Research Article - Der Pharma Chemica ( 2018) Volume 10, Issue 7
Analytical Method Development and Validation of Amlodipine in Human Plasma by using Lc-Ms/Ms
Akiful Haque M*, Shanthi Priya DK and Bakshi V
Department of Pharmaceutical Analysis, Anurag Group of Institution (formerly), Lalitha College of Pharmacy, Venkatapur, Hyderabad Telangana, India
- *Corresponding Author:
- Akiful Haque M
Department of Pharmaceutical Analysis
Anurag Group of Institution (formerly)
Lalitha College of Pharmacy
Venkatapur, Hyderabad Telangana, India
Abstract
A touchy and precise excessive-overall performance liquid chromatography combined with triple quadrupole mass spectrometry (lc-ms/ms) method, working in the positive ionization mode, for quantifying of Amlodipine in human plasma using Amlodipine maleate d4 as inner widespread (IS) was advanced and confirmed. The analyte and internal standard were extracted by using solid-section extraction with a aggregate of 5 mm ammonium acetate in 0.1% formic acid: methanol: acetonitrile (40: 30: 30%). The Chromatographic separation was performed on zorbax sb, c18, 50*4.6 mm, 3.5 mm. The analytical approach is legitimate for the estimation of amlodipine, in human plasma over a number 0.100 ng/ml to 9.990 ng/ml with the detection of amlodipine m/z-409.10 (determine) and 238.00 (product) and internal standard amlodipine maleate d4 m/z-413.20 (determine) and 238.00 (product) in positive ion mode. The results of carryover test, matrix impact, linearity, precision and accuracy, stabilities, dilution integrity, run size assessment check provided in this document are inside the popularity range. The robust and rapid lc-ms/ms approach has been efficaciously carried out for ordinary assay to aid bioequivalence or pharmacokinetics studies of amlodipine administered as oral dose to wholesome volunteers.
Keywords
Amlodipine, Amlodipine maleate d4, LC-MS/MS, Human plasma, Validation, Stability studies.
Introduction
Amlodipine is a three-ethly-5-methly-2-[(2-amino ethoxy) methyl]-four-(2-chlorophenyl)6-methyl,1,4-dihydro pyridine-3,five- dicarboxylate (Figure 1). It is a calcium channel blocker that dilates (widens) blood vessels and improves blood waft. It's so far used to treat chest pain (angina) and different situations due to coronary artery illnesses. It is also used to treat excessive blood pressure. It is also used alone or together with other drug treatments to treat chest pain and high blood pressure. It affects the movement of calcium into the cells of the heart and blood vessels. As Amlodipine is a calcium antagonist it reduces coronary and peripheral vascular resistance, decreases blood pressure and myocardial oxygen intake [1]. Amlodipine is norm frequent (with everyday heart rate) and has 24 h long impact. Chemical formulation of amlodipine is C20H25ClN2O5, molecular weight is 567.1 and it is barely soluble in Water and sparingly soluble in ethanol.
Amlodipine is a dihydropyridine calcium antagonist (Calcium ion antagonist or slow channel blocker) that inhits the transmembrane inflow of calcium ions into vascular smooth muscle and cardiac muscle. After oral management of therapeutic doses of Amlodipine, absorption produces height plasma concentrations between 6 and 12 h. Amlodipine is considerably (approximately 90%) transformed to inactive metabolites through hepatic Metabolism with 10% of the determine compound and 60% of the metabolites excreted within the urine. Amlodipine is slowly and nearly absolutely absorbed from the gastrointestinal tract. Peak plasma attention is reached 6-12 h following oral management [2]. Its predicted bio-availability is 64%-90%. Absorption is not stricken by meals.
Inner standard decided on for the estimation of Amlodipine is Amlodipine Maleate d4 (Figure 2).
Materials and Methods
Amlodipine and Amlodipine maleate d4 have been procured from vivan life technology pvt ltd. acetonitrile (Hplc grade), ammonium acetate and formic acids were obtained from merck and methanol (Hplc grade) is from JT baker [3]. The water was purified using milli-q device (Rankem).
