Full Length Research Article - Der Pharma Chemica ( 2022) Volume 14, Issue 9
Analytical Determination of Imiquimod in Various Solvents by Uv Spectroscopical Technique
Bhupinder Kaur*Bhupinder Kaur, University Institute of Pharmaceutical Sciences, Chandigarh University, Punjab, India, Email: bhupinder21990@gmail.com
Received: 09-Sep-2019, Manuscript No. dpc-21-02; Editor assigned: 13-Sep-2019, Pre QC No. dpc-21-02 (PQ); Reviewed: 27-Sep-2019, QC No. dpc-21-02; Revised: 20-Jul-2022, Manuscript No. dpc-21-02 (R); Published: 05-Sep-2022, DOI: 10.4172/0975-413X.14.9.24-27
Abstract
UV spectrophotometric method is the promising method which is developed for the programmed approximation of Imiquimod in bulk drug and different delivery systems. The absorption maxima of drug is shown at 244 nm and molar absortivity of 1.5899 ×104 l/mol×cm. Drug completely obeyed Beer-Lambert’s law within the concentration range of 0-2.5μg/ml. Correlation coefficients (R2) was found to be 0.998, 0.999. 0.999 in 0.1N HCl, Phosphate buffer pH 6.8 and methanol respectively.
Keywords
Imiquimod; Beer-Lambert’s law; Spectrophotometric method; Linear
INTRODUCTION
UV Analysis is one of the most conventional but unsurpassed method of drug estimation and quality control in divergent drug formulations when already many of the hitech chromatographic techniques and other hyphenated techniques are available till date [1]. Recent strives in research are initiated to develop such a UV analytical technique which should be simple, sensitive, accurate, precise and economical so that routine estimation of Imiquimod could be done in bulk and pharmaceutical dosage form using 0.1 N HCl, Phosphate Buffer 6.8, Methanol. The γ max was observed at 244.0 nm. It was seen that in the concentration 0-2.5 μg/ml beer law was followed. Imiquimod belong to class Quinolines. Imiquimod is available in market by the brand name of Aldara [2]. It is most commonly used antiviral drug. It is basically used to cure many types of out-growths on the skin. These may be the early signs of cancerous growths (actinic keratoses), certain type of skin cancer, and outward pertusions like warts on the outside of the genitals/ anus. Imiquimod are the type of drugs called immune response modifiers [3,4]. Imiquimod is an immune responser modifier which will play the role of toll-like receptor 7 agonist. Imiquimod can be used to cure certain types of skin cancer called superfacial basal cell carcinoma.
Mechanism of action
The mechanism of action of Imiquimod is through triggering of innate and acquired immune responses, which will finally lead to inflammatory cell infiltration with-in the area where drug is applied followed by apoptosis of diseased tissue. Imiquimod is also responsible for inducing the expression of interleukin (IL)-6, IL-8, and tumor necrosis factor alpha genes. During the treatment of basal cell carcinoma, Imiquimod resembles to act as a toll-like receptor-7 agonist, and exert its anti-tumor effect via modifying the immune responses and triggering of apoptosis in BCC cells. During the treatment of BCC Cells it may increase the infiltration of lymphocytes, dendritic cells and macrophage into the tumor lesion [5-7] (Figure 1).
MATERIALS AND METHODS
Materials
UV-visible double beam spectrophotometer, Systonics, Ahmadabad (Gujarat) – 2201, Imiquimod was bought as pure drug from Glenmark Pharmaceuticals, Baddi India.
Solvent selection
The solubility of the drug is accessed in different solvents and after that 0.1 N HCl, Phosphate Buffer 6.8, methanol were selected as suitable solvents for analysis of spectral characteristics.
Methods
Preparation of 0.1 N HCl
Add 8.3 ml of concentrated HCl in a 1000ml volumetric flask and make up the remaining volume with the help of distilled water [7].
Phosphate buffer 6.8 pH
In a 200 ml volumetric flask 50 ml of 0.2 M potassium dihydrogen phosphate was dissolved and 22.4 ml of 0.2 m sodium hydroxide was added to it when total volume was made up to 200 ml [8-10].
Construction of standard calibration curve for Imiquimod
Suitable dilutions were prepared to produce a solution of Imiquimod in the concentration range of 0, 0.5, 1, 1.5, 2, 2.5 μg/ml in 0.1N HCl, phosphate buffer 6.8 and methanol [11]. 244 nm was selected as the value to check the absorbance of Imiquimod in 0.1 N HCl, Phosphate buffer pH 6.8 and methanol by UV-visible spectrophotometer and the graphical plot was plotted by taking concentration in X-axis and absorbance in Y-axis to get the standard curve.
Procedure for Calibration curve of Imiquimod in 0.1 N HCl
• An accurately weighed 100mg of Imiquimod was mixed in 100ml of 0.1 N HCl from which the further test dilutions for drug having concentration 0, 0.5, 1, 1.5, 2, 2.5 μg/ml were prepared.
