Review - Der Pharma Chemica ( 2023) Volume 15, Issue 1
ADVANCED DEVELOPMENT ON MARBURG VIRUS AND ITââ¬â¢S VACCINATION: A REVIEW
Lopamudra Saha and Dipanjan Mandal*Dipanjan Mandal, Department of Pharmacology, Guru Nanak Institute of Pharmaceutical Science and Technology, Sodepur Kolkata-700114, India, Email: dipanjan.mondal@gnipst.ac.in
Received: 24-Dec-2022, Manuscript No. dpc-22-84619; Editor assigned: 26-Dec-2022, Pre QC No. dpc-22-84619; Reviewed: 09-Jan-2023, QC No. dpc-22-84619; Revised: 11-Jan-2023, Manuscript No. dpc-22-84619; Published: 18-Jan-2023, DOI: 10.4172/0975-413X.15.1.7-12
Abstract
Marburg Virus (MARV) belongs to the family of animal viruses and is the reason for a deadly and severely troubling viral hemorrhagic fever. The fatality rate of the virus ranges from 24.0 to 88.0%, demonstrating its deadly nature and also the need for its widespread information. The first case of the Marburg virus sickness (MARD) was reported in 1967 when science laboratory personnel operating with African inexperienced monkeys got infected in the Federal Republic of Germany and Belgrade at the same time. Following the initial case, many more outbreaks occurred around the world, such as in Uganda, Angola, Congo, African countries, and even within the United States in 2008. The Egyptian chiropteran (Rousettus aegyptiacus) is thought to be one in every one of the significant sources of infection, and tourists visiting caves inhabited by these haywire or employees accessing mines populated by the haywire are at an exaggerated risk of contracting the health problem. The primary target cells for this virus infection are macrophages and nerve fibre cells. In nerve fibre cells, infection ends up in "paralysis" of the innate response and dysregulation of stimulation of lymphocytes. Disease-modifying agents and inhibitors of microorganism replication show constructive outcomes. While abundant is being investigated to plot an immunising agent, it is important to coach healthcare employees (HCWs) and close contacts facing the health problem. Stopping the transmission remains the most effective action that may be taken.
Keywords
Marburg virus; Filovirus; Nonhuman primate; Animal modeling; Fatal infections; Vaccines; Viral haemorrhagic fever
INTRODUCTION
Marburg virus (MARV) could also be a viral hemorrhagic fever (VHF) with a fatality magnitude relation of up to half a mile. It's inside an equivalent family as a result of the virus that causes animal virus malady. A pair of large outbreaks that occurred at an equivalent time in Marburg and Frankfurt, the main European countries, and in the capital of Yugoslavia, Serbia, in 1967, semiconductor diode to the initial recognition of the malady. The event was related to laboratory work using African inexperienced monkeys (Cercopithe cusaethiops) imported from the Republic of Uganda [1]. Afterward, outbreaks and irregular cases were reported in Angola, the Democratic Republic of the Congo, Kenya, an African nation (in somebody with recent travel history to Zimbabwe) and the Republic of Uganda. In 2008, two freelance cases were reported among travellers A UN agency visited a cave haunted by Rousettus bat colonies in the Republic of Uganda. The morphological traits of MARV once studied at a lower place by a transmission magnifier showed pleomorphism. Thirty-one of us became ill as a result of twenty-five laboratory workers, medical personnel, and a fan UN agency caring for them [2]. MARV could also be a disease (animal-borne) virus and its reservoir is the Egyptian eutherian mammal (Rousettus aegyptiacus).The United National agency reports that the case fatality rate of the Ebola virus ranges from 25.0 to 90.0%, whereas that of the MARV ranges from twenty-four to a mile [4]. As of March 2018, there were thirteen outbreaks of MARV malady, most occurring in geographical areas. The largest of those occurred in the state throughout 2004-2005 and had a case-fatality rate of nineteen. Rather than the MARV movement being a possible and severe threat to public health and safety, general police investigation is required to beat its return and rising mortality rates. Considering the regular epidemics and a very recent pandemic, it's vital to spotlight the burden of even the rare diseases, primarily diseases like MARD that do not have a definitive treatment with a high case-fatality rate [3].
