antibody formation even after the vaccination like Covaxin and Covishield in India. Soon we will have the best vaccine all over the world as scientists are working tirelessly to address all the complications related to the vaccine. There are many drugs available for the cure of infection in covid 19 patients. For instance, a drug named Ivermectin which is a prescribed drug used against parasites mostly for treatment of diseases like Scabies, treatment against head lice, etc. is found to kill COVID-19 within 48 hours in in-vitro studies [84], the clinical trials of this drug are still going on. There are various allopathic, ayurvedic, homeopathic medications, and therapies available to alleviate SARS-CoV-2 infection yet none found suitable completely for the disease as most of them are under clinical trials. The FDA just approved the remdesivir, an anti-viral drug for the treatment of this virus. It is found that SARS-CoV2 infection is linked with an exaggerated immune response like inflammatory response against the virus that might reduce the oxygen level in our body where the use of a specified amount of steroid is recommended at the right time that’s why the WHO allows the use of corticosteroids to reduce the inflammatory response in covid patients. In India, the misuse of steroids is a major concern as it causes another deadly disease called Mucormycosis or black fungus. It has been studied that the ignorance of early infection symptoms leads to high fatality rates. The DGHS has given the guidelines for the use of drugs along with supplements for covid suspected patients shown in the table below (Table 4).
| Name of the Drug/supplement | Quantity | Duration |
|---|---|---|
| Tab Ivermectin | 12 mg once a day | 3 days |
| Tab Zinc | 50 mg once daily | 3 weeks |
| Tab Vitamin C | 500 mg once daily | 3weeks |
| Tab Vitamin D 60,000 IU | Once a week | 3 weeks |
| Tab Paracetamol | 500-650 mg (6-hour gap between two doses) | Till fever subsides |
Along with these medicines steam inhalation, intake of plenty of fluids, and cough relieving syrups are also recommended. The DGHS (Directorate General of Health Sciences, India) removed Ivermectin, zinc, multivitamins, hydroxychloroquine, and favipiravir (anti-viral) from the list for the treatment of mild cases of coronavirus infection on June 7, 2021, and instead placed an emphasis on a well-balanced diet and a positive attitude for a quick recovery [85]. Still lot of changes can be expected to come up in the near future to perfectly control and eradicate this virus completely from the world.
Acknowledgments
The authors are thankful to Chandigarh University, Gharuan for providing them with all types of help to complete this review.
References
- Lauer SA. Ann Intern Med. 2020. 172(9): p. 577–582.
- Zoonosis. Merriam-Webster Dictionary. 2011.
- Zoonoses. WHO. 2014.
- The National Centre for Foreign Animal Diseases. 2019.
- Coral-Almeida M, Gabriël S, Abatih EN et al., PLoS Negl Trop Dis, 2015, 9(7): p. e0003919.
- Taylor LH and Latham SM. Philos Trans R Soc Lond B Biol Sci. 2001, (356): p. 983–989.
- Marx PA, Apetrei C and Drucker E. J Med Primatol. 2004, 33: p. 220–226.
- Zoonosis. Medical Dictionary. 2013.
- Messenger A, Barnes A and Gray G. J Exot Pet Med. 2014, 23(4): p. e89055.
- Humphrey T, Brien SO and Madsen M. Int J Food Microbiol. 2007, 117(3): p. 237–257.
- Gelderblom HR, Medical Microbiology. 1996.
- Van Regenmortel MHV. Serology and Immunochemistry of Plant Viruses. 1982, p. 174–192.
- Cascella M, Rajnik M, Cuomo A et al., StatPearls. 2020.
- Schoeman D and Fielding BC. Virology journal. 2019, 16(1): p. 1–22.
- Laurie Barclay MD and Esther Nyarko. Medscape.2020.
- Kuba K et al. Nature medicine. 2005, 11(8): p. 875–879.
- Tai W et al. Cell Mol Immunol. 2020, 17(6): p. 613–620
- Hoffmann M et al. cell. 2020, 181: p. 271–280.
- Park Y. Atlas of Genetics and Cytogenetics in Oncology and Haematology, 2010.
- Grove J and Marsh M. J. Cell Biol. 2011, 195(7): p. 1071-1082.
- Lodish H, Berk A, Zipursky SL et al., Molecular Cell Biology. 4th edition, WH Freeman, 2000.
- Al-Bari MAA. Pharmacol res perspect. 2017, 5(1): p. e00293.
- Fear G, Komarnytsky S and Raskin I. Pharmacology & therapeutics. 2007, 113(2): p. 354–368.
- Alberts B, Johnson A, Lewis J et al., Molecular Biology of the Cell. 4th edition, Garland Science, 2002.
- Talwar S, Sood S, Kumar J et al., Curr Pharmacol Rep. 2020, 6(6): p. 354–363.
- Su H, Xu Y and Jiang H. Fundamental Research. 2021, (2): p. 151–165.
- Gil C et al., J Med Chem. 2020, 21: p. 12359-12386.
- Wu Y, Li Z, S Y et al., Med Res Rev. 2021, 41(3): p. 1775-1797.
- Good SS et al., Antimicrob Agents Chemother. 2021, 65: p. 1-12.
