Research Article - Der Pharma Chemica ( 2018) Volume 10, Issue 8
Analytical Method Development and Validation for Simultaneous Estimation of Cinitapride Hydrogen Tartrate and Pantoprazole Sodium in Pharmaceutical Dosage Form by RP-HPLC
Sravanthi Macharla1,2* and Ravindar Bairam1,2
1Department of Pharmaceutical Analysis, School of Pharmacy Anurag Group of Institutions, Venkatapur (V), Ghatkesar (M), Ranga Reddy (Dist), Telangana- 501301, India
2Department of Pharmaceutical Chemistry, Vignan Institute of Pharmaceutical Sciences, Deshmukhi, Yadadribhuvanagiri (Dist), Telangana- 508284, India
- *Corresponding Author:
- Sravanthi Macharla
Department of Pharmaceutical Analysis
School of Pharmacy Anurag Group of Institutions
Venkatapur (V), Ghatkesar (M), Ranga Reddy (Dist), Telangana- 501301, India
Abstract
The aim of the research is method development and validation of Reversed-Phase High-Performance Liquid chromatography (RP-HPLC) method for simultaneous determination of Cinitapride hydrogen tartrate and Pantoprazole sodium in its pharmaceutical dosage form. The method is simple, precise, economic, less time consuming and suitable for routine quality control analysis of both the drugs in formulation. The chromatographic separation was achieved on Thermoscientific BDS Hypersil C18 (250 × 4.6 mm, 5μl) column using a mixture of methanol and 0.1% v/v triethylamine (pH 6) in the ratio 85: 15 % v/v at a flow rate of 1.0 ml/min and UV detection at 264 nm. The retention times of Cinitapride and Pantoprazole were found to be 4.73 and 2.86 min respectively. The method shows linearity in the concentration range of 0.5-1.3 μg/ml for both the drugs with r2=0.9922 for Cinitapride and r2 =0.9974 for Pantoprazole. The LOD of Cinitapride and Pantoprazole were found to be 0.00164 μg/ml and 0.00042 μg/ml respectively. The LOQ of Cinitapride and Pantoprazole were found to be 0.00496 μg/ml and 0.00126 μg/ml respectively. The percentage recovery was found to be within the limits. The method for the determination of assay was below 2.0% RSD. Hence the developed HPLC method was applied for the estimation of Cinitapride and Pantoprazole in its pure form as well as in tablet dosage form and results was found to be in good agreement with the labeled claim. The developed method was found to be simple, accurate, precise, and specific and is useful in the quality control of bulk and pharmaceutical formulations.
Keywords
RP-HPLC, Cinitapride hydrogen tartrate, Pantoprazole sodium, Simultaneous estimation, Validation.
Introduction
Cinitapride hydrogen tartrate (Figure 1) is chemically 4-Amino-N-[1-(cyclohex-3-en-1yl methyl) piperidin-4-yl]-2-ethoxy-5-nitrobenzamide. It is gastroprokinetic agent and anti-ulcer agent of Benzamide class [1]. It acts as an agonist of 5HT1 and 5HT4 receptors and as an antagonist of 5HT2 receptors. Cinitapride is indicated for gastrointestinal disorders associated with motility disturbances such as gastrooesophageal reflux disease, non-ulcer dyspepsia and delayed gastric emptying.
Pantoprazole (Figure 2) is chemically 6-(diflouromethoxy)-2- {[(3,4-dimethoxypyridine-2yl)methane]sulfinyl}-1H-1,3-benzodiazole. It is a proton-pump inhibitor that inhibits gastric acid by blocking H+/K+ adenosine triphosphate enzyme system (proton pump) of gastric parietal cells. It is substituted benzimidazole indicated for stomach ulcers, intestinal ulcers, gastrooesophageal disease (GERD) by reducing amount of acid production in stomach. It is used to treat stomach ulcers caused due to medication with NSAIDs and by bacteria called H. pylori. Cinitapride and Pantoprazole are available in combined dosage form as hard gelatin capsule (CINTODAC). Each capsule contains 3 mg of Cinitapride hydrogen tartrate equivalent to Cinitapride (as extended release pellets) and 40 mg of Pantoprazole sodium equivalent to Pantoprazole (as enteric coated tablet) [2-6].
