Research - Der Pharma Chemica ( 2021) Volume 13, Issue 11
Absorption of Lanthanum oxide nanoparticles and its distributive effects on some elements in femur bone of male Wistar rats after acute oral exposure
OT Ogunmodede1*, GM Heidrich2, V. L. Dressler2 and E. Ogola-Emma32Department of Chemistry, Federal University Santa Maria, RS, Brazil
3Department of Chemistry, Bayelsa Medical University, Nigeria
OT Ogunmodede, Department of Chemistry, Afe Babalola University- Ado-Ekiti, Ekiti State, Nigeria, Email: taiwokehindelolade@gmail.com
Abstract
Lanthanum oxide Nanoparticles (La2O3NPs ) absorption and its distributive effects on elements in male Wistar rats femur bone was investigated on four groups of 4-week-old Wistar rats orally administered different doses (1, 10, or 100 mg/kg per day) for 31days. The animals were sacrificed, the femur bones were collected, digested by Microwave-Induced Combustion (MIC), and the metal content was measured by Inductively Coupled Plasma-Mass Spectrometry (ICP-MS) and Inductively Coupled Plasma-Optical Emission Spectrometry (ICP-OES). The results revealed that there are differences in the elemental concentrations of the femur bone sampled and the La2O3NPs concentration. The La2O3NPs concentration treatment was moderately (R = 0.0004952–0.8361) positively correlated with the trace elements in the femur diaphysis. High (R = 0.001021–0.9741) positive correlation was found in the epiphysis. Hence, a comparison of femur Epiphysis and Diaphysis metal concentrations in the region assessed by correlation analysis can be used to determine the natural elemental concentration in bones of mammalian organism.
Keywords
Lanthanum oxide; Nanoparticles; Epiphysis bone; Diaphysis bone; ICP-MS; ICP-MS
Introduction
The bone trace elements and the quantities vary significantly between individuals or bone types. Distribution of elements in the bone matrix is influenced by different variables such as bone location and bone type, growth environment, and taphonomic modifications. The human femoral bone population is often used as a biomarker by chronic exposure of metal to the environment [1]. The trace elements distribution in the bones of the body is not even [2]. In bone, some elements are found in higher concentration in epiphysis regions than diaphysis region whereas in the central portion of the diaphysis region elements such as calcium, strontium, sodium, and potassium are found more often [3]. The relative densities of bones are one of the important characteristics used to differentiate between epiphyseal bone and diaphyseal bone. The spongy bone component of epiphyseal bone is higher than diaphyseal bone, which leads to increase rate of remodelling. This also makes epiphyseal bone to be more prone to chemical change [4]. In the works of Aaseth living organisms tissue is analyzed using highly sensitive chemical analytic methods, specific trace elements both essential and/or non-essential for the living organism were found.
Bone’s affinity for some trace elements shows lower concentrations of trace elements in modern tissues compared to bones of dead bodies [5]. Radioactive trace elements that are produced from nuclear processes pose serious health risks. These elements such as Ca, P, Sr, Pb, Zn, lanthanides, Ba and actinides concentrate in bone seek residual placement before elimination from the body. Hence bones act as bio-archives for hydroxyapatite, collagen, water, and elements such as Na, Mg, Zn, St, Ba, and Pb which are found in food diet. In particular, analyses of femurs and tibiae have been proven very effective because they can give long-term information and fix lifetime metals [6].
In addition, individual ions tend to bond with different parts of Calcium phosphate matrix. Calcium ions can be replaced by metals such Na, Li, St, Mg, La while phosphate group (PO4) can be substituted by citrate, phosphate esters, carbon tetraoxide (CO4), diphosphates and amino acids. The hydroxyl group (OH) can be substituted by halogens [7].
According to Priest, bone valence levels of the radio-nucleotides can remove radio-nucleotides from the blood and deposit them at different locations in the bone system. Depending on the deposition location, bone turn-over rate and environmental conditions, elements can be transferred within or filter out from bone matrix [8]. However, metal substitutions do not occur frequently and there is variation in the occurrence from one to other. This, in addition to bone’s element homeostasis role, results in elemental formation within and outside of the calcium phosphate phase. Hence, in this study, the main objectives were to determine the absorption of La2O3NPs and its distributive effects on some femur elements in the epiphysis and diaphysis of male Wistar rats’ femur bone after acute oral administration of La2O3NPs.
Experimental Procedures
Materials and Methods
Twenty-four (24) femur bone containing lanthanum content after acute oral administration of La2O3 nanoparticles on male Wistar rats were selected.