Objectives
Everyone knows that there's no specific treatment for Marburg hemorrhagic fever. Confirming hospital treatment ought to be used, which includes levelling the patient's fluids and electrolytes, maintaining gas standing and pressure level, replacement of lost blood and natural process factors, and treatment for any complicating infections (Table 1).
| SL NO. | YEAR | COUNTRY | CASES AND DEATH |
|---|---|---|---|
| 1 | 1967 | GERMANY AND YUGOSLAVIA | 31 CASES, 7 DEATHS |
| 2 | 1975 | SOUTH AFRICA | 3 CASES, 1 DEATH |
| 3 | 1980 | KENYA | 2 CASES, 1 DEATH |
| 4 | 1987 | KENYA | 1 CASE, 1 DEATH |
| 5 | 1998-2000 | CONGO | 154 CASES, 128 DEATHS |
| 6 | 2004 - STILL | ANGOLA | 266 CASES, 244 DEATHS |
Structure
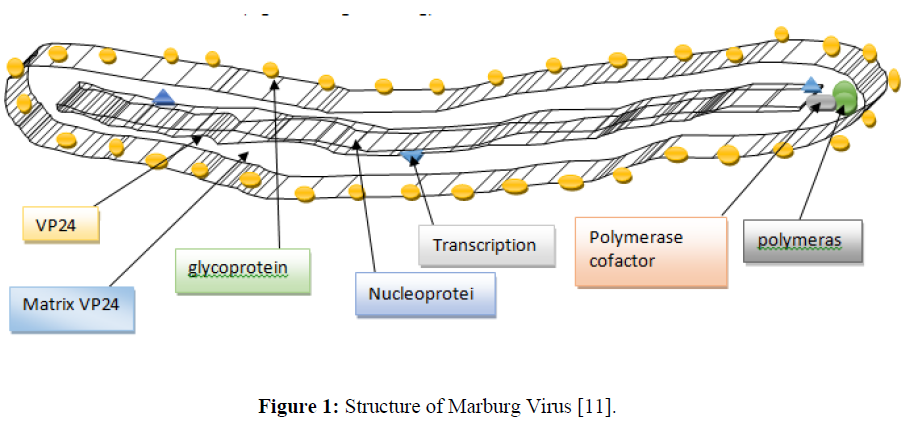
Like all Mononega viruses, Marburg virions contain non-infectious, linear, non-divided, single-stranded RNA genomes of negative polarity that possess inverse-complementary 3' and 5' termini, do not possess a 5' cap, don't seem to be polyadenylated, and don't seem to be covalently joined to a super molecule [6]. Marburg virus genomes are roughly nineteen kbp long and contain seven genes within the order 3'-UTR-NP-VP35-VP40-GP-VP30-VP24-L-5'-UTR.
The breadth of Marburg viruses is 80 nm; the median particle length ranges from 795 to 828 nm; however, particles as long as fourteen thousand nm have been detected in tissue culture. Marburg virions incorporate seven structural proteins. Marburg is an associate-degree enclosed, fiber-bound, united, negative-sense polymer virus. It's the filamentous structure that will seem like aU, a6, or a spiralled sort of snail and might typically be branched. They tend to incorporate long noncoding regions at their 3' and/or 5' ends that, in all probability, contribute to the stability of the infective agent transcript. The infective agent fragment is an organic phenomenon [7]. The coiled ribonucleic capsid consists of the genomic polymer wrapped around a polymer of nucleoproteins. Related to the ribonucleoprotein is the polymer-dependent RNA enzyme (L) with the polymerase compound (VP35) and a transcription matter (VP30) [8]. The ribonucleoprotein is embedded in a matrix, fashioned by the key (VP40) and minor (VP24) matrix proteins. These particles are encircled by a lipoid membrane derived from the host plasma membrane. The membrane anchors a compound protein (GP1, 2) that has seven to ten nm spikes removed from its surface [9]. While nearly clones of viral hemorrhagic fever virions in structure, Marburg virions are antigenically distinct. Human infection with Marburg virus unwellness initio results from prolonged exposure to mines or caves inhabited by Rousettus bat colonies [10]. Once a person is infected with the virus, it will spread through human-to-human transmission via direct contact (through broken skin or secretion membranes) with the blood, secretions, organs, or different bodily fluids of infected people and with surfaces and materials (e.g. bedding, clothing) contaminated with these fluids (Figure 1).
