Research Article - Der Pharma Chemica ( 2025) Volume 17, Issue 2
Advanced Analytical Methods: LC-MS/MS with Multiple Reaction Monitoring (MRM Mode) For the Quantification of Two Potential Genotoxic Impurities in Emtricitabine Drug Substance
Pantula Nagendra Srinivas1*, P Shyamala2, K Rama Srinivas1 and Ch Nagaraju12Department of Chemistry, Andhra University, Visakhapatnam, India
Pantula Nagendra Srinivas, Department of Chemistry, Aurobindo Pharma Limited, Andhra Pradesh, India, Email: pnsrinivas70@gmail.com
Received: 20-Dec-2023, Manuscript No. DPC-23-123113; Editor assigned: 23-Dec-2023, Pre QC No. DPC-23-123113; Reviewed: 08-Jan-2024, QC No. DPC-23-123113; Revised: 21-Feb-2025, Manuscript No. DPC-23-123113; Published: 28-Feb-2025, DOI: 10.4172/0975-413X.17.2.638-643
Abstract
To detect Possible Genotoxic Impurities (PGIs) in Emtricitabine drug substance 2-Methoxy 4-Amino-5-Fluro pyramidine and L-Methyl glyoxalate monohydrate, two sensitive LC-MS/MS techniques were developed and verified. 2-Methoxy 4-Amino-5-Fluro pyramidine and L menthyl glyoxalate contaminants in the Active Pharmaceutical Ingredient (API) of Emtricitabine. Multiple Reaction Monitoring (MRM) mode was employed with the LC-MS/MS method on acquity UPLC CSH phenyl hexyl, 150 x 2.1mm, 1.7µm and X-Select CSH C18, 150 x 4.6 mm, 3.5 µm respectively, using Electrospray Ionization (ESI). The suggested approach was exact, robust, accurate, linear, and specific. Over the concentration range of 0.6 µg/g to 9.5 µg/g and 0.6 µg/ml to 9.4 µg/ml, the calibration curves demonstrated satisfactory linearity; the correlation coefficients were.0.999 and.0.999 in each case. The method's very Low Limit of Quantification (LOQ) and Limit of Detection (LOD) were 0.3 µg/g and 0.6 µg/g, respectively.
Keywords
L-Methyl glyoxalate monohydrate and 2-Methoxy 4-Amino-5-Fluro pyramidine; LC-MS/MS; Emtricitabine; Genotoxic impurities; Trace analysis
Introduction
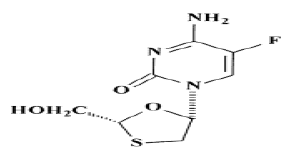
A nucleoside reverse transcriptase inhibitor called emtricitabine is used to treat HIV (human immunodeficiency virus) infection in adults. The medication copies HIV RNA (ribonucleic acid) into new virus DNA (deoxyribonucleic acid) by suppressing the reverse transcriptase enzyme. Tenofovir disoproxil fumarate is frequently given in conjunction with emtricitabine; the daily maximum dose of emtricitabine is 0.2 g. Because they are reactive by nature, synthetic starting materials and intermediates could appear as contaminants in the finished API. These substances are frequently carcinogens or mutagens due to the biological reactivity that commonly results from the chemical reactivity [1-4]. It has been repeatedly demonstrated that the significant chemical reactivities of the various alkylating agents prevented their retention within the final API (Figure 1).
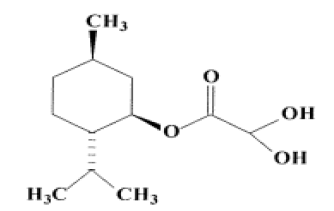
L-methyl glyoxylate monohydrate (LMGH) is a crucial step in the production of lamivudine and emtricitabine 1, two significant antiretroviral medications. These two commonly prescribed medications serve as cytidine reverse transcriptase inhibitors in combination therapy for the treatment of HIV-1, HIV-2, and Hepatitis B. Since Sub-Saharan Africa has a higher rate of AIDS cases than any other region in the world, these generic medications are especially crucial in these nations (Figure 2).
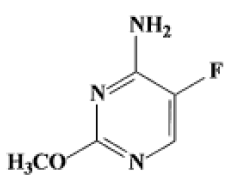
2-methoxy 4-amino-5-fluro pyramidine introduction part (Figure 3).
Based on the maximum daily dosage of Emtricitabine L -Menthyl glyoxylate monohydrate (LMGH) and 2-Methoxy 4-Amino-5-Fluro pyramidine (MAFP) are required to be controlled at a limit of NMT 6.25 µg/g and NMT 6.25 µg/g respectively in the API.