The combination of Cinitapride and Pantoprazole is used to treat gastro intestinal disorders in particular hyperacidity associated with gastro-intestinal dismotility [7]. Extensive literature survey reveals that several analytical methods have been reported for the estimation of Cinitapride and Pantoprazole in pharmaceutical dosage form.
The aim of the current research is to develop simple and accurate RP-HPLC method for simultaneous determination of Cinitapride and Pantoprazole and extend it for their determination in formulation and validate as per the ICH guidelines [8-10].
Materials and Instruments
Chemicals and solvents used [11-15]
• Acetonitrile
• Water
• Methanol
• Orthophosphoric acid
• Triethylamine
All reagents and chemicals were used of HPLC grade.
Pure drug samples (Table 1) [16]
| Drugs | Supplier | Quantity | Purity |
|---|---|---|---|
| Cinitapride hydrogen tartrate | Comprime Labs | 10.0 g | 99.98% w/w |
| Pantoprazole sodium | Comprime Labs | 10.0 g | 99.98% w/w |
Table 1: Pure drug information
The drugs used for the present investigation were donated as gift samples.
Marketed formulation available (Table 2) [17]
| Brand Name | Mfg By | Content | Quantity |
|---|---|---|---|
| CINTODAC hard gelatin capsule | Zydus Cadila Healthcare Ltd. | Cinitapride hydrogen tartrate | 3 mg |
| Pantoprazole sodium | 40 mg |