Reagents and standards
The La2O3NPs were bought from Nanoamor (Houston, USA), with a diameter of 15-30 nm and 99.9% purity. MilliQ water with 18.2 MΩcm resistivity purity was used. The concentrated tri oxo nitrate V acid (HNO3) (65%, Merck, Darmstadt, Germany) used was purified by sub-boiling system. A range of 0.025 to 10 μg L−1 for ICP-MS and 5 to 100 μgL−1 for ICP-OES was used to prepare a standard multi-element solution containing 10 mgL−1 of As, Cd, and Pb (SCP33MS, SCP Science, Quebec, Canada). A stock suspension of La2O3NPs was prepared in MilliQ water with 18.2 MΩcm resistivity purity. The suspension was sonicated in an ultrasonic bath for 10 min before dilution and administration to the animals.
Characterization of La2O3NPs
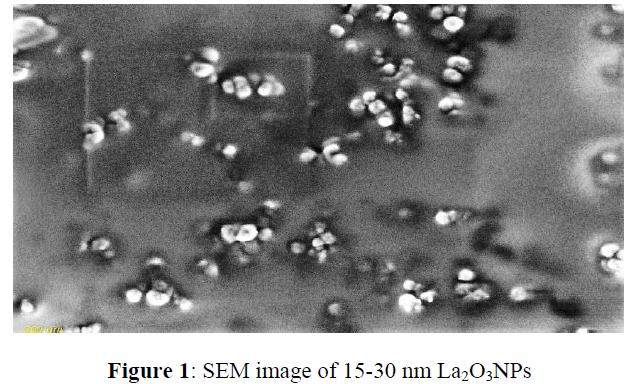
Characterization of La2O3NPs was carried out to assess the size and morphology using Scanning Electron Microscope (SEM) (JEM-2100, JEOL, Japan).
Animal study
A total of twenty-four (24) male Wistar rats of four (4) weeks old, were acquired from Biotério Central of Federal University Santa Maria, Brazil. The body weight of the animals at the beginning of the study was between 200 to 270g. Before submitting it to La2O3NPs treatment, the animals spent 10 days to adapt in four group in a polypropylene cage. The wistar rats were placed under 23ºC ± 1 temperature, 50% to 60% relative humidity and 12 h light/ dark cycles. The feeding of the animals was provided in ad lib. The study was approved by the Animals Ethics Committee of the Federal University Santa Maria, Brazil (protocol number 4250170317).
After ten (10) days of the stabilization period, acute oral toxicity of La2O3NPs was realized according to the OECD guideline 425. The Wistar rats were randomly distributed into 4 groups as described: Group1 (6 rats) is the control group; Group2 (6 rats) were administered with1mg/kg of La2O3 nanoparticles; Group3 (6 rats) were administered with 10mg/kg of La2O3 nanoparticles and Group 4 (6 rats) were administered with 100 mg/kg of La2O3 nanoparticles. The animals were weighed at the end of every week. Control animals received single dose of water only. The solutions were in volume equal to 1.0ml/kg body and between 16 and 17 hrs. The animals were understudied after the treatment for 14 days, and the symptoms associated with intoxication were monitored during this period. Without of an intoxication sign, the animals are weighed and euthanized. Bioaccumulation study of La2O3NPs in heart, lung, spleen, liver, pancreas, kidney, brain, hair and histopathology studies were done. The femurs were wet weighed immediately and stored dry at −80ºC for bulk lanthanum content analysis using ICP-MS and ICP-OES.
Bone preparation for bone material analysis
The bone samples were prepared twenty-four hours after the last treatment. The femoral bones were removed from the male Wistar rats and cleaned of adhering tissue. Chemical analysis was done on two materials: (i) the bone epiphysis/metaphysis part, and (ii) the diaphysis part of the bone. The bone samples were dried to 60ºC temperature and latter were grounded using pestle and mortar.
Digestion in closed vessels method
The method recommended Flores et al was used for the microwave assisted digestion. About 1 mg of the grounded bone sample was digested with 3 mL of concentrated HNO3. Microwave digestion was done for approximately 20 min and at the temperature of 180 ± 5ºC. The digest was cool for 20 minutes and milliQ water was used to dilute the solution to 30 mL in polypropylene vials. The solution in polypropylene vials were diluted 20 times or more to prevent the damage of ICP-MS and ICP-OES nebulizer, chamber, and plasma torch from being damage. Before the determination step, digests were diluted twenty times or more to avoid Trace elements and La in the diluted sample solution was determined by ICP-MS and ICP-OES.