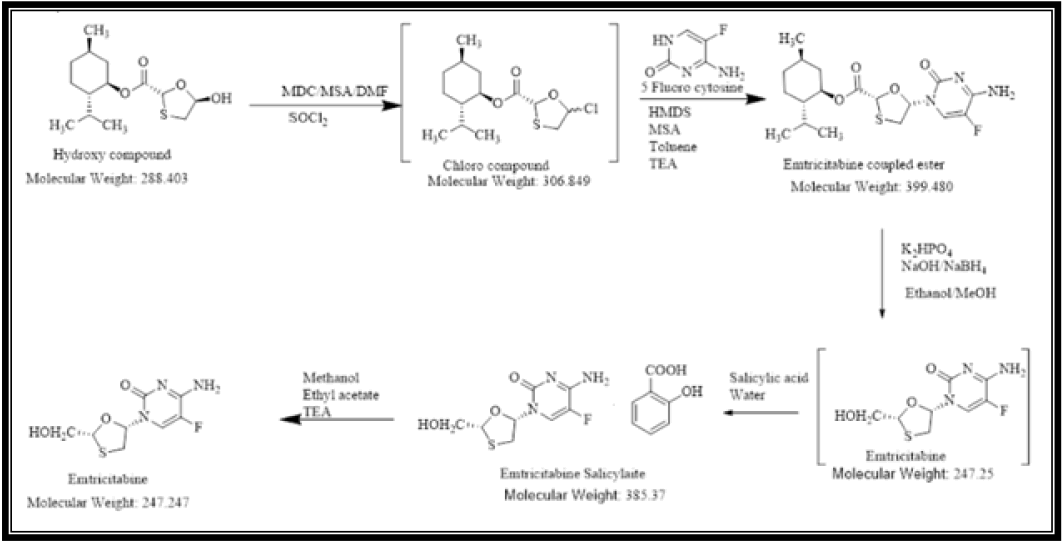
L-Menthyl glyoxylate monohydrate and 2-Methoxy 4-Amino-5-Fluro pyramidine are used in the synthesis of Emtricitabine.Route of synthesis shown below (Figure 4).
Materials and Methods
The variety of analytical procedures has expanded and there has been a general revival due to the growing regulatory awareness on the potential risks. For the determination of L-Menthyl glyoxylate monohydrate (LMGH) and 2-Methoxy 4-Amino-5-Fluro pyramidine (MAFP), the suggested LC-MS/MS (liquid chromatography/mass spectrometery/mass spectrometery) approach provides a straightforward, robust, and labor-saving alternative that requires no time-consuming sample preparation steps. When comparing this method to arduous sample preparation techniques, it has numerous advantages over the method documented in the literature in terms of specificity, accuracy, and reproducibility involving direct analysis [5-7]. In order to deal with matrix interference, a diluent that makes the emtricitabine API insoluble and allows for the extraction of analytes from the API matrix is used. The suggested technique uses electrospray ionization in the MRM mode to quantify LMenthyl glyoxylate monohydrate (LMGH) and 2-Methoxy 4-Amino-5-Fluro pyramidine (MAFP).
Experimental
Chemical and Reagents
List of chemicals, reagents, chemicals, sample, standards and impurities show in Table 1.
| S. No | Name of the materials | Grade | |
|---|---|---|---|
| 1 | LMHG | MAFP | |
| 2 | Formic acid | Formic acid | LCMS |
| 3 | Methanol | Ammonia | LCMS |
| 4 | Sodium hydroxide | Ammonium Formate | AR & LCMS |
| 5 | Water | Water | Milli-Q |
| 6 | L-Menthyl glyoxylate monohydrate | NA | |
| 7 | 2-Methoxy 4-Amino-5-Fluro pyramidine | NA | |
| 8 | Salicylic acid | NA | |
| 9 | Emtricitabine coupled ester | NA | |
| 10 | Emtricitabine sulfoxide | NA | |
| 11 | Emtricitabine acid | NA | |
| 12 | Desfluoro Emtricitabine | NA | |
| 13 | 5-Fluorouracil Analog | NA | |
| 14 | Emtricitabine | NA | |
Table 1: List of Chemicals and Reagents.
Instrumentation
For L -Menthyl glyoxylate monohydrate (LMHG) and 2-Methoxy 4-Amino-5-Fluro pyramidine (MAFP): An ultra-performance liquid chromatography, acquity H-class system, with a quaternary solvent manager, gradient mixer assembly, sample manager-F.T.N (Flow through needle) auto injector, with a column oven coupled to Xevo TQ-S Triple Quadrupole LC/MS/MS Mass Spectrometer, (Make Waters).Column was employed in the method was acquity UPLC CSH Phenyl Hexyl 1.7 µm. (150 mm × 2.1 mm) and X-Select CSH C18 150 × 4.6 3.5 µm mm respectively. All the weighing in the experiments was done with Sartorius balance capable of measuring with an accuracy of 0.01 mg.