Calibration curve requirements and quality control samples:
Calibration curve trendy together with a set of 9 non-zero concentrations ranging from zero. 100 ng/ml to nine. 990 ng/ml ofAmlodipine were organized. Organized high-quality manage samples consisted of concentrations of 0.101 ng/ml (lloq quality control), 0.287 ng/ml (lqc), 1.198 ng/ml (mqc1), 4.991 ng/ml (mqc2), and 7.799 ng/ml (hqc) for Amlodipine. Those samples have been saved at –70°c until use. Optimized Bioanalytical Conditions is explained in Table 1.
| Chromatographic conditions | |
|---|---|
| Column | Zorbax SB, C18, 50*4.6 mm, 3.5 µm |
| Mobile Phase | 5 mm Ammonium acetate in 0.1% Formicacid: methanol : Acetonitrile (30: 30: 40) |
| Rinsing Solution | Acetonitrile: Milli Q water |
| (60: 40) | |
| Flow Rate | 0.7 ml/min |
| Sampler Cooler Temperature | 10°C |
| Injection Volume | 20 µl |
| Split ratio | 50: 50: 00 |
| Needle Rinsing Volume | 1000 µl |
| Retention Times | 1.35 ± 0.5 (Amlodipine) |
| 1.35 ± 0.5 (Amlodipine Maleate d4) | |
| Run Time | 2.50 min |
Table 1: Optimized chromatographic conditions
Sample preparation
The samples were thawed at room temperature and vortexed to make certain entire blending of the contents. 300 μl of the plasma sample was pipetted 5 ml polypropylene ria vial tubes, 30 μl of internal standard dilution (49.985 ng/ml of Amlodipine Maleate d4) became delivered to it and vortexed, besides in blank plasma samples where 30 μl diluent was introduced and vortexed. Then, 500 μl of Hplc grade water turned into introduced and vortex. The sample aggregate was loaded onto strata x 33 μm polymeric sorbent (30 mg/1 cc) cartridges that have been pre-conditioned with 1.0 ml of hplc grade methanol followed through 1.0 ml milli q/hplc grade water (New cartridge for each pattern). After making use of the most pressure the extraction cartridge become washed with 1 ml of 50 mm ammonium acetate and 2 ml of milli q/hplc grade water (1 ml every time) [4]. then the samples were eluted with 2 ml of methanol and evaporated to dryness at 45ºC beneath gentle movement of nitrogen and the dried extract changed into reconstituted with 300 μl of the Cellular segment. The samples had been transferred to sampler loading vials (Ambered coloration) and loaded.
Validation
Method validation consists of all of the techniques required to demonstrate that a method to quantify the attention of amlodipine in plasma is dependable for the intended software.
Carry over check
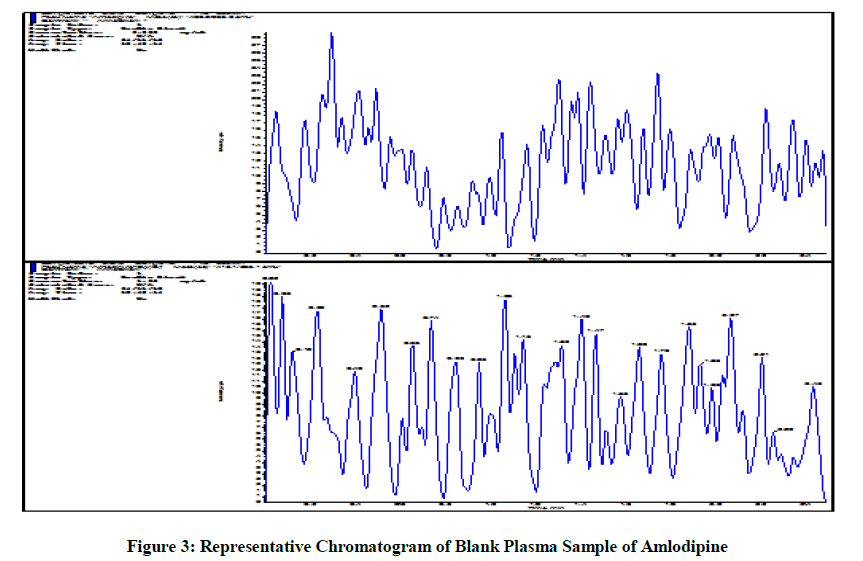
Certainly organized extracted matric clean (cot clean), excessive (cot uloq) and low (lloq) attention of analyte whilst injected, the effects Display that there's no deliver over impact of analyte and internal preferred. Figure 3 is the representative chromatogram of extracted blank plasma sample analyzed for carry over test [5].