Instrumentation
Spectro Arcos ICP-OES (SPECTRO Analytical Instruments GmbH, Kleve, Germany) and a NexION 300X ICP-MS were used to determine the total metal content of the sample solution. The ICP-OES and ICP-MS instrument were daily optimized to give the highest intensity. NexION 300X ICP-MS in combination with the Syngistix Nano Application Module 1.0 (Perkin-Elmer, Shelton, CT, USA) was used for the NPs analysis in single-particle mode). Tables 1 show the operating conditions of the systems.
| Parameters | ICP-MS | ICP-OES |
|---|---|---|
| Generator power RF, W | 1300 | 1400 |
| Main Argon flow, L.Min-1 | 15 | 15 |
| Auxiliary Argon flow, L.Min-1 | 1.2 | 0.20 |
| Nebulizer Argon flow, L.Min-1 | 1.00 | 0.70 |
| Dwell time ms | 40 | - |
| Reading by replicate | 3 | 3 |
| Reading | 5 | |
| Isotope monitored m/z | 139La,55Mn,65Cu,13C,82Se,98Mo | - |
| Spectra wave lengths nm (Atomic line) (Ionic line) |
- - |
Ca(393,366), Fe(238,204). K(766,490), Mg(285,213), P(214,914), Zn(206,200). |
Statistical analysis
Statistically one-way ANOVA method was used to analyzed significant changes between treated and control animal group. The results of the analysis were in mean and standard deviation. Graph Pad Instat Prism 3 Software package for Windows was used for the statistical analyses. The statistical significance for the tests was set at p < 0.05.
Results
Characterization of La2O3-NPs
The several applications of La2O3NPs have made it to be one of the most interesting NPs. The physicochemical characteristics of La2O3 particles were determined by SEM analysis. Table 2 shows the data obtained while Figure 1 show the size and shape of the La2O3-NPs. The particles were of polyhedron crystals.
| Particles | Size using SEM | Morphology | Purity |
|---|---|---|---|
| La2O3-NPs | 15 - 30nm | Polyhedron crystal | 99.99% |
Measurement by ICP-OES and ICP-MS
The influence of La2O3NPs on element levels in Epiphysis and Diaphysis region of the male Wistar rats’ femoral bone was investigated. The aliquots obtained after digestion of the femoral Epiphysis and Diaphysis of the wistar rats were analyzed in respect of their elemental bulk composition by using ICP-OES and ICP-MS. The obtained quantitative values as shown in Table 3 and 4 revealed the overview of the variable range of different elements in the studied group. The ICP-OES was used to analyse Calcium, Potassium, Phosphorus, Sulphur, and Sodium, because of there usually present in large concentrations and their interference with classical ICP-MS. The La, Mn, Mg, Zn, As, Ni, Se, Pb, Cr, Cd, and Cu were analysed using ICP-MS due to their low concentration. But Mn, As, Ni, Se, and Cu were analysed using both ICP-OES and ICP-MS and the obtained results were compared. The instrumental limits of detection (LDs) and quantification (LQs) was determined by the 3σ and 10σ criteria. Methodological LDs obtained for Diaphysis femur bone were in the range and 0.001-9.737 μg/g for ICP-MS and 0.569- 69.59 μg/g for ICP-OES as shown in Tables 3.
Element levels did not differ significantly between the bone epiphysis and diaphysis of the male Wistar rats tested together at α = 0.05. Thus, differences in element levels between La2O3NPs treated and the control was done for both epiphysis and diaphysis. The elemental concentration in Diaphysis and Epiphysis bone region is shown in Table 3 and 4. The elemental determination in diaphysis region of male Wistar rats femoral bone shown in Table 3 revealed differences in La2O3NPs treated groups. The mean concentration of Ca, P, S, Mn, As, and Se in bones were found lower in the La2O3NPs treated groups compare to control group, whereas La, K, Zn, Ni, Pb, Cr, Cd, and Na concentration were higher in the La2O3NPs treated compare to the control group. However, there is a positive correlation between the La2O3NPs treatment and the element level as shown in table 5.