Chromatographic conditions for LC
| Chromatographic Conditions for LC | |||
|---|---|---|---|
| S. No | Parameters | L-Menthyl glyoxylate monohydrate (Method-A) | 2-Methoxy 4-Amino-5-Fluro pyramidine (Method-B) |
| 1 | Mobile phase-A | Mix 1.0 mL of formic acid 1000 ml of water | Weigh 126 mg of ammonium formate in 1000 mL of water and add 1.0 ml ammonia to the solution |
| 2 | Mobile phase-B | Methanol | Acetonitrile |
| 3 | Column | Acquity UPLC CSH phenyl hexyl 1.7 µm. (150 mm × 2.1 mm) | X-select CSH C18 150 × 4.6 mm,3.5 µm |
| 4 | Flow rate | 0.25 mL/min | 0.3 mL/min |
| 5 | Injection volume | 5.0 µl | 5.0 µl |
| 6 | Column oven temperature | 40°C | 45°C |
| 7 | Auto sampler temperature | 10°C | 10°C |
| 8 | Run time | 20 min | 20 min |
| 9 | Pump mode | Gradient | Gradient |
| Time (min) | Mobile Phase A (%, v/v) | Mobile Phase B (%, v/v) | |
| T0 | 90 | 10 | |
| T0 | 90 | 10 | |
| T10.0 | 10 | 90 | |
| T13.0 | 10 | 90 | |
| T13.5 | 90 | 10 | |
| T20.0 | 90 | 10 | |
| Time (min) | Mobile Phase A (%, v/v) | Mobile Phase B (%, v/v) | |
| T0.01 | 85 | 15 | |
| T2.0 | 85 | 15 | |
| T5.0 | 65 | 35 | |
| T10.0 | 65 | 35 | |
| T12.0 | 10 | 90 | |
| T14.0 | 10 | 90 | |
| T14.1 | 85 | 15 | |
| T20.0 | 85 | 15 | |
Table 2: Chromatographic Conditions for LC.
Mass spectroscopic conditions
Source type: ESI
Mode of ionization: Positive
Source parameters
Capillary: 3.9 kv
Source temperature: 150°C
Desolvation temperature: 500°C
Cone gas flow: 150 L/Hr
Desolvation gas flow: 1000 L/Hr
Nebulizer (Bar): 7.0
Condition for MRM:
Scan type: MRM
Function type: MRM of 1 channel
| Name | Q1 mass (amu) | Q3 mass (amu) | Cone(v) | Collision energy (v) |
|---|---|---|---|---|
| L-methyl glyoxalate monohydrate | 253.2 [M+Na]+ | 115 | 26 | 10 |
| 2-methoxy 4-amino-5-fluro pyramidine | 143.9 | 112.8 | 48 | 18 |
Table 3: MRM of 1 channel.
Preparation of diluent for L-methyl glyoxalate monohydrate
Prepare a degassed mixture of Methanol, Water and methanolic sodium hydroxide solution in the ratio of 800:200:0.1 v/v/v.
Preparation of diluent for 2-methoxy 4-amino-5-fluro pyramidine
Prepare a degassed mixture of Acetonitrile, Water and Formic acid in the ratio of 500:500:0.1 v/v/v.
Preparation of standard solution for L-methyl glyoxalate monohydrate
Primary standard stock solution
Accurately weigh and transfer about 11.0 mg of L-Menthyl Glyoxalate reference sample into a 50 mL clean, dry volumetric flask, add 30 mL of diluent sonicate to dissolve and make up to volume with diluents (0.22 mg/mL).
Pipette 3.0 mL of Primary standard stock solution into 50 mL of volumetric flask make up to the mark with diluents (528.0 µg/g). Further pipette 3.0 mL of solution into 50 mL of volumetric flask make up to the mark with diluents (31.68 µg/g). Further pipette 2.0 mL of solution into 10 mL of volumetric flask make up to the mark with diluent (6.34 µg/g).
Preparation of standard solution for 2-methoxy 4-amino-5-fluro pyramidine
Primary standard stock solution
Accurately weigh and transfer about 9.8 mg of 2-Methoxy 4-Amino-5-Fluro pyramidine reference sample into a 50 mL clean, dry volumetric flask, add 30 mL of diluent sonicate to dissolve and make up to volume with diluents (0.196 mg/mL).
Pipette 2.0 mL of primary standard stock solution into 50 mL of volumetric flask make up to the mark with diluents (784.0 µg/g). Further pipette 2.0 mL of solution into 50 mL of volumetric flask make up to the mark with diluents (31.36 µg/g). Further pipette 2.0 mL of solution into 10 mL of volumetric flask make up to the mark with diluent (6.27 µg/g).
Preparation of sample solution for L-methyl glyoxalate monohydrate
Accurately weigh and transfer 250 mg of sample into a clean, dry 10 mL volumetric flask/Centrifuge tube, add 5.0 mL of diluent sonicate to dissolve and then add 5.0 mL of diluent and mix well. Total volume is 10.0 mL [8-10].
Preparation of sample solution for 2-methoxy 4-amino-5-fluro pyramidine
Accurately weigh and transfer 100 mg of sample into a clean, dry 10 mL volumetric flask/Centrifuge tube, add 5.0 mL of diluent sonicate to dissolve and then add 5.0 mL of diluent and mix well. Total volume is 10.0 mL [11-13].