Figure 1: Structure of Marburg Virus [11].
Two variants: LAKE VICTORIA filovirus and RAVN filovirus
Host response to Marburg virus infection
Transcriptional analysis revealed distinct immune signatures associated with each virus and suggested an additional pronounced immune dysregulation in MARV infection 96% of the time. MARV-specific organic phenomenon profile included complement system gene up regulation, genes involved in white corpuscle and white corpuscle enlistment, and innate immune signal genes 96 [12]. In each human survivor and macaque infected with MARV, distinct immune responses that are prognostic of clinical outcomes are detected: while fatal infection of Macaca mulatta macaques with MARV Angola was associated with T-helper cell sort a pair of (Th2)-skewed responses, 98 human survivors of MARV infection exhibited Th1-skewed CD4 + T-cell responses. Fourteen powers to spot these prognostic responses in patients may lead to more practical patient sorting and administration of targeted therapies [13].
MARV human pathologic process
• Transmission and virus unfold within the physical structure area unit delineate.
• On 2–21 days-several alternative infectious diseases, cases of MVD begin with flu-like symptoms like chills, fever, headache, inflammatory disease, myalgia, joint pain, and uneasy [14].
• On days 5–7, the intensity of the sickness will increase, and embrace a maculopapular rash spreading from the body to the limbs, pinkeye, sustained fever, and symptoms of viral haemorrhagic fever, petechiae, blood within the stool and excreta, and haemorrhage from puncture sites [15].
• The neutralizing protein concentration diminished over time, with the decrease starting at 21 months post infection and dropping below detectable limits at twenty seven.
• Patients either live through their unhealthiness or die of dehydration, internal haemorrhage, organ failure, or some combination of general factors assisted by a dysregulated immune reaction to the virus [16].
• The maculopapular rash begins as little, redness spots around hair follicles of the trunk and typically upper arms, developing into a diffuse rash, and may become a dark erythroderma that covers the face, neck, chest, and arms.
• Harm to the liver tissue was severe, and there was intensive hepatocellular swelling and degeneration. Stainability living substance inclusions were found in eosinophils close to areas with death and were positive for microorganism substance.
• The kidneys were swollen, pale, and trauma, and there was tube-shaped structure death and parenchymal damage [17].
• Within the humour organs and mucosa membranes of the abdomen and intestines, there was a high range of plasma cells and monocytes. There was a marked depletion of lymphocytes, currently thought to be the merchandise of looker caspase-mediated cell death rather than direct infection.
• Autopsies of RAVV-infected patients with deadly outcomes showed swelling of the centre, brain, spleen, kidneys, and humour nodes, as well as haemorrhage of mucosa membranes, soft tissues, and varied alternative organs [18].
• Macrophages within the intestines and excretory organ contained what gave the look of microorganism inclusions. The alveoli of the lungs were engorged, haemorrhaged, and contained alveolar macrophages surrounded by protein, and infrequently stained positive for microorganism substance [19].
Newly developed animal models to check Marburg virus infection
In 2012, a variety of recent animal models of MVD were developed. Elaborate comparisons of those and established MVD animal models have recently been revealed [20]. Recently developed animal models that permit the study of deadly MVD while not virus adaptation embrace STAT2 (signal transducer and activator of transcription 2) knockout hamsters, humanised mice, and marmosets. Infection of humanised mice with MARV was related to lower overall weight loss, whereas the microorganism titters were similar. MARV infection models for small-animal infection, including mice, hamsters, and guinea pigs, need virus adaptation [21]. Recent virus-adapted systems embrace a gnawer model for MARV African country that recapitulates the illness ascertained in humans and NHPs and MARV African country mouse models. Just like what was ascertained before for mouse-adapted MARV isolates Ci67, deep sequencing of the MARV African country order throughout mouse adaptation unveiled, among alternative mutations, adaptive changes within the VP40 open reading frame, accentuating the importance of VP40 as a species-specific virulence issue [22].