| Diaphysis Sample with concentration of NP-La2O3 | Elements | Element (µg/g) | Elements | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| La | Ca | P | Mn | S | Mg | K | Zn | As | Ni | Se | Pb | Cr | Ba | Na | Cu | |
| a Control b |
0.01 ± 0.00 n.a |
n.a
319969.03 ± 146.02 |
n.a
119542.6 ± 380.67 |
0.55 ± 0.01 0.32 ± 0.05 |
n.a
1745.01 ± 17.22 |
1704.23 ± 24.83 5176.41 ± 43.33 |
n.a
3402.80 ± 5.09 |
174.34 ± 2.49 247.54 ± 1.74 |
0.53 ± 0.01 15.88 ± 1.37 |
1.35 ± 0.06 2.05 ± 0.57 |
0.66 ± 0.09 15.47 ± 4.63 |
0.09 ± 0.00 15.16 ± 5.54 |
0.44 ± 0.01 n.a |
6.09 ±0.01 n.a |
n.a
7914.82 ±34.49 |
0.94 ±0.01 5.63 ±0.92 |
| a 1mg/kg b |
0.07 ± 0.00 n.a |
n.a
183633.67 ± 241.78 |
n.a ± 128087.1 ± 430.76 |
0.49 ± 0.00 0.65 ± 0.07 |
n.a
1418.50 ± 39.64 |
1941.26 ± 24.56 4752.30 ± 95.26 |
n.a
3379.22 ±11.70 |
180.43 ± 1.16 252.68 ± 1.42 |
0.35 ± 0.02 14.78 ± 2.65 |
1.23 ± 0.05 1.84 ± 0.72 |
0.31 ± 0.03 11.65 ± 4.30 |
0.21 ± 0.03 7.94 ± 6.68 |
0.17 ± 0.02 n.a |
5.39 ±0.02 n.a |
n.a
8405.46 ±89.29 |
1.23 ±0.03 3.58 ±0.97 |
| a 10mg/kg b |
0.01 ± 0.00 n.a |
n.a
152466.38 ± 151.12 |
n.a
130135.1 ± 764.76 |
0.43 ± 0.02 0.61 ± 0.05 |
n.a
1602.63 ± 24.59 |
2008.80 ± 10.16 4787.59 ± 22.30 |
n.a
3679.88 ±12.23 |
158.56 ± 1.25 260.93 ± 1.28 |
0.32 ± 0.01 7.39 ± 2.15 |
1.13 ± 0.02 0.64 ± 1.32 |
0.38 ± 0.04 3.69 ± 2.56 |
0.04 ± 0.00 5.77 ± 2.35 |
<LD n.a | 4.78 ±0.01 0.23 ±0.01 |
n.a
9050.81 ±109.42 |
0.69 ±0.01 1.92 ±0.62 |
| a 100mg/kg b |
0.03 ± 0.00 n.a |
n.a
132784.49 ± 120.69 |
n.a
123085.6 ± 354.90 |
0.35 ± 0.01 0.52 ± 0.02 |
n.a
1640.89 ± 24.59 |
1784.74 ± 16.33 4511.54 ± 34.29 |
n.a
3443.24 ± 1.33 |
145.79 ± 3.67 221.75 ± 3.43 |
0.37 ± 0.01 8.64 ± 0.60 |
1.05 ± 0.05 2.83 ± 0.40 |
0.43 ± 0.06 3.15 ± 5.33 |
0.04 ± 0.00 12.09 ± 4.08 |
0.19 ± 0.00 n.a |
4.53 ±0.01 0.08 ±0.07 |
n.a
8107.92 ±81.41 |
0.72 ±0.01 4.23\ ±0.90 |
| LD ICP-MS LQ |
0.010 0.031 | - - | - - | 0.147 0.187 | - - | 6.951 9.737 | - - | 5.337 6.524 | 0.001 0.01 | 0.010 0.010 | 0.257 0.612 | 0.046 0.066 | 0.056 0.169 | 0.015 - | - - | 0.152 0.347 |
| LD ICP-OES LQ |
- - | 52.759 49.375 | - - | 0.569 1.591 | 48.401 69.59 | - - | - - | 15.918 21.616 | - - | - - | 15.459 22.851 | 21.021 49.553 | - - | - - | - - | 3.268 7.23 |
| a- Concentration determined by Inductively Coupled Plasma Mass Spectrometry (ICP-MS); b- Concentrations determined by Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES); n.a.- not analyzed; LD and LQ- Methodological were determined considering the instrumental Limit of Detection (LD) and Limit of Quantification (LQ); T1- treatment with 1mg/kg La2O3NPs at 31days; T2- treatment with 10mg/kg La2O3NPs at 31days; T3- treatment with 1mg/kg La2O3NPs at 31days; CTR- control sample at 31 days. | ||||||||||||||||
| Epiphysis Sample with concentration of NP-La2O3 | Element (µg/g) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| La | Ca | P | Mn | S | Mg | K | Zn | As | Ni | Se | Pb | Cr | Cd | Na | Cu | |
| a Control b |
0.04 ± 0.01 n.a |
n.a
216461.77 ± 133.82 |
n.a
119542.6 ± 489.34 |
0.47 ±0.01 0.32 ± 0.07 |
n.a
2195.26 ± 42.41 |
1646.81 ± 24.84 4975.92 ± 8.57 |
n.a