Results and Discussion
Validation
System precision: Prepared the standard solution of L-menthyl glyoxalate and 2-methoxy 4-amino 5-fluoropyrimidine as per methodology.
| Sample preparation ID | Area of L-menthyl glyoxalate | Area of 2-methoxy 4-amino 5-fluoropyrimidine |
|---|---|---|
| 1 | 78439 | 39706 |
| 2 | 80464 | 37454 |
| 3 | 78524 | 38826 |
| 4 | 79475 | 38255 |
| 5 | 76820 | 36404 |
| 6 | 77051 | 37046 |
| Mean | 78462 | 37949 |
| SD | 1394.68 | 1216.33 |
| %RSD | 1.8 | 3.2 |
Table 4: L-menthyl glyoxalate and 2-methoxy 4-amino 5-fluoropyrimidine.
%RSD for six standard solution injections were observed below 10.0% for both impurities and results are 1.8% for L-menthyl glyoxalate and 3.2% for 2-methoxy 4-amino 5-fluoropyrimidine. System precision met the acceptance criteria.
Identification and specificity
The identification and specificity is defined as the ability to assess and ensure that the impurities and diluent do not affect the sample analyzed.
For L-methyl glyoxalate monohydrate
Inject the blank (as diluent), standard solution, and control sample, spiked sample with all related compounds with and without LMGM and at specification level. Check the interference at the retention time and mass of analyte.
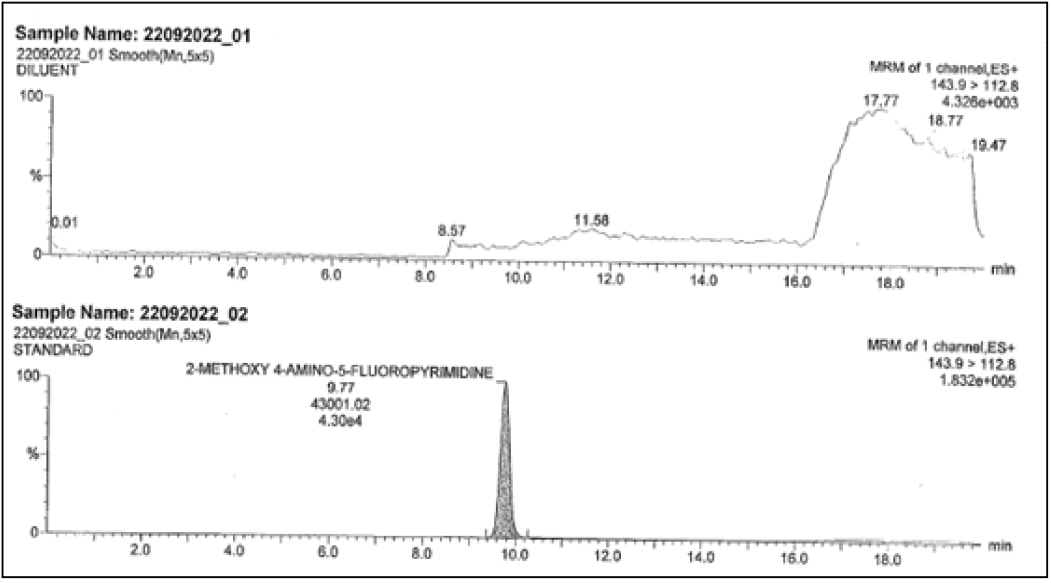
For 2-methoxy 4-amino-5-fluro pyramidine
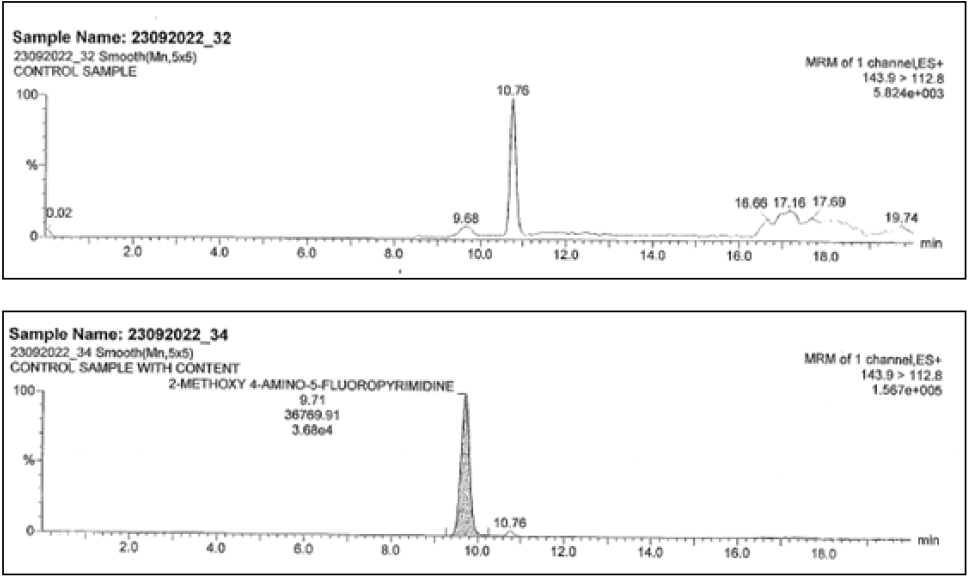
Inject the blank (as diluent), standard solution, and control sample, spiked sample with all related compounds with and without MAFP and at specification level. Check the interference at the retention time and mass of analyte.