- Qiao Y et al., Proceedings of the National Academy of Sciences of the United States of America. 2020,118(1).
- Chakravarty D et al., Communications Biology, vol. 3, no. 1, pp. 1–12, 2020, doi: 10.1038/s42003-020-1088-9.
- Rice MA, Malhotra SV and Stoyanova T. Front Oncol. 2019, (10): p. 1-12.
- Kregel S et al., Neoplasia. 2020, 22(2): p. 111-119.
- Asangani IA et al., Nature. 2014, 510(7504): p. 278-282.
- Wettstein L et al., Nat Commun. 2021, 12(1): p. 1-10.
- Salle V. Clin Immunol. 2021, 226.
- Al-kuraishy HM, Al-Gareeb AI, Qusty N et al., Pulm Pharmacol Ther. 2021, 67: p. 102008.
- Martínez GJ, Celermajer DS and Patel S, Atherosclerosis. 2018, 269: p. 262-271.
- Gendrot M et al., Molecules (Basel, Switzerland), 2020, 25: p. 1-10.
- Ou T, Mou H, Zhang L et al., PLoS Pathog. 2021, 17: p. 1–15.
- Ive EC, Couchman IMS and Reddy L. Int J Mol Sci. 2012, 13: p. 3979-3987.
- Бевз Т Society. Document Communication. 2021, p. 11-32.
- Joshi JA and Puthiyedath R. J Ayurveda Integr Med. 2020.
- Girija PLT and Sivan N. J Ayurveda Integr Med. 2020.
- Venkat Kumar G, Jeyanthi V and Ramakrishnan S. New Microbes New Infect. 2020, 35: p. 100682
- Zhao Q and He Y. J Clin Virol. 2020, 127: p. 104358
- Zhao Q and He Y. J Clin Virol. 2020, 127: p. 104358
- Yu et al., Immunology Letters. 2005, 100(2): p. 177-181.
- Walls AC, Park YJ, Tortorici MA et al., Cell. 2020, 181(2): p. 281-292.
- Rodriguez JH and Gupta A. Scientific Reports. 2021, 11(1): p. 1-12.
- Wanga C et al., BioRxiv. 2020.
- Copin R et al., Emergence of Viral Escape in Humans, 2021.
- Jones BE et al., Science Translational Medicine. 2021.
- Tian X et al., Emerg Microbes Infect. 2020, 9(1): p. 382-385.
- Liu W and Li H. Am Chem Soc, 2020.
- Zasloff M et al., Proceedings of the National Academy of Sciences. 2011, 108(38): p. 15978–15983.
- Tan CWet al., medRxiv. 2020, 12.
- Name JJ, Souza ACR, Vasconcelos AR et al., Front Nutr. 7: 2020.
- Shakoor H et al., Maturitas. 2021, 144: p. 108–111.
- Shakoor H et al., Maturitas. 2020, 143: p. 1–9.
- Simoneaux R and Shafer SL. ASA Monitor. 2020, 84(8): p. 17-18.
- Jeff C. covid-19-vaccine-tracker. 2021.
- Krammer F. Nature. 2020, 586: p. 516-527.
- National Intitutes of Health. 1895, p. 1–2.
- Shakoor H et al., Maturitas. 2021, p. 108–111.
- Tang FC et al., Eur J Pharmacol. 2020, p. 886.
- Polack FP et al., NEJM. 2020, 383(27): p. 2603-2615.
- Baden LR et al., NEJM. 2021, 384(5): p. 403-416.
- AZD1222 US Phase III trial met primary efficacy endpoint in preventing COVID-19 at interim analysis.
- Jones I and Roy P. The Lancet. 2021, 397(10275): p. 642-643.
- Sadoff J et al., NEJM. 2021, p. 1–15.
- UAE says Sinopharm vaccine has 86% efficacy against COVID-19.
- Biotech B, Council I and Ella K. 2021, p. 3-5.
- Xia S et al., JAMA. 2020, 324: p. 951-960.
- Yang S et al., The Lancet Infectious Diseases. 2021.
- Gibran Naiyyar Peshimam UF. CanSinoBIO’s COVID-19 vaccine 65.7% effective in global trials, Pakistan official says.
- Meyer D. A new vaccine on the scene: Kazakhstan begins rollout of homegrown QazVac. 2021.
- Catalona WJ. Cancer Research, 2009, p. 202-206.
- Macklem PT, Albert RK and Surgery E. Correspondence Susceptibility to Exacerbation in COPD. 2010, p. 2670-2671.
- Kyosuke Kita KY, Atsushi Ishida, Kosei Tanaka et al., crossm Complete Genome Sequence of a Novel. 2015, 5(15): p.2015-2016.
- Li Q et al., Cell. 2020, 182(5): p. 1284-1294.
- Marina T Alamanou. Towards Data Science.2020.
- Cherian S, Potdar V, Jadhav S et al., CSH. 2021.
- PTI. US scientists link BCG vaccination with fewer coronavirus cases, Indian scientists hopeful but cautious.
- Hospital C. Comprehensive Guidelines for Management of COVID-19 patients Symptoms. 2021, p. 1-9.