4801.26 ± 3.43 |
191.59 ± 2.49 259.18 ± 1.72 |
0.53 ± 0.01 21.48 ± 1.97 |
1.35 ± 0.06 0.95 ± 1.05 |
0.66 ± 0.09 18.88 ± 3.90 |
0.10 ± 0.02 6.42 ± 4.79 |
0.61 ± 0.01 <LD |
0.02 ± 0.01 0.48 ± 0.09 |
n.a
7440.72 ± 27.36 |
1.45 ± 0.01 3.53 ± 1.01 |
| a 1mg/kg b |
0.06 ±0.01 n.a |
n.a
183871.61 ± 178.90 |
n.a
113487.6 ± 431.10 |
0.45 0.03 0.61 ± 0.07 |
n.a
2486.26 ± 38.83 |
1491.27 ± 96.61 4456.93 ± 21.52 |
n.a
4214.33 ± 0.00 |
220.87 ± 1.76 312.64 ± 3.02 |
0.52 ± 0.01 13.79 ± 0.89 |
1.48 ± 0.00 2.51 ± 0.75 |
0.52 ± 0.00 8.18 ± 6.43 |
0.20 ± 0.05 <LD |
0.24 ± 0.01 <LD |
<LD
0.73 ± 0.04 |
n.a
7617.85 ± 42.70 |
1.43 ± 0.02 5.81 ± 0.84 |
| a 10mg/kg b |
0.02 ±0.01 n.a |
n.a
154878.74 ± 112.51 |
n.a
113137.3 ± 467.54 |
0.41 ±0.01 0.46 ± 0.06 |
n.a
2812.88 ± 25.33 |
1443.38 ± 31.39 4554.85 ± 19.82 |
n.a
4620.62 ± 14.64 |
195.28 ± 1.19 300.67 ± 4.84 |
0.38 ± 0.07 23.46 ± 1.78 |
0.84 ± 0.03 2.07 ± 0.16 |
0.59 ± 0.03 9.00 ± 0.85 |
0.07 ± 0.01 18.97 ± 2.76 |
0.39 ±0.02 <LD |
<LD
0.80 ± 0.03 |
n.a
7816.94 ± 36.89 |
1.16 ± 0.04 5.63 ± 1.07 |
| a 100mg/kg b |
0.11 ±0.02 n.a |
n.a
123897.04 ± 102.33 |
n.a
121986.0 ± 53.29 |
0.60 ± 0.02 0.66 ± 0.05 |
n.a
3353.83 ± 40.74 |
1418.40 ±37.84 4714.39 ± 73.50 |
n.a
5625.35 ± 21.27 |
295.07 ±6.97 327.03 ± 4.97 |
0.72 ±0.03 22.15 ± 0.55 |
1.79 ±0.03 3.68 ± 0.89 |
0.83 ±0.07 20.82 ±4.32 |
0.09 ±0.01 14.89 ±3.53 |
0.65 ±0.02 <LD |
0.02 ±0.01 0.66 ± 0.11 |
n.a
8863.47 ± 37.65 |
1.99 ± 0.03 9.52 ± 0.18 |
| LD ICP-MS LQ |
0.010 0.031 | - - | - - | 0.147 0.187 | - - | 6.951 9.737 | - - | 5.337 6.524 | 0.001 0.01 | 0.010 0.010 | 0.257 0.612 | 0.046 0.066 | 0.056 0.169 | 0.001 0.002 | - - | 0.152 0.347 |
| LD ICP-MS LQ |
- - | 52.759 49.375 | - - | 0.569 1.591 | 48.401 69.59 | - - | - - | 15.918 21.616 | - - | - - | 15.459 22.851 | 21.021 49.553 | - - | - - | - - | 3.268 7.23 |
| a- Concentration determined by Inductively Coupled Plasma Mass Spectrometry (ICP-MS); b- Concentrations determined by Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES); n.a.- not analyzed; LD and LQ- Methodological were determined considering the instrumental Limit of Detection (LD) and Limit of Quantification (LQ); T1- treatment with 1mg/kg La2O3NPs at 31days; T2- treatment with 10mg/kg La2O3NPs at 31days; T3- treatment with 1mg/kg La2O3NPs at 31days; CTR- control sample at 31 days. | ||||||||||||||||
In Table 4, analysis of data from epiphysis, revealed lower levels of Ca, P, Mn, Zn and Cd than the control and a higher level of Mn, S, K, As, Ni, Se, Pb, Na, and Cu. Table 5 show that there is positive correlation between the La2O3NPs treatment and the element level show La2O3NPs concentration-related increase in La, As, Cr, Cu, Se and S levels, respectively in both diaphysis and epiphysis, but decrease in Pb and Ca. While there is an increase in Mn, Mg, K, Na, Zn, P and Ni content of epiphysis and decreases in diaphysis of male Wistar rat bone. The La2O3NPs concentration treatment was moderately (R = 0.0004952–0.8361) positively correlated with trace elements in femur bone diaphysis. High (R = 0.001021–0.9741) positive correlation was found in the epiphysis.