Results table for identification
| Impurity Name | Impurity stock solution (RT in minutes) | Spiked sample solution (RT in minutes) |
|---|---|---|
| L-methyl glyoxalate monohydrate | 10.94 | 11.01 |
| 2-methoxy 4-amino-5-fluro pyramidine | 9.77 | 9.71 |
Table 5: Table for Identification.
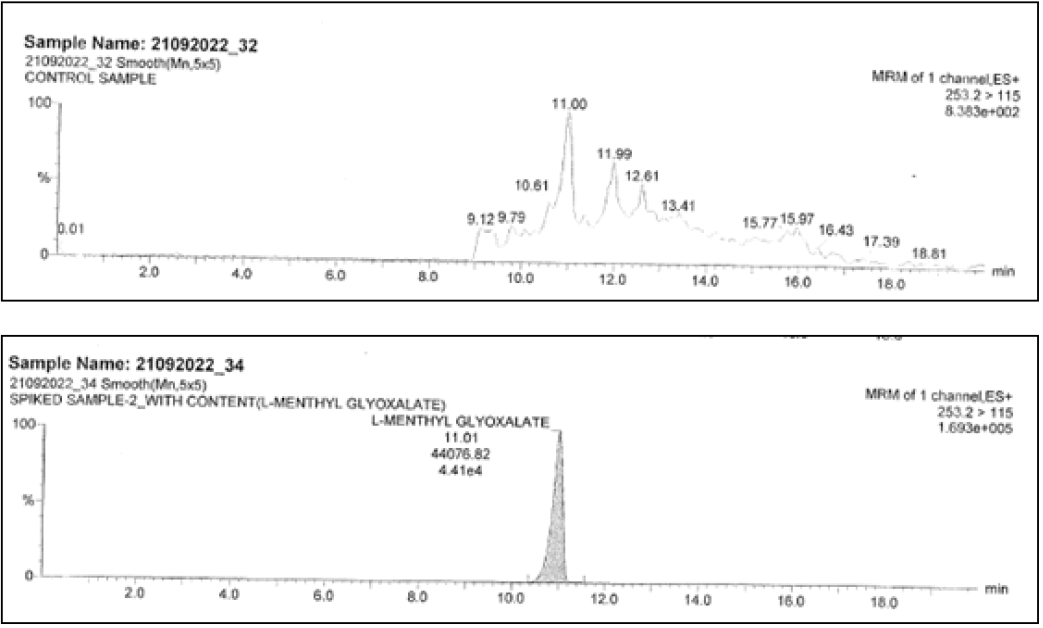
Results table for Specificity
| Impurity name | Area | MRM trace | |
|---|---|---|---|
| Control sample | Spiked sample | ||
| L-methyl glyoxalate monohydrate | ND | 44077 | 253.2→115.0 |
| 2-methoxy4-amino-5-fluro pyramidine | ND | 36770 | 143.9 →112.8 |
Table 6: Table for Specificity.
Spiked sample Retention Times (RT) were comparable to that of reference sample for both impurities and both methods. Did not show any response at the retention time of the L-methyl glyoxalate monohydrate and 2-methoxy 4-amino-5-fluro pyramidine respective methodologies. Hence methods can be capable for the identification and proved the specificity.
Limit of Detection (LOD) and Limit of Quantitation (LOQ)
Prediction of LOD and LOQ
Prepared a series of diluted solutions of L-menthyl glyoxalate and 2-methoxy 4-amino-5-fluro pyramidine impurities with respect to analyte concentration were prepared and injected into the LC-MS/MS system until to get the signal to noise (USP S/N) ratio is more than 3 for LOD and more than 10 for LOQ and achieved the target. The Signal to Noise (S/N) ratio of L-menthyl glyoxalate and 2-methoxy 4-Amino-5-Fluro pyramidine peaks were recorded using system data software. The LOQ and LOD results were captured in the below tables. Results Limit of Quantitation and Limit of detection.
| Impurity name | LOQ concentration (ÃÂ?µg/g) | USP S/N | LOD concentration (ÃÂ?µg/g) | USP S/N |
|---|---|---|---|---|
| L-menthyl glyoxalate | 0.63 | 222.7 | 0.32 | 82.6 |
| 2-methoxy 4-amino-5-fluro pyramidine | 0.63 | 298.8 | 31 | 171.8 |
Table 7: Limit of quantitation and limit of detection.