• All the prepared drug concentrations were scanned in a 10mm quartz cell on a Systronics 2201 UV spectrophotometer between range 200-600 nm.
• The scan of the drug was plotted on U.V spectrophotometer between range 200-400 nm when at 244.0 nm which shows good agreement to European Pharmacopoeia and referred articles.
• The drug was scanned and γ max of the drug was found within the range which states that drug follows Beer lamberts’ law. The drug gave best fitting with the reference value.
• As the concentrations 0, 0.5, 1, 1.5, 2, 2.5 μg/ml were found to fit in the equation of linearity so the given concentrations were selected and calibration curve was drawn in triplicate and S.D was calculated for the same.
Calibration curve of Imiquimod in Phosphate buffer pH 6.8
• An accurately weighed 100mg of Imiquimod was dissolved in 100ml of 6.8 pH buffer from which the further working standard solutions of drug having concentration 0, 0.5, 1, 1.5, 2, 2.5 μg/ml were prepared by diluting 6.8 pH buffer .
• All the prepared drug concentrations were scanned in a 10mm quartz cell on a Systronics 2201 UV spectrophotometer between range 200-600 nm.
• The scan of the drug was plotted on U.V spectrophotometer between range 200-400 nm when at 244.0 nm which shows good agreement to European Pharmacopoeia and referred articles.
• The drug was scanned and γ max of the drug was found within the range which states that drug follows Beer lamberts’ law. The drug gave best fitting with the reference value.
• As the concentrations 0, 0.5, 1, 1.5, 2, 2.5 μg/ml were found to fit in the equation of linearity so the given concentrations were selected and calibration curve was drawn in triplicate and S.D was calculated for the same. Procedure for the preparation of calibration curve in methanol
• An accurately weighed 100mg of Imiquimod was dissolved in 100ml of methanol from which the further working standard solutions of drug having concentration 0, 0.5, 1, 1.5, 2, 2.5 μg/ml were prepared.
• All the prepared drug concentrations were scanned in a 10mm quartz cell on a Systronics 2201 UV spectrophotometer between range 200-600 nm.
• The scan of the drug was plotted on U.V spectrophotometer between range 200-400 nm when at 244.0 nm which shows good agreement to European Pharmacopoeia and referred articles.