Differential virulence of Marburg virus variants passaging of viruses in cell culture is thought to lead to the build-up of defective busy microorganism particles and better particle/plaque-forming unit magnitude relations. This was additionally shown for MARV Angola; a virus passed multiple times in cell culture was found to be associated with redoubled survival and delayed illness progression in NHPs [23]. Comparative studies in small-animal models that need virus adaptation revealed a spread in pathogenicity, with MARV African countries being more infective than alternative Marburg virus variants. Completely different results were obtained exploitation the STAT2-deficient gnawer infection model that doesn't need virus adaptation. A fast, however equally deadly infection was ascertained with MARV Musoke. A delayed, however equally deadly infection was ascertained with MARV African country, which resulted in a very symptomatic, however non-lethal infection [24]. The pathogenicity of various Marburg virus variants was additionally studied in NHP models that recapitulate the disease in humans most reliably. Because of advancements in clinical data measurement and analysis, disease symptoms can now be more easily monitored during this infection model [25].
Marburg virus infection in NHPs (nonhuman primates): Because the primary natural event of MHF was caused by wild-caught African inexperienced monkeys, this species was a natural choice for the MHF animal model. At that point, Macaca mulatta macaques (Macaca mulatta) were found to be equally prone to infection and showed symptoms when immunised with MARV [26]. The Associate in Nursing MHF model was also well characterised in Cynomolgus macaques (Macaca fascicularis). When associated with a nursing period of 2–6 days, the monkeys showed symptoms of unwellness, anorexia, diarrhoea, skin rash, and hemorrhagic manifestations by any route of MARV-inoculation. Death occurred 6-13 days after infection due to a sharp decrease in blood heat, and mortality was nearly 100% [27]. It was shown that reducing the virus matter light-emitting diode delayed the onset of the illness and longer time to death without reducing mortality. Within the macaques, petechial rashes on the forehead, chest, axillae, and groynes were distinguished and resembled the rashes that appeared in patients with MHF, but intriguingly, the rashes weren't seen in inexperienced African monkeys (Simpson). A marked blood disorder was ascertained at the beginning of the illness. Blood disorder and blood disease thanks to redoubled neutrophilia were prominent on 5-6 days when infection was redoubled. Changes in natural action systems, like a decrease in current levels of supermolecule C, a rise in current D-dimer, and protein deposition in tissues, were noted at late stages of the illness. The pathological changes in the liver as well as multifocal gangrene of the parenchyma cells and white corpuscle cell death in liquid body substance tissues were distinguished. Monocytes and DCs within the liquid body substance tissues, similarly as Kupffer cells and sinusoids lining cells within the liver, were the first target cells for infections with MARV. The infection then progressed to parenchymal cells within the liver, endocrine gland, and high epithelium venules in liquid body substance tissues. Finally, the infection spreads to epithelium cells in a very specific organ tissue. The virus or microorganism substance was detected in the liver, body fluid nodes, spleen, adrenal gland, kidney, and blood in infected cynomolgus macaques. The onset of viraemia occurred on Day three, and the maximum titre in cynomolgus macaques and African inexperienced monkeys was 1078 pfu/ml on Day eight, when infection occurred. Under experimental conditions, the possibility of aerosol transmission of MARV was shown in catarrhine models, though such a transmission route has not been delineated in human outbreaks [28].