Precision at LOD and LOQ and accuracy at LOQ
Six different solutions were prepared to contain L-menthyl glyoxalate impurity and 2-methoxy 4-amino-5-fluro pyramidine impurity separately as per methodology at proposed LOD and LOQ level and each solution was injected once into the L-MS/MS, area of L-menthyl glyoxalate impurity and 2-methoxy 4-amino-5-fluro pyramidine impurity in each solution was recorded. %RSD for the content of L-menthyl glyoxalate impurity and 2-methoxy 4-amino-5-fluro pyramidine impurity in each preparation was calculated. The % recovery of each solution was calculated.
| Injection ID | Area of L-menthyl glyoxalate | Area of 2-methoxy 4-amino-5-fluro pyramidine | ||
|---|---|---|---|---|
| LOD | LOQ | LOD | LOQ | |
| 1 | 5308 | 8329 | 2818 | 4750 |
| 2 | 5271 | 8147 | 2793 | 4716 |
| 3 | 5311 | 8235 | 2771 | 4712 |
| 4 | 5239 | 8245 | 2713 | 4701 |
| 5 | 5271 | 8153 | 2763 | 4706 |
| 6 | 5255 | 8134 | 2744 | 4804 |
| Mean | 5276 | 8207 | 2767 | 4732 |
| SD | 26.67 | 76.1 | 36.75 | 39.5 |
| %RSD | 0.5 | 0.9 | 1.3 | 0.63 |
Table 8: Area of L-menthyl glyoxalate.
| Sample Preparation ID | Area of Spiked Sample (L-Menthyl Glyoxalate) | Area of Neat Standard (L-Menthyl Glyoxalate) | % Recovery |
|---|---|---|---|
| LOQ Accuracy-1 | 8237 | 8284 | 100 |
| LOQ Accuracy-2 | 8284 | 8171 | 100.7 |
| LOQ Accuracy-3 | 8272 | 8257 | 100.4 |
Table 9: Area of Spiked Sample (L-Menthyl Glyoxalate).
| Sample Preparation ID | Area of spiked sample (2-methoxy 4-amino-5-fluro pyramidine) | Area of Neat Standard (2-Methoxy 4-Amino-5-Fluro pyramidine) | % Recovery |
|---|---|---|---|
| LOQ accuracy-1 | 4992 | 4768 | 105.3 |
| LOQ accuracy-2 | 4994 | 4822 | 105.3 |
| LOQ accuracy-3 | 4904 | 4633 | 103.4 |
Table 10: Area of Spiked Sample (2-Methoxy 4-Amino-5-Fluro pyramidine).
S/N ratio, %RSD and Accuracy results were met the acceptance criteria.
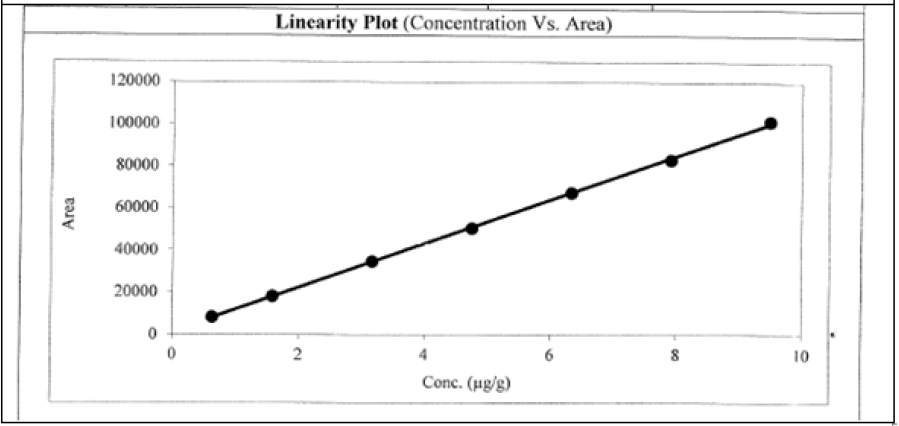
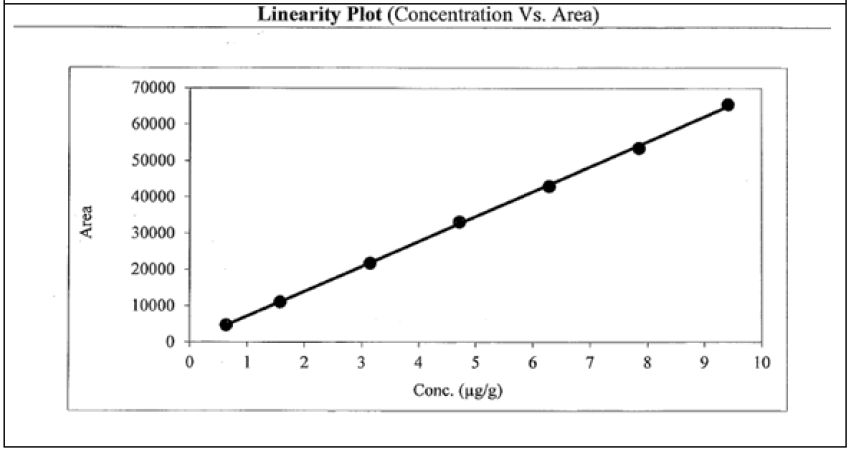
Linearity
Seven linearity solutions were prepared for L-Menthyl Glyoxalate and 2-Methoxy 4-Amino-5-Fluro pyramidine impurities from LOQ to 150% with respect to test concentration (Table 11 and Figure 5, Table 12 and Figure 6).