| Diaphysis region | Epiphysis region | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Correlation | R | P | Correlation | R | P | Correlation | R | P | Correlation | R | P | |||||
|
0.3171 | 0.4368 |
|
0.3052 | 0.4476 |
|
0.6753 | 0.1783 |
|
0.1195 | 0.6543 | |||||
| Conc vs. P | 0.05313 | 0.7695 | Conc vs. Ni | 0.4375 | 0.3386 | Conc vs. P | 0.4972 | 0.2949 | Conc vs. Ni | O.6853 | 0.1733 | |||||
| Conc vs. Mn | 0.0004952 | 0.9777 | Conc vs. Se | 0.4328 | 0.3421 | Conc vs. Mn | 0.4111 | 0.3588 | Conc vs. Se | 0.3998 | 0.3677 | |||||
| Conc vs. S | 0.03578 | 0.8108 | Conc vs. Pb | 0.05128 | 0.7736 | Conc vs. S | 0.8121 | 0.0988 | Conc vs. Pb | 0.2064 | 0.5457 | |||||
| Conc vs. Mg | 0.5565 | 0.2540 | Conc vs. Cd | 0.6209 | 0.2120 | Conc vs. Mg | 0.005600 | 0.9252 | Conc vs. Cd | 0.001021 | 0.9680 | |||||
| Conc vs. K | 0.004631 | 0.9320 | Conc vs. Na | 0.07191 | 0.7318 | Conc vs. K | 0.8305 | 0.8887 | Conc vs. Na | 0.9741 | 0.0130 | |||||
| Conc vs Zn | 0.8361 | 0.0856 | Conc vs Cu | 0.008802 | 0.9062 | Conc vs Zn | 0.4169 | 0.3543 | Conc vs Cu | 0.8567 | 0.0744 | |||||
Discussion
Trace elements determination in bone tissue and other living organisms is an indirect way of assessing environmental pollution using bio-indicators. The level of bone element value and importance as bio-maker in the monitoring of inorganic contaminants stem from the bone tissue physiological characteristic [9]. The analysis of male wistar rat’s epiphysis and diaphysis after oral administration of La2O3NPs for 30days showed an increase in the content of some toxic metals and reduction in the content of calcium, which affects the bones’ strength, was observed [10]. The mineralized bone matrix contains majorly of hydroxyapatite, whose major constituents are Ca, P and much less K, Mg and Na that are important for bone metabolism. Potassium and Sodium relate closely meyabolim of calcium to Ca metabolism, and Mn, Cu and Zn as enzymes cofactors [12]. An increase in the content of As, Ni, Cu, and Pb in both diaphysis and epiphysis part of the bone resulted in calcium ions replacement in hydroxyapatite, leading to a change in its mineral structure [13].
Calcium content was lower in the diaphysis and epiphysis of femur bone with La2O3NPs as compared control sample, this may be the reason why there are more intensive elemental replacement with hydroxyapatite. The sustenance mineral homeostasis, bone metabolism, and biomarker suitability for the assessment of bone health makes Ca and P relative content critical [14].
In this study, the changes in bone P and Ca contents were strongly connected to La2O3NPs treatment, although the content mass ratios of P and Ca in femur bone did not vary significantly, being approximately the same as those described for mammalian bone bulk [15]. The variation of S, K and Zn contents in bone are influenced by bone Epiphysis or Diaphysis region and bone type associated with variations in P and Ca content after La2O3NPs treatment did not lead to serious alterations elemental ratio.
The mineral, organic constituents' equilibrium, and organic bone bulk content, can be indicated by S content in the bone matrix [16]. The variation in S contents for Diaphysis or Epiphysis regions, in La2O3NPs treated rats’ compare to controls, indicates a change of bone mass as Ca and P contents vary. In the La2O3NPs treated male Wistar rat femur bone, there are common correlations between Ca and S in the epiphysis (R= 0.9746) and in the diaphysis (R= 0.5275). The higher content of nickel in La2O3NPs treated bone may be due to the fact that the osseous tissue hydroxyapatite resistance has been affected by this element [17,18]. Furthermore, the increased Lanthanum content is accompanied by an increase in the contents of elements that can substitute themselves for calcium in hydroxyapatite structures. Changes in the structure of hydroxyapatite, which then change the strength and increased susceptibility to bone defect are due to high content of toxic elements [19].