Table 2: Marketed formulation information
The marketed formulation was purchased from local market.
Instrumentation (Table 3) [18]
| Name of Equipment | Make | Model |
|---|---|---|
| HPLC | Shimadzu | 996 PDA Detector |
| LC Solutions Software | ||
| pH Meter | Lab India® | SAB 5000 |
| Digital Electronic Balance | Shimadzu | BL 220H |
| Column | Thermoscientific (5 µm) | BDS HYPERSIL C18 [4.6 x 250 mm (id)] |
Table 3: Required Instruments information
Experimental
HPLC method [19]
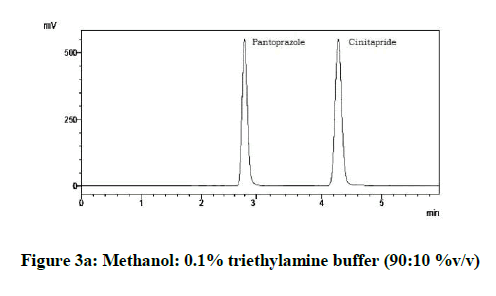
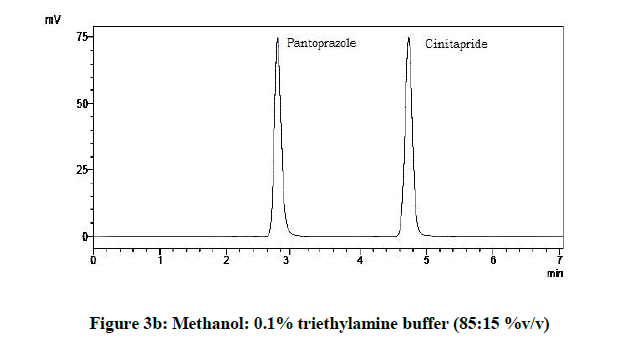
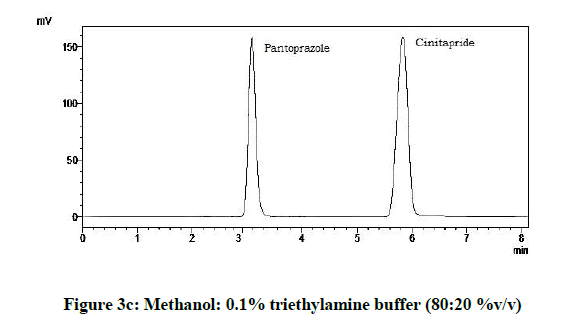
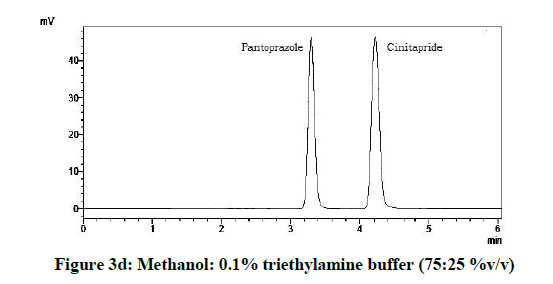
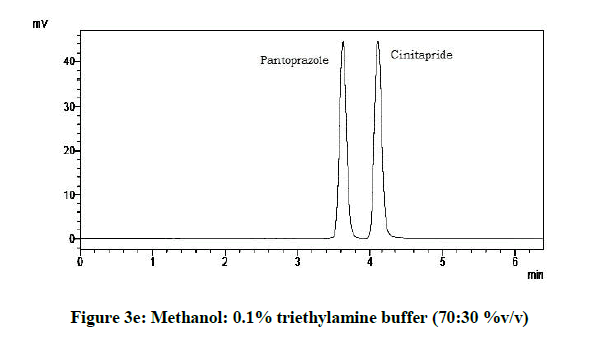
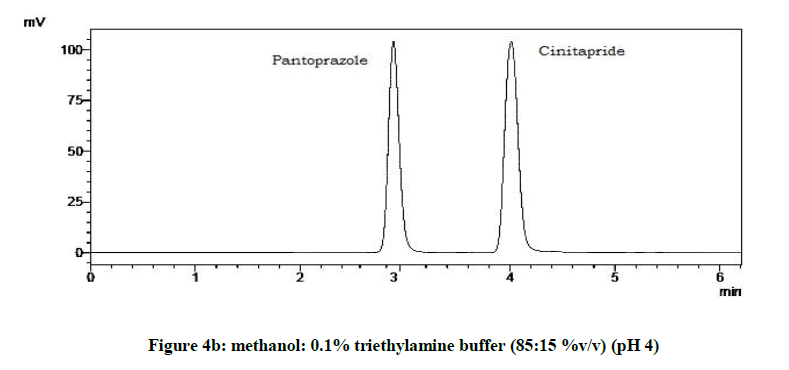
From the various trials (Figures 3a-3e) (Table 4), chromatograms using mobile phase containing different ratios of methanol and 0.1% triethylamine such as 90: 10, 85: 15, 80: 20, 75: 25 and 70: 30 %v/v were recorded at 264 nm at a flow rate of 1.0 ml/min. The ratio of methanol and 0.1% triethylamine in 85: 15 %v/v (Figure 1b) gave good resolution with symmetric peaks.
Effect of pH of mobile phase
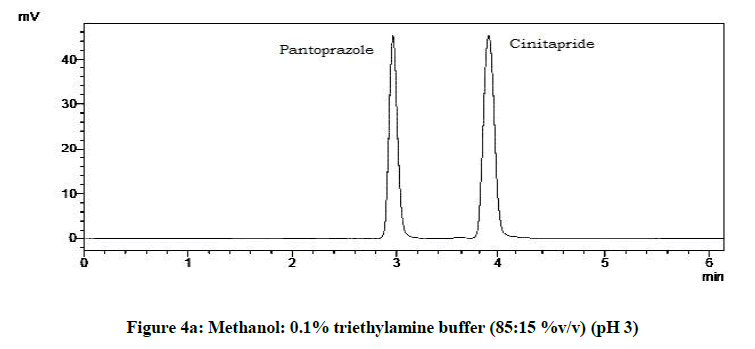
The mobile phase consisting methanol and 0.1 % triethylamine in ratio 85:15 %v/v was adjusted to different pH such as 3, 4, 5, and 6 using 1% Orthophosphoric acid.