| L-menthyl glyoxalate | ||
|---|---|---|
| % Level | Concentration (ÃÂ?µg/g) | Area |
| LOQ | 0.63 | 8179 |
| 25 | 1.58 | 18135 |
| 50 | 3.16 | 34542 |
| 75 | 4.75 | 50457 |
| 100 | 6.33 | 67243 |
| 125 | 7.91 | 82933 |
| 150 | 9.49 | 101450 |
| Slope | 10426 | |
| Intercept | 1429.942 | |
| Correlation coefficient | 0.9998 | |
Table 11: L-menthyl glyoxalate
| 2-methoxy 4-amino-5-fluro pyramidine | ||
|---|---|---|
| % Level | Concentration (ÃÂ?µg/g) | Area |
| LOQ | 0.63 | 4736 |
| 25 | 1.57 | 11110 |
| 50 | 3,14 | 21872 |
| 75 | 4.71 | 33167 |
| 100 | 6.28 | 43059 |
| 125 | 7.85 | 53542 |
| 150 | 9.41 | 65466 |
| Slope | 6857 | |
| Intercept | 376.77 | |
| Correlation Coefficient | 0.9998 | |
Table 12: 2-methoxy 4-amino-5-fluro pyramidine.
The correlation coefficient is more than 0.99 for L-menthyl glyoxalate and 2-methoxy 4-amino-5-fluro pyramidine impurities in both methodologies. Hence the response of for L-menthyl glyoxalate and 2-methoxy 4-amino-5-fluro pyramidine impurities is linear LOQ to 150% of specification level in both methods.
Method precision
Six sample solutions were prepared individually by spiking the impurity into the Emtricitabine as per test method at specification level and injected into LC-MS/MS as per methodologies (Tables 13 and 14).
| Sample preparation ID | Area of L-menthyl glyoxalate | Area of 2-methoxy 4-amino-5-fluro pyramidine |
|---|---|---|
| 1 | 71543 | 45275 |
| 2 | 72164 | 44776 |
| 3 | 71999 | 45304 |
| 4 | 70869 | 45001 |
| 5 | 71076 | 45341 |
| 6 | 71901 | 45270 |
| Mean | 71592 | 45161 |
| SD | 525.3 | 224.3 |
| %RSD | 0.7 | 0.5 |
Table 13: Sample Preparation.
| Preparation ID | Added ofÃÂ? L-menthyl glyoxalate in ÃÂ?µg/g | Found of L-menthyl glyoxalate in ÃÂ?µg/g | Added of 2-methoxy 4-amino-5-fluro pyramidine in ÃÂ?µg/g | Found of 2-methoxy 4-amino-5-fluro pyramidine in ÃÂ?µg/g |
|---|---|---|---|---|
| 1 | 6.33 | 5.77 | 6.28 | 6.68 |
| 2 | 6.33 | 5.82 | 6.28 | 6.61 |
| 3 | 6.33 | 5.81 | 6.28 | 6.69 |
| 4 | 6.33 | 5.72 | 6.28 | 6.64 |
| 5 | 6.33 | 5.73 | 6.28 | 6.69 |
| 6 | 6.33 | 5.8 | 6.28 | 6.68 |
| Mean | 5.78 | Mean | 6.67 | |
| SD | 0.04 | SD | 0.03 | |
| %RSD | 0.7 | %RSD | 0.4 |
Table 14: Preparation Method precision.
%RSD of area and obtained concentration for method precision met the acceptance criteria as per set criteria is not more than 20.0% for both L-menthyl glyoxalate and 2-methoxy 4-amino-5-fluro pyramidine impurities.
Accuracy
Prepared the samples spiked with L-menthyl glyoxalate and 2-methoxy 4-amino-5-fluro pyramidine impurities at 100% level and 150% level in presence of Emtricitabine drug substance (prepared in triplicates) against L-menthyl glyoxalate and 2-methoxy 4-amino-5-fluoropyrimidine impurities at 100% level and 150% level in absence of Emtricitabine drug substance and injected into LC-MS/MS as per methodologies (Tables 15-18).
| Sample Preparation ID | Area of Spiked Sample (L-Menthyl Glyoxalate) | Area of Neat Standard (L-Menthyl Glyoxalate) | % Recovery |
|---|---|---|---|
| 100% accuracy-1 | 69244 | 69451 | 100.7 |
| 100% accuracy-2 | 69438 | 68026 | 100.9 |
| 100% accuracy-3 | 69809 | 68883 | 101.5 |
Table 15: 100% of spiked sample (L-menthyl glyoxalate).
| Sample preparation ID | Area of spiked sample (L-menthyl glyoxalate) | Area of neat standard (L-menthyl glyoxalate) | % Recovery |
|---|---|---|---|
| 150% accuracy-1 | 100660 | 100096 | 100.2 |
| 150%ÃÂ? accuracy-2 | 99903 | 100545 | 99.5 |
| 150%ÃÂ? accuracy-3 | 102057 | 100656 | 101.6 |
Table 16: 150% of spiked sample (L-menthyl glyoxalate).