In this research work, a correlation analysis was used to determine the relationship between calcium with the changes in the contents of other element. The correlation coefficient ‘‘R’’ identifies the correlation behaviour between variables, hence it enables an analysis of changes in the occurrence and distribution of each metal ions in the femur (Epiphysis and Diaphysis) bone. According to Brodziak-Dopierala,et al the occurrences of other ions affect the biological effect of changes in the impact of particular elements [20-23]. The positive correlation coefficients show that synergetic trends and inter relationship between various elements. Table 6 show the correlation analysis result conducted for calcium and trace elements in the Epiphysis and Diaphysis region of male Wistar rat bone after treatment with different concentrations of La2O3NPs. In the correlation coefficients comparison of the selected metals in the bone, it was concluded that the element of the bone characterized by the highest number of significant correlations was in the epiphysis region of the femur bone and that these correlations occurred between Ca and S (0.97), Zn (0.74), Ni (0.79), Na (0.81) and Cu (0.86). These results suggest that the differences in the metal contents are low in the epiphysis of the femur bone. The correlations between calcium and other elements were also found in diaphysis, where calcium was additionally shown to a high correlation with Mg (0.89), Se (0.79) and Cd (0.89). In comparing the correlations in epiphysis region with diaphyseal, the epiphyseal region has a higher correlation coefficient.
| Epiphysis femur bone | Diaphysis femur bone | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Correlation | R | P | Correlation | R | P | Correlation | R | P | Correlation | R | P | |||||||||
|
0.03776 | 0.8057 |
|
0.7997 | 0.1057 |
|
0.4522 | 0.3275 |
|
0.004667 | 0.9317 | |||||||||
| Ca vs. Mn | 0.5529 | 0.2564 | Ca vs. Se | 0.01413 | 0.8811 | Ca vs. Mn | 0.6627 | 0.1859 | Ca vs. Se | 0.7984 | 0.1065 | |||||||||
| Ca vs. S | 0.9746 | 0.0128 | Ca vs. Pb | 0.4342 | 0.3411 | Ca vs. S | 0.5275 | 0.4925 | Ca vs. Pb | 0.4527 | 0.3271 | |||||||||
| Ca vs. Mg | 0.1656 | 0.5931 | Ca vs. Cd | 0.3386 | 0.4181 | Ca vs. Mg | 0.8958 | 0.0536 | Ca vs. Cd | 0.8894 | 0.0569 | |||||||||
| Ca vs. K | 0.3791 | 0.3843 | Ca vs. Na | 0.8141 | 0.0977 | Ca vs. K | 0.1756 | 0.5810 | Ca vs. Na | 0.3085 | 0.4446 | |||||||||
| Ca vs. Zn | 0.7369 | 0.1415 | Ca vs. Cu | 0.8575 | 0.0740 | Ca vs. Zn | 0.05367 | 0.7683 | Ca vs. Cu | 0.5241 | 0.2760 | |||||||||
| Ca vs As | 0.1057 | 0.6748 | Ca vs As | 0.6313 | 0.2055 | |||||||||||||||
| Calcium in the diaphysis part of femur bone have low correlation with S (R = 0.5275), Zn (R = 0.5367), Ni (R = 0.0004667), Na (R = 0.3085), K (R= 0.1756) but moderate correlation with P (R= 0.4522), Mn (R= 0.6627), As (R= 0.6313), Pb (R= 0.4527), Cu (R= 0.5241) and high correlation with Mg (R= 0.8958), Se (R= 0.7984), Cd (R= 0.8894). | ||||||||||||||||||||
Tables 5 showed similarities in the correlations between La2O3NPs concentration and other elements. Studies have shown that both human and animal has differential distribution of bone elements depending on the type bone (e.g., tibia, rib and femur) [24], section of bone (proximal and distal bone part; Cretacci and Parsons, 2010) or region of bone [25,26]. Results presented the differences between epiphysis and diaphysis trace element content after La2O3NPs treatment, which may follow from the presence of ossification centers in epiphysis [27]. The results of this study reveal that concentration of trace elements: Ca, K, P, S, Na, La, Mn, Mg, Zn, As, Ni, Se, Pb, Cr, Cd, and Cu differ between various La2O3NPs treatment concentration as well as regions of the femur bone, what seems to be associated with bone functions and its different parts, especially during bone development [28]. This shows the importance of differences between bone region and the effect of La2O3NPs concentration on bone tissues.