• The drug was scanned and γ max of the drug was found within the range which states that drug follows Beer lamberts’ law. The drug gave best fitting with the reference value.
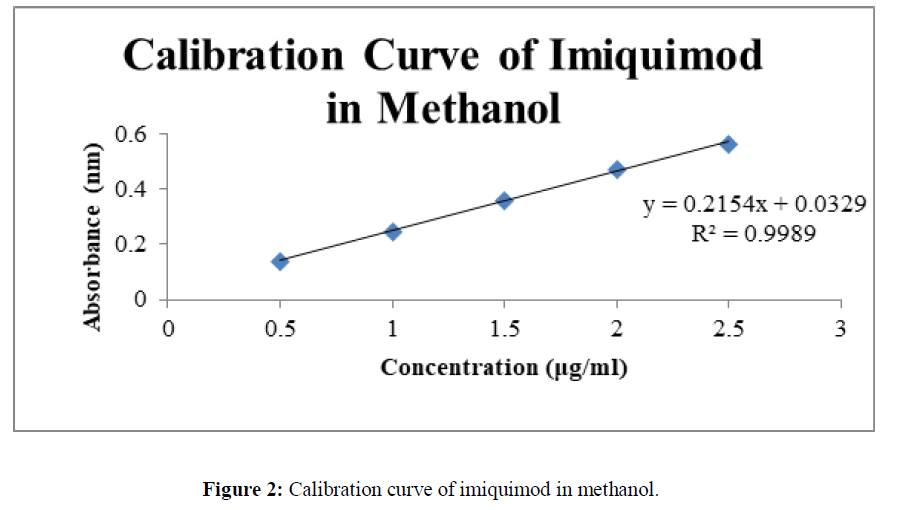
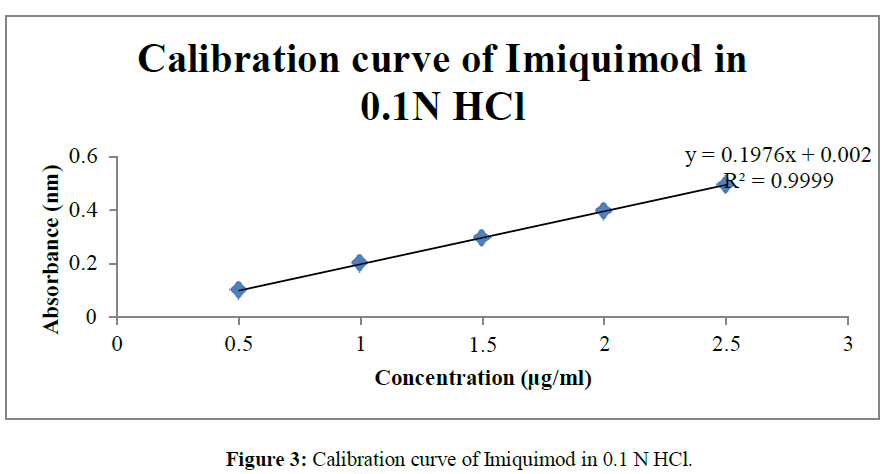
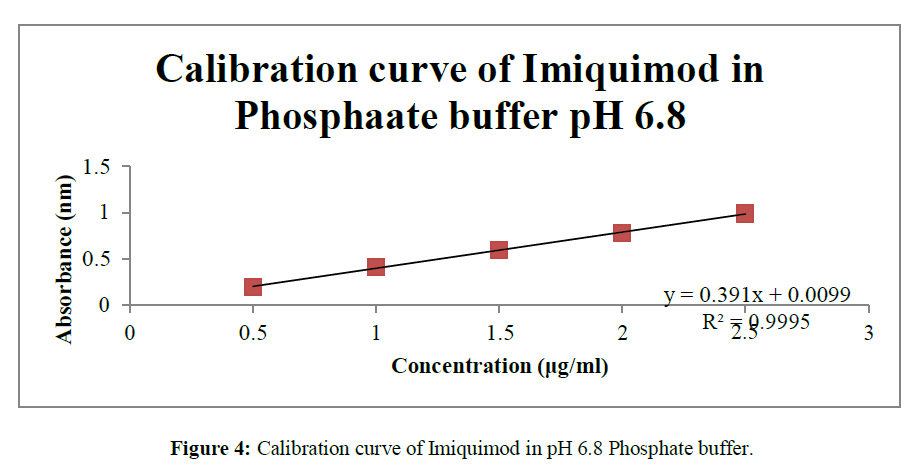
• As the concentrations 0, 0.5, 1, 1.5, 2, 2.5 μg/ml were found to fit in the equation of linearity so the given concentrations were selected and calibration curve was drawn in triplicate and S.D was calculated for the same (Table 1) (Figure 2-4).
| Sr.No. | Concentration(mg/ml) | Absorbance | ||
|---|---|---|---|---|
| In Methanol | In pH 6.8 Phosphate Buffer |
In 0.1 N HCl | ||
| 1 | 0 | 0 | 0 | 0 |
| 2 | 0.5 | 0.14 ± 0.018 | 0.101 ± 0.007 | 0.201 ± 0.016 |
| 3 | 1 | 0.245 ± 0.005 | 0.201 ± 0.014 | 0.409 ± 0.021 |
| 4 | 1.5 | 0.358 ± 0.013 | 0.296 ± 0.002 | 0.598 ± 0.027 |
| 5 | 2 | 0.472 ± 0.009 | 0.397 ± 0.006 | 0.782 ± 0.018 |
| 6 | 2.5 | 0.565 ± 0.004 | 0.497 ± 0.001 | 0.992 ± 0.026 |
RESULTS AND DISCUSSION
The method which was proposed for the UV analysis was simple, rapid and precise and do not suffer from any kind of interference and disturbance. Various readings from the calibration curve are depicted in the Table No.1. The validated analytical method were found to be linear in the range of 0-2.5μg/ml at 244 nm with correlation coefficients (R2) 0.998, 0.999. 0.999 in methanol, 0.1N HCl and Phosphate buffer pH 6.8respectively.
CONCLUSION
A new validated accurate and precise spectrophotometric method were built for the analysis of Imiquimod in APIs and different drug formulations.
REFERENCES
- Kaur B, Goswami M. J Inf Comput Sci. 5(9): p. 99-105.
- Gupta V, Chuttani K. Trivedi P, J Labelled comp Radiopharm, 2014, 1: p. 1-4.
- Oumata N, Nguyen PH, Beringue V. 2013, 8: p. 1-11.
- Padamwar MN, Pawar AP. Indian Drugs. 2003, 9: p. 526-531.
- Khan AW, Kotta S, Ansari SH. 2014, 4: p. 1-8.
- Lachman L, Lieberman IA. 3rd edition Varghese publication house. 1991, 3: p. 536-537.
- Isenberg G, Isenberg G, 1995.
- Indian Pharmacopeia. 2007, 2: p. 67-70.
- Holkar GS, Rokade MD. IJTAS. 2012.
- Ghada A, Rania HF. AAPS Pharm Sci Tech. 2009, 10.
- Nesalin JAJ, Babu CJG, Kumar GV, et al., EJ Chem. 2009, 6(3): p. 780-784.