| Sample preparation ID | Area of spiked sample (2-methoxy 4-amino-5-fluro pyramidine) | Area of neat standard (2-methoxy 4-amino-5-fluro pyramidine) | % Recovery |
|---|---|---|---|
| 100% accuracy-1 | 44497 | 42879 | 104.8 |
| 100% accuracy-2 | 44288 | 42452 | 104.8 |
| 100% accuracy-3 | 43803 | 42029 | 103.2 |
Table 17: 100% of spiked sample (2-methoxy 4-amino-5-fluro pyramidine).
| Sample preparation ID | Area of Spiked Sample (2-Methoxy 4-Amino-5-Fluro pyramidine) | Area of Neat Standard (2-Methoxy 4-Amino-5-Fluro pyramidine) | % Recovery |
|---|---|---|---|
| 150% accuracy-1 | 63279 | 64281 | 99.7 |
| 150% accuracy-2 | 64376 | 62791 | 101.4 |
| 150% accuracy-3 | 65428 | 63390 | 103.1 |
Table 18: 150% of Spiked Sample (2-Methoxy 4-Amino-5-Fluro pyramidine).
The individual percentage recovery for each sample at 100% level & 150% specification level are meeting set criteria of between 80.0% to 120.0%.
Range
Range of analytical method can be obtained from linearity and recovery data of L-menthyl glyoxalate and 2-methoxy 4-amino-5-fluro pyramidine impurities in both methodologies. Reported the range in LOQ to 150% with respect to specification level.
Robustness
Prepared standard solution as per test methods for L-menthyl glyoxalate and 2-methoxy 4-amino-5-fluro pyramidine impurities at specification level. Injected to in to LC-MS/MS at deliberately varied conditions to evaluate the system suitability and methods ability to remain unaffected (Tables 19 and 20).
| Parameter | Variation | L-menthyl glyoxalate | |
|---|---|---|---|
| RT (minutes) | %RSD | ||
| Flow rate | -10% | 11.49 | 2.1 |
| 10% | 10.76 | 1.2 | |
| Source cleaning | Before | 10.96 | 0.7 |
| After | 10.98 | 0.9 | |
Table 19: L-menthyl glyoxalate.
| Parameter | Variation | 2-methoxy 4-amino-5-fluro pyramidine | |
|---|---|---|---|
| RT (minutes) | %RSD | ||
| Flow rate | -10% | 10.66 | 0.9 |
| 10% | 8.9 | 9.6 | |
| Source cleaning | Before | 9.77 | 2.9 |
| After | 9.71 | 3.2 | |
Table 20: 2-Methoxy 4-amino-5-fluro pyramidine.
The system suitability results at each of the varied conditions complied the requirements as per the test procedure. Hence it can be concluded that the test method is robust across the extent of changes studied for each of the above parameters.
Specimen chromatograms
The result show in Figures 7-10.
Conclusion
Analytical test method for determination of L-menthyl glyoxalate and 2-methoxy 4-amino-5-fluro pyramidine in emtricitabine by LC-MS/MS was validated for system suitability, identification, specificity, linearity, method precision, accuracy, range (linearity, precision and accuracy) and robustness (flow rate and source cleaning) and meets all the pre-established acceptance criteria.
References
- E de Clercq. Biophys Acta. 2002; 1587: p.258â??275.
- JW Beach. Clin Ther. 1998; 20: p. 2â??25.
- JE Gallant. J Clin Virology. 2002; 25: p.317â??333.
- Darbyshire J. Trop Med Int Health. 2000; 5: p. A26â??A31.
- Clevenbergh P, Mouly S, Sellier P, et al. Curr HIV Res. 2004; 2(4):309-321.
[Crossref] [Google Scholar] [PubMed]
- Boffito M, Acosta E, Burger D, et al. Antivir Ther. 2005; 10(3): p.375-392.
[Crossref] [Google Scholar] [PubMed]
- SC Piscitelli, K D Gallicano. N Engl J Med. 2001; 344: p. 984â??996
- Aarnoutse RE, Schapiro JM, Boucher CA, et al. Drugs. 2003; 63: p.741-753.
[Crossref] [Google Scholar] [PubMed]
- Saxena D, Damale S, Joshi A, et al. IJLBPR. 2014; 3(3):196.
- NH Supriya, AM Ashis, C Meena. RJPT. 2012; 5(1): p.133-137.
- Delahunty T, Bushman L, Robbins B, et al. J Chromatogr B Analyt Technol Biomed Life Sci. 2009; 877(20-21): p.1907-1914.
[Crossref] [Google Scholar] [PubMed]
- BS Rao, S Nagaraju, BV Kiran. AJRC. 2013; 6(10): p.936-944.
- Nagaraju PT, Channabasavaraj KP, Shantha Kumar PT. Int J Chemtech Res. 2011; 3(1):23-28.