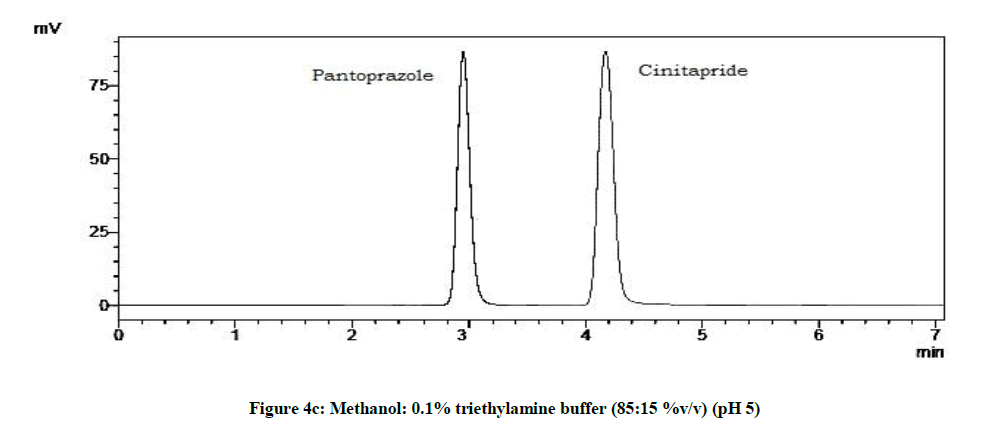
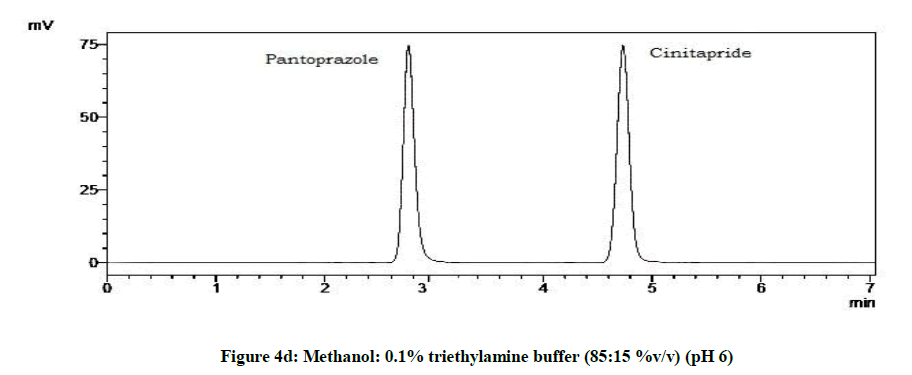
From the trials (Figures 4a-4d and Table 5), at the pH of 6 (Figure 4d), both the drugs showed symmetric peaks and hence selected for the study. Chromatographic condition also determined (Table 6).
Preparation of standard solutions
Accurately weighed 10 mg of Cinitapride and 10 mg of Pantoprazole were transferred into a separate clean, dry 100 ml volumetric flasks and dissolved with sufficient volume of mobile phase. The volume was made up to 100 ml with mobile phase to get 100 μg/ml concentration of each drug.