Inactivated virus and fractional monetary unit vaccines
According to 2018 studies, FAVIPIRAVIR: Vero E6 cells were taken from the Yankee kind of culture assortment and maintained at 37°C with five-hitter dioxide in Dulbecco’s changed Eagle’s medium and supplemented with 100% heat-inactivated foetal bovine body fluid (FBS) and I Chronicles Penicillin-Streptomycin. The mouse was propagated on Vero E6 cells. The favipiravir was dissolved in Tween 80 (0.5%) and DPBS to ten mg/ml for orally administered treatment in vivo. In case of vitro work, favipiravir was dissolved in dimethyl sulfoxide at concentration of ten mg/ml and keep at -20ºC. The ultimate concentration of dimethyl sulphoxide within the cells that were civilised supernatant was 0.1% [29].
In vitro study
In 96-well plates, Vero E6 cells were adult to ninety-fifth confluence and infected with virus at an MOI (multiplicity of infection) of 0.01. Diluted favipiravir solutions were an extra one post-infection. The substance was removed one hour after infection and mixed with 100 l of modern DMEM and two FBS. At seventy-two hours post-infection, cell supernatants were collected and virus polymers were analysed by RT-qPCR wherever the supreme effective concentration of restrictive operation had to be determined and therefore the ninetieth effective restrictive concentration. By titration, cell culture supernatant was tested with infectious viruses [30].
In vivo study
Groups of 9/10 feminine mice for 6–8 weeks got a dose of 1000X the five hundred dose (LD50=0.01) with two hundred l of DPBS (pH 7.4) by intraperitoneal injection. On day 1, mice were treated with either three hundred mg/kg of weight of favipiravir. On the second day, two, three, or four post-infection, or seventy-five or one hundred fifty mg/kg solely orally. The drug was administered as per day until a continuous eight days. Management animals are treated in the same manner with PBS rather than drugs. Daily the animal’s square measure treated and vi mice are taken for treatment for weight loss and survival. On the sixth day, blood and tissue were collected from 3/4 mice per treatment cluster to see infectious agent polymer levels and infectious agent hundreds. Then they evaluated organic chemistry markers and counted somatic cell numbers. Quantification of infectious agent polymer levels by RT-qPCR. Infectious agent polymer was extracted from mouse blood using the QIAamp infectious agent polymer minikit (Qiagen), and total polymer was extracted from mouse tissues using the RNeasy mini Kit according to the manufacturer's instructions. The infectious agent polymer levels were resolute by reverse transcription quantitative PCR (RT-qPCR) with the assistance of the Light Cycler 480 thermal cycler and the Light Cycler 480 polymer Master reaction Probes kit together with the primers [31].
Infectious virus titrations: virus sublimates from blood or harvested organs that were diluted 10-fold in DMEM by supplementing with two heat-inactivated FBS. Cells were inoculated with a hundred l of every dilution in triplicate and incubated at 37ºC for one hour. Then the supernatant was replaced with a hundred l of contemporary DMEM with two FBS and once more incubated for fourteen days. Once that was scored for the presence of cytopathic effects and volumetric analysis were calculated by the Reed and Muench methodology [32].
According to 2021 studies on Remdisivir, Marburg virus may be an animal virus with documented human case-fatality rates of up to ninetieth. Here, we have a tendency to evaluate the therapeutic efficacy of remdesivir (GS-5734) in bloodless primates through an experiment infected with MARV. Starting four or five days post immunisation, cynomolgus macaques were treated once daily for 12 days with vehicle, 5 mg/kg remdesivir, or a 10-mg/kg loading dose followed by 5 mg/kg remdesivir. All vehicle-control animals died, whereas eighty-three of animals receiving a 10-mg/kg loading dose of remdesivir survived, as did five hundredths of animals receiving a 5-mg/kg remdesivir regime. Remdesivir-treated animals exhibited improved clinical scores, lower plasma infective agent ribonucleic acid, and improved markers of excretory organ function, liver function, and coagulopathy versus vehicle-control animals [33]. The tiny molecule remdesivir showed therapeutic effect in this filovirus malady model with treatment initiation five days post immunisation, supporting additional assessment of remdesivir for the treatment of filovirus malady in humans. Vaccination, which will be deoxyribonucleic acid, DNA/rAd5 boost or rAd5 alone, protects the animal from fatal disease once challenged with a dose of MARV African country. Whereas none of the animals developed MARV African country viraemia, the deoxyribonucleic acid/rAd5 combination cluster moreover DNA solely developed gentle clinical signs like rash, white corpuscle depletion, and eating disorder. The results represented by Geisbert and colleagues give insight into the importance of CD4 and CD8 T-cell responses; a qualitative difference in T-cell responses looks to correlate with protection of the NHPs from each mortality and morbidity exploitation of the deoxyribonucleic acid and/or rAd5 vaccinum, whereas antigen-specific antibodies alone aren't sufficient for cover from MHF [34].