Conclusion
It can be deduced from this study that a higher content of La, Mn, S, Zn, Ni, Cu, and Na were seen in the femur (epiphysis and diaphysis) bone of Wistar rats with La2O3NPs than in those without treatment. But of Ca, P, Mg, K, As, Se, Pb, Cr, and Cd were observed to be lower than the control. The concentration of Calcium in the epiphysis and diaphysis correlate with the concentrations of K, P, S, Na, La, Mn, Mg, Zn, As, Ni, Se, Pb, Cr, Cd, and Cu. The La2O3NPs may be an important factor in decreasing the content of calcium in the femoral bone. The concentration of calcium differs in particular elements of the femur bone with the highest value in the epiphysis region. Femoral (epiphysis) bone showed a higher level of Cd, Cr, and Pb, which may be dangerous to hydroxyapatite mineral structure. There is also significant decrease in the calcium content of epiphysis as compared with diaphysis region of the femoral bone. The higher content of cadmium, chromium, and zinc were detected in diaphysis region of the femoral bone.
Acknowledgements
The authors acknowledge the support of Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Proc. Nr. 120230/ 2017-8 and Proc. nr. 306052/2017-2) for fellowship and support toward this project.
Conflict of Interests
The authors declare that they have no competing interests.
References
- Wiechuła D, Jurkiewicz A and Loska K. Sci Total Environ. 2008, 406: p. 161–167.
- Rautray TR, Mishra S, Patnaik SK et al. Indian J. Physiol. 2007, 81(1): p. 99-102.
- Smrčka V, Mihaljevič M, Zocová J et al., Anthropologie, 2008, 46(3): p. 219-226.
- Allmäe R, Limbo-Simovart J, Heapost L et al., Papers on Anthropology, 2012, 21: p. 27-49.
- Aaseth J, Boivin G and Andersen O. J Trace Elem Med Biol. 2012, 26,149-52.
- Trueman CN and Tuross N. Rev Mineral Geochemistr. 2002, 48: p. 489–521.
- International Commission on Radiological Protection, (1993). Ingestion Dose Coefficients, Pergamon Press, Oxford, United Kingdom.
- Carvalho ML, Marques AF, Lima MT et al., Acta B. 2004, p. 1251-1257.
- Pate D. J Archaeol Method Theory. 1994, 1(2): p. 161-209.
- Priest ND. CRC Press. 2000, p. 84-139.
- Bronner F. Principles of bone biology. 2008, p 515-531.
- Flores EMM, Barin JS, Paniz JNG et al., Analytical Chemistry. 2004, 76: p. 3525-3529.
- Rodríguez J and Mandalunis PM. J. Toxicol. 2018, p. 1–11.
- Okrajni J and Jasik A. Journal of Medical Information and Technology. 2002, 4: p. 19–26.
- Dermience M, Lognay G, Mathieu F et al., J Trace Elem Med Biol. 2015, 32: p. 86–106.
- Sergey V Dorozhkin. Prog Biomater. 2016, 5: p. 9–70.
- Kourkoumelis N, Balatsoukas I and Tzaphlidou M. J Biology and Phys, 2012, 38: p. 279-291.
- Zaichick V and Tzaphlidou M. Appl Radiat Isot, 2003, 58: p. 623–627.
- Miller LM, Novatt JT, Hamernan D et al., Bone. 2003, 35: p. 498–506.
- Branca F and Vatuena S. Public Health Nutr. 2001, 4: p. 117–123.
- Liam CP, Christina JN, Stuart RK et al., Chemical Review. 2008, 108(11): p. 4754-4783.
- Brodziak-Dopierala B, Kwapulin ski J, Kusz D et al., Arch Environ Contam. 2009, 57: p. 203-210.
- Nganvongpanit K, Buddhachat K, Piboon P et al., Biol Trace Elem Res. 2016, 174: p. 93-104.
- Cretacci Y and Parsons PJ. Environ. Res. 2010, 110(1): p. 26–32.
- Takata MK, Saiki M, Sumita NM et al., J Radioanal Nucl Chem. 2005, 264(1): p. 5-8.
- Lanocha N, Kalisinska E, Kosik-Bogacka DI et al., Acta Theriol, 2012, 57: p. 233-244.
- Setiawati R and Rahardjo P. Google Scholar. 2018.
- Hirayama M, Iijima S, Iwashita M et al., J Trace Elem Med Bio. 2011, 25: p.73-78.