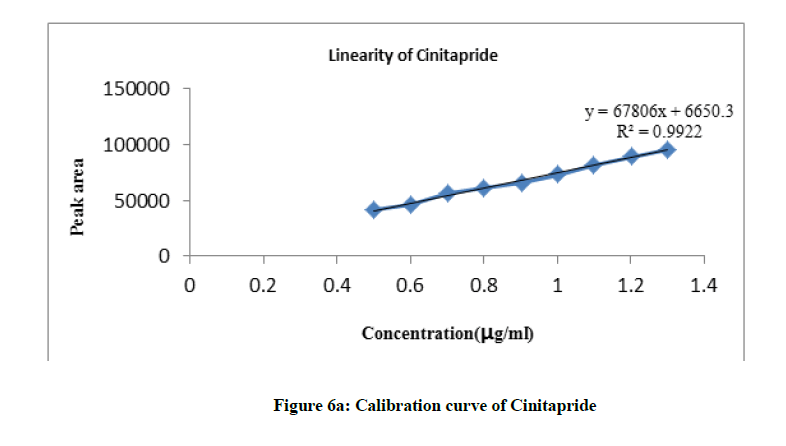
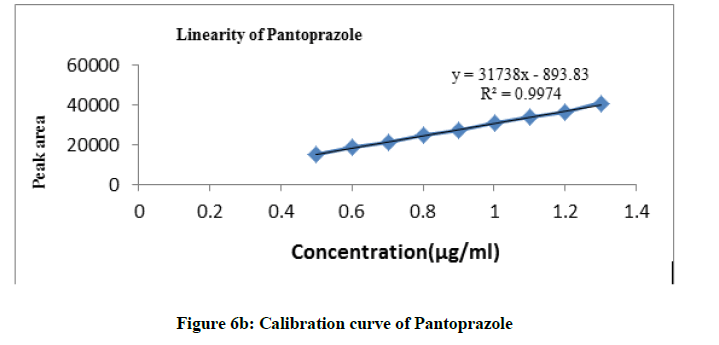
From the stock solution, dilutions were made in the concentration range of 0.5-1.3 μg/ml for both the drugs with mobile phase and chromatograms were recorded at 264 nm. The peak areas were plotted against concentration and calibration graphs were constructed for both the drugs. Concentration range of 0.5-1.3 μg/ml was found to be linear and obeys Beer’s law.
Analysis of marketed formulation [20-23]
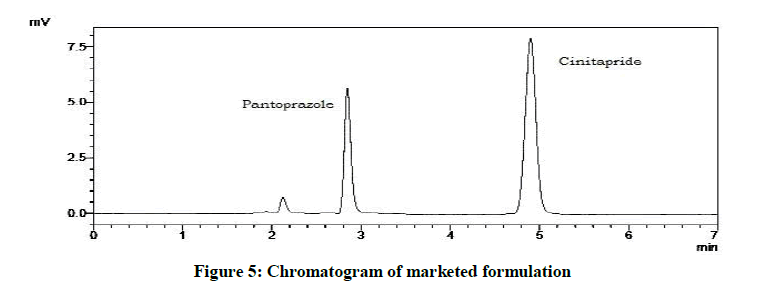
20 capsules of Cintodac (Label claim: 40 mg Pantoprazole and 3 mg Cinitapride) were weighed, emptied in a glass mortar and powdered. Average weight of capsule was calculated and amount of powder equivalent to 20 mg of Pantoprazole and 18.5 mg of Cinitapride was accurately weighed and added to 50 ml volumetric flask so that sample contains 20 mg equivalent of each drug. It is dissolved in mobile phase, sonicated and made up to the mark and filtered through 0.45 μm membrane filter. Appropriate aliquot of this standard stock solution of formulation (400 μg/ml) was taken into a 10 ml volumetric flask and volume was made up to mark with mobile phase to obtain desired concentration. A 20 μl of sample was injected into injector of liquid chromatographic system and chromatogram was recorded (Figure 5 and Table 7).
Method validation
The method was developed and validated according to ICH guidelines.
Linearity
The calibration curves for Cinitapride and Pantoprazole were constructed by plotting peak area against concentration and regression equations were calculated. Both the drugs showed linearity in the concentration range of 0.5-1.3 μg/ml. Aliquots 20 μl of each solution injected under the operating chromatographic condition (Figures 6a, 6b and Table 8).
Accuracy
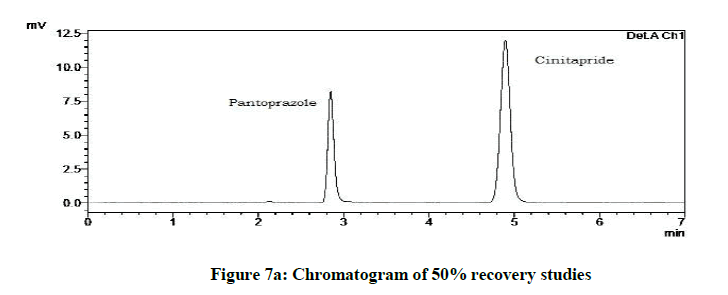
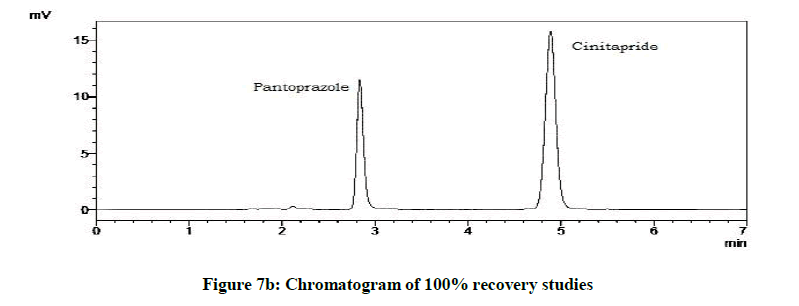
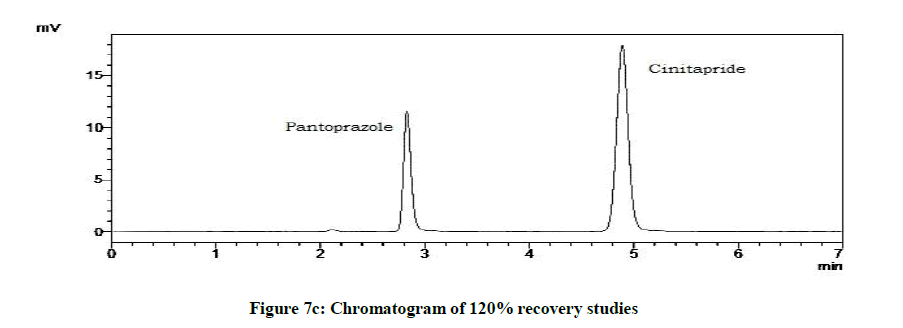
Accuracy studies were expressed as recovery (%), which is determined by the standard addition method at 50%, 100%, 120% of the label claim according to ICH guidelines. The %Recovery and %RSD were calculated and reported (Figures 7a-7c and Table 9).
Precision
The intraday and interday precision of the proposed method was evaluated by analyzing samples of two different concentration of Cinitapride (0.8, 0.9 μg/ml) and Pantoprazole (0.8, 0.9 μg/ml) in triplicates on same and different days (Tables 10 and 11).
Repeatability
Repeatability was determined by analyzing standard solutions of 0.8 μg/ml concentration of Cinitapride and Pantoprazole by injecting six times. The precision and % RSD were calculated and reported (Table 12).
Limit of detection and limit of quantitation (LOD and LOQ)
The LOD and LOQ of Cinitapride and Pantoprazole were calculated based on standard deviation of the response and slope values of two drugs according to ICH guidelines.
System suitability studies
System suitability is a pharmacopoeial requirement and used to verify, whether the resolution and reproducibility of chromatographic system are adequate for analysis to be done. The parameters like theoretical plate count, retention time, tailing factor and resolution of both the drugs were reported (Table 13).
Results and Discussion
Mobile phase
Effect of ratio of mobile phase
From the above five trials the observations are follows:
| S. No. | Methanol: 0.1%triethyl amine (% v/v) | Retention time | |
|---|---|---|---|
| CIN | PAN | ||
| 1 | 90:10:00 | 4.29 | 2.88 |
| 2 | 85:15:00 | 4.73 | 2.86 |
| 3 | 80:20:00 | 5.82 | 3.1 |
| 4 | 75:25:00 | 4.27 | 3.29 |
| 5 | 70:30:00 | 4.12 | 3.62 |
Table 4: Observations for trial of effect of ratio of mobile phase
Effect of pH of mobile phase
| S. No. | pH | Observation |
|---|---|---|
| 1 | pH 3 | Asymmetric peaks |
| 2 | pH 4 | Asymmetric peaks |
| 3 | pH 5 | Slight tailing |
| 4 | pH 6 | Symmetric peaks |
Table 5: Observations for trial of effect of pH of mobile phase
Chromatographic conditions
| Column | Thermoscientific (250 x 4.6 mm, 5 μ) |
| Particle size packing | 10 µm |
| Stationaray phase | BDS HYPERSIL C18 (5 µm) |
| Mobile phase | Methanol: 0.1% triethylamine (pH 6) |
| Detection wavelength | 264 nm |
| Flow rate | 1 ml/min |
| Run time | 07 min |
| Temperature | Ambient |
| Sample size | 20 µl |
| Diluent | Methanol |
Table 6: Chromatographic condition
| Drugs | Labeled amount, mg tablet-1 | Amount found, mg tablet-1 | % Label claim |
|---|---|---|---|
| Cinitapride hydrogen tartrate | 3 | 3.06 | 102 |
| Pantoprazole sodium | 40 | 41.05 | 102.87 |
Table 7: Analysis of marketed formulation
Method validation
Linearity
| Parameters | Cinitapride | Pantoprazole |
|---|---|---|
| λmax, nm | 264 | 264 |
| Beer’s Law limit (µg/ml) | 0.5-1.3 | 0.5-1.3 |
| Regression equation (Y*) | Y = 67806x+6650 | Y = 31738x-893.8 |
| Correlation coefficient (r2) | 0.992 | 0.997 |
| Slope (b) | 67806 | 31738 |
| Intercept (a) | 6650 | 893.8 |
Table 8: Linearity observation
Accuracy studies
| Drugs | % Level | % Recovery | % RSD |
|---|---|---|---|
| Cinitapride | 50 | 103.18 | 0.72 |
| 100 | 102.1 | 1.18 | |
| 120 | 102 | 1.49 | |
| Pantoprazole | 50 | 102.59 | 0.94 |
| 100 | 101.09 | 1.42 | |
| 120 | 102.41 | 1.09 |
Table 9: Accuracy studies
Precision
| Concentration ( (µg/ml) | Injection | Peak area | %RSD | |||
|---|---|---|---|---|---|---|
| CIN | PAN | CIN | PAN | CIN | PAN | |
| 0.8 | 0.8 | 1 | 74340 | 23988 | 0.22 | 0.72 |
| 2 | 74822 | 23668 | ||||
| 3 | 74549 | 23992 | ||||
| 4 | 74654 | 23882 | ||||
| 5 | 74734 | 23789 | ||||
| 6 | 74594 | 23567 | ||||
Table 10: Intraday precision
| S. No. | Concentration (µg/ml) | Peak area | %RSD | |||
|---|---|---|---|---|---|---|
| CIN | PAN | CIN | PAN | CIN | PAN | |
| 1 | 0.8 | 0.8 | 73450 | 22452 | 0.13 | 1.08 |
| 73650 | 22942 | |||||
| 73522 | 22752 | |||||
| 2 | 0.9 | 0.9 | 80290 | 26941 | 0.22 | 0.38 |
| 80652 | 26851 | |||||
| 80428 | 26734 | |||||
Table 11: Interday precision
Repeatability
| Concentration (µg/ml) | Peak area | %RSD | ||||
|---|---|---|---|---|---|---|
| S. No. | CIN | PAN | CIN | PAN | CIN | PAN |
| 1 | 0.8 | 0.8 | 74340 | 23988 | 0.32 | 0.77 |
| 74822 | 23668 | |||||
| 74549 | 23992 | |||||
| 2 | 0.9 | 0.9 | 81590 | 27941 | 0.18 | 0.12 |
| 81312 | 27984 | |||||
| 81341 | 28009 | |||||
Table 12: Observation for Repeatability
System suitability studies
| Drug | Theoretical plate count | Retention time (min) | Tailing factor | Resolution (Rs) |
|---|---|---|---|---|
| Cinitapride | 6806.03 | 2.86 | 1.03 | 17.14 |
| Pantoprazole | 5682.03 | 4.73 | 1.21 |
Table 13: Observation for System suitability studies
Conclusion
For the simultaneous estimation of Cinitapride hydrogen tartrate and Pantoprazole sodium, RP-HPLC method was developed and validated according to ICH guidelines. The proposed method was found to be simple, precise, economic, less time consuming and proved to be superior to most of the reported methods. The mobile phase was simple to prepare and economical. The sample recovery in the formulation was in good agreement with their respective label claims and suitable for routine quality control analysis of both the drugs in formulation.
Acknowledgement
The authors are grateful to Comprime Labs Pvt Ltd, Kukatpally, Hyderabad for providing gift samples of Cinitapride and Pantoprazole. The authors are also thankful to Principal Lalitha College of Pharmacy, Anurag Group of Institutions for providing all the facilities to support the research work.
References
- P.D. Sethi, Quantitative Analysis of Drugs in Pharmaceutical Formulation, 3rd (Edn.), CBS Publishers and Distributors, 1997, 346.
- A.I. Vogel, Elementary Practical Organic Chemistry Part 2: Qualitative Organic Analysis, 3rd (Edn.), 1956, 241.
- A.D. Skoog, Principles of Instrumental Analysis, 1992, 376, 399.
- A.H. Beckett, J.B. Stenlake, Practical Pharmaceutical Chemistry (4th Edn.), CBS Publishers and Distributors, 1997, 281.
- Willard. Dean. Settle. Instrumental Method of Analysis (6th Edn.), 1988, 77.
- S. Joseph, J. Kirkland, J.L. Glajc, John Wiley and Sons Practical HPLC Method Development 2nd (Edn.), 1997, 7, 234.
- G.R. Chatwal, S.K. Anand, Instrumental Methods of Chemical Analysis, Himalaya Publishing House, 1998, 2, 624.
- Food and Drug Administration, Guidance for Industry: Analytical Procedures and Methods Validation (Draft guidance), 2000, 501.
- ICH, Q2 (R1), Validation of Analytical Procedures, Text and Methodology, 2005, 472.
- B. Thangabalan, P.V. Kumar, Asian J. Pharmaceut. Clin. Res., 2012, 5(1), 117.
- N.M. Jagani, V.D. Prajapati, J.S. Shah, P.B. Patel, Inventi Rapid: Pharma Analysis and Quality Assurance., 2012, 285.
- H. Syeda, D. Akalank, Raju, S. Appala, S. Syed, J. Chem., 2011, 8(3), 1424.
- S. Ashok Reddy, K.B. Chandra Shekar, C.M. Murali, J. Global Trends in Pharmaceut. Sci., 2012, 3(2), 619.
- M.N.S. Roy, H.M. Santos, V.Y.S. Chavan, D.R. Pradhan, S.S. Joshi, J. Chem., 2008, 5(3), 453.
- R. Kumar, H. Singh, P. Singh, J. Chemical and Pharmaceut. Res., 2011, 3(2), 113.
- V. Saini, V.B. Gupta, Int. J. Pharm. Tech. Res., 2009, 1(4), 1094.
- B.P. Reddy, N.K.K. Reddy, Int. J. Chem. Tech. Res., 1998, (2), 195.
- Y.V. Rami Reddy, M. Rama Chandraiah, Bullet. Environ. Pharmacol. Life Sci., 2012, 1(8), 39.
- Q.B. Cass, A.L.G. Degani, N.M. Cassiano, J.R. Pedrazolli, J. Chromatogr. B., 2002, 766(1), 153.
- N. Zalak, Mevada, N. Kalsariya, R. Chauhan, S. Shah, D. Shah, Int. J. Pharmaceut. Sci. Rev. Res., 2012, 14(2), 102.
- G.H. Patel, S.T. Prajapati, C.N. Patel, Res. J. Pharm. Technol., 2011, 4(9), 1428.
- V. Niraimathi, P.V. Hemalatha, A. Jerad Suresh, Int. J. Pharm. Pharmaceut. Sci., 2012, 4(3), 279.
- J. Varsha, J. Patel, Int. J. Chem. Tech. Res., 2012, 4(4), 1396.