Symptoms
Fever/Severe headache, Joint and muscle aches, Chills/Weakness, Nausea and vomiting, Diarrhoea, Red eyes (Bleeding, usually from the eyes, and bruising), Raised rash, Chest pain and cough, Stomach pain, Severe weight loss etc [35].
Treatment
The area unit currently has no vaccines or medication approved for human use as a safeguard against the Marburg virus. Since the first recognised irruption of the Marburg virus in 1967, the common case mortality rate has been eighty percent. The study was conducted at level (BSL)-4 at UTMB's Town National Laboratory. BSL-4 may be a highly-restricted space where scientists wear positive pressure protective suits and study pathogens that cause severe and sometimes fatal diseases. UTMB has the sole functioning BSL-4 laboratory settled on an associate's degree Yankee university field. The A spread rate of NHP candidate Marburg vaccines is incontestable, with favourable survival and immunogenicity parameters, to incorporate VSV, VLP, and adenoviral vectored vaccines. Elevated binding antibodies are perceived to be systematically related to protection across the NHP challenging studies [36]. Any human trials to advance vaccines to limit the spread of this highly lethal virus are required.
DISCUSSION
Many outbreaks of the MARD have been documented ever since the first-ever case in 1967, following the initial contact with wild animals and contraction of the malady. Despite a large variety of medicines being tried thus far, no constructive outcome has been achieved. Therefore, the most reliable methodology of treatment remains subsidiary with careful watching and isolation of the patient. Disease-modifying medicine and infective agent super molecule matter have shown some promising leads for several patients and may benefit those affected; however, they may not be the ultimate solution to the deadly virus. There's a sheer want for production of a far better possibility, such as a secure and reliable immunising agent to shield individuals particularly at risk of the malady. Though much has been done to develop a totally credible vaccination, there are still several aspects concerning it that require to be dealt with before we tend to proceed to the good invention. Thus, by providing an in-depth examination of MARD, we hope to specialise in its related developments as well as its impact on the health-care system. It adds value to medical literature by collecting the required data about MARD and helping guide health policies to constraint. In view of the recent natural event within the Democratic Republic of Congo, it's imperative for health authorities to plan an inspiration keeping in sight the current times, where an uncontrolled endemic or pandemic will speedily transgress a virus, inflicting mayhem on the already restricted health facilities and innumerable challenges.
CONCLUSION
Marburg virus impact on earth could cause epidemic impact in future. Currently days for Corona virus it's terribly difficult to steer a standard life style, thus in future if Marburg unfold like corona then it should cause famine or destroy the life from earth as a result of it not solely unfold in human additionally in non-human perimates (NHPs). From 1967 when the natural event of this virus in European nation, still there's no vaccines. It takes fifty four years, still the absence of the vaccines it's terribly harmful for human. Reviewing on this virus is that the solely explanation for knowing that not only from corona virus we've to remain safe there are thousands of thousands viruses from that we've to protect our world.
CONFLICT OF INTEREST
There is no potential conflict of interest associated with this review work.
REFERENCES
- Rougeron V, Feldmann H, Grard G, et al., J Clin Virol. 2015, 64: p. 111-119.
- Song JD, Qu JG, Hong T. Bing Du XueBao. 2014, 30(3): p. 292-297.