Accuracy and precision (Tables 2-4)
Inside-batch, intraday, and between-run/inter day precision and accuracy have been evaluated at three nice control samples concentrations (lqc, mqc1, mqc2, hqc). Inside-batch, intraday and between-run/inter day assay precision were determined as coefficient of variation (cv%),and within-batch, intraday and between-run/ inter day assay accuracies have been expressed as percent nominal.
Linearity
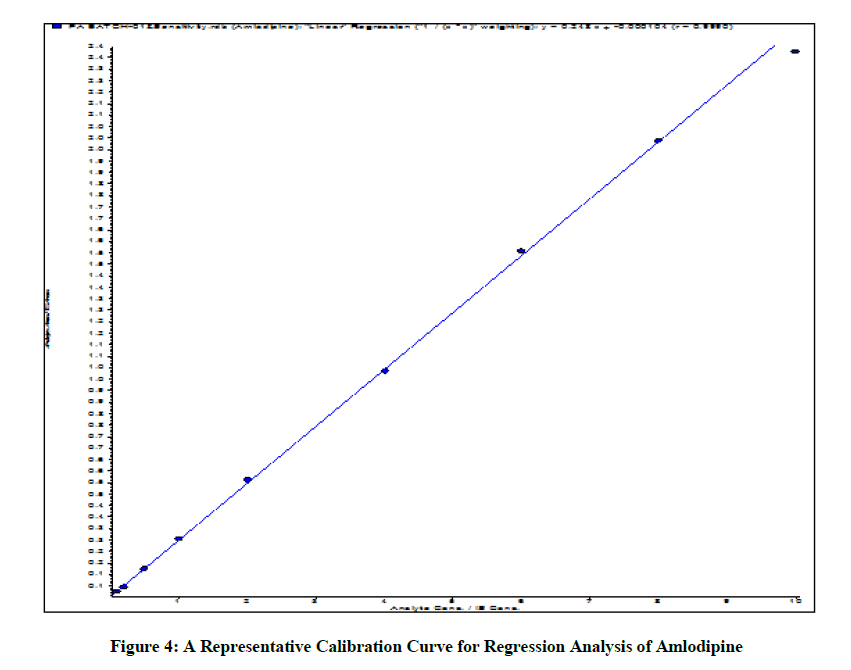
Use a regression equation with the perfect weighting thing for determining the detector reaction/ concentration courting. Encompass trendy and widespread in training of calibration curve. Figure 4 suggests representative calibration curve for regression evaluation of Amlodipine
Sensitivity (Table 5)
Decide the sensitivity in phrases of lloq in which the reaction of the lloq need to be atleast five times greater than the reaction of interference in clean matrix on the retention time or mass transitions of the analyte (s)
Dilution integrity (Table 6)
Put together twelve units of qcs spiked with approximately 1.5-1. Eight instances the concentration of the highest preferred (uloq) method six units of above qcs with the aid of diluting them two times and another six sets by using diluting four instances prior to extraction by addition of screened blank matrix. inject these quality control samples along with the calibration curve requirements processed without dilution and calculate the quality control concentrations using multiplication issue as 2 (for two times diluted samples) and four (for 4 times diluted samples).
Stabilities
Freeze and thaw stability (Table 7): The samples have been saved at -20°c and thawed at room temperature (≤ 27°C). The stability of quality controls concentrations was envisioned after three cycles of freezing and thawing of samples. The samples have been analyzed underneath a fresh calibration curve and the concentrations obtained have been as compared with the nominal attention of exceptional managing samples [6,7].
Re-injection stability (Table 8): In having access to the reinjection balance, six sets of quality control samples (lqc and hqc) had been processed and analyzed with calibration curve general. The qc samples have been retained within the vehicle sampler and re-injected after a period of 46 h 30 min and quantified in opposition to the preliminary calibration curve statistics. The mean concentration of re-injected qcs become in comparison against the nimply of the qcs whilst injected for first time [8].
Wet extract stability (Table 9): Moist extract balance is finished to evaluate the integrity of analyze samples which had been kept at room temperature Over a time period after processing. Good enough wide variety of low and excessive quality control samples (6 replicates) to facilitate injection at proposed balance durations. Maintain the processed samples in vehicle sampler at room temperature/ special temperature. At the day of balance, put together sparkling trendy inventory solution of analyte(s). Inject quality controls samples in replicates (6 samples each) in conjunction with the freshly spiked calibration standards of awareness variety equal to that used for the calculation of Precision and accuracy. Calculate the auto sampler stability intervals at the time of injection of first quality