- Brauburger K, Hume AJ, Mühlberger E, et al., Viruses. 2012, 4(10): p. 1878-927.
- Languon S, Quaye O. Virology (Auckl). 2019.
- Amman BR, Swanepoel R, Nichol ST, et al., Curr Top Microbiol Immunol. 2017, 411: p. 23-61.
- Mehedi M, Groseth A, Feldmann H, et al., Future Virol. 2011, 6(9): p. 1091-106.
- Nyakarahuka L, Shoemaker TR, Balinandi S, et al., PLoS Negl Trop Dis. 2019, 13(3): p. e0007257.
- Brainard J, Hooper L, Pond K, et al., Int J Epidemiol. 2016, 45(1): p. 102-116.
- Schmidt KM, Mühlberger E. Viruses. 2016, 8(6).
- Shifflett K, Marzi A. Virol J. 2019, 16(1): p. 1650.
- Martines RB, Ng DL, Greer PW, et al., J Pathol. 2015, 235(2): p. 153-174.
- Yang Y, Greenough K, Wilson JM. J Virol. 1995, 69: p. 2004-2015.
- Kass-Eisler A, Leinwand L, Gall J, et al., Gene Ther. 1996, 3: p. 154-162.
- Kobinger GP, Feldmann H, Zhi Y, et al., Virology. 2006, 346(2): p. 394-401.
- Sullivan NJ, Hensley L, Asiedu C, et al., Nat Med. 2011, 17(9): p. 1128–1131.
- Ledgerwood JE, Costner P, Desai N, et al., Vaccine. 2010, 29(2): 304-313.
- Zhu F-C, Hou L-H, Li J-X, et al., Lancet. 2015, 385(9984): p. 2272–2279.
- Zhu FC, Wurie AH, Hou LH, et al., Lancet. 2017, 389 (10069): p. 621-628.
- Li J-X, Hou L-H, Meng F-Y, et al., Lancet Glob Health. 2017, 5(3): p. e324–e334.
- Heald AE, Iversen PL, Saoud JB, et al. Antimicrob Agents Chemother. 2014, 58: p. 6639-6647.
- Feldmann H, Sanchez A, Geisbert T. Filoviridae: Marburg and Ebola viruses. 2013, p. 923-956.
- Meltzer MI, Atkins CY, Santibanez S, et al., 2014, 63: p. 1-14.
- Geisbert TW, Geisbert JB, Leung A, et al., J Virol. 2009, 83: p. 7296 -7304.
- Matassov D, Marzi A, Latham T, et al., J Infect Dis. 2015, 212: p. S443-S451.
- Slenczka W, Klenk HD. J Infect Dis. 2007, 196: p. S131-S135.
- Siegert R, Shu HL, Slenczka W, et al., Ger Med Mon. 1968, 13: p. 1-2.
- Kunz C, Hofmann H, Kovac W, et al., Wien Klin Wochenschr. 1968, 80: p. 161-162.
- Kissling RE, Robinson RQ, Murphy FA, et al., Science. 1968, 160: p. 888-890.
- Smith CE, Simpson DI, Bowen ET, et al., Lancet. 1967, 7526: p. 1119-1121.
- WHO. Ebola haemorrhagic fever in Sudan, 1976. Bull. World Health Organ. 1978, 56: p. 247-270.
- WHO. Ebola haemorrhagic fever in Zaire, 1976. Bull World Health Organ. 1978, 56: p. 271-293.
- Gear JS, Cassel GA, Gear AJ, et al., Br Med J. 1975, p. 489-493.
- Bausch DG, Nichol ST, Muyembe-Tamfum JJ, et al., N Engl J Med. 2006, 355: p. 909-919.
- Towner JS, Khristova ML, Sealy TK, et al., J Virol. 2006, 80: p. 6497-6516.
- Centers for Disease Control and Prevention. Mortal Wkly Rep. 2009, 58: p. 1377-1381.
- Timen A, Koopmans MP, Vossen AC, et al., Emerg Infect Dis. 2009, 15: p. 1171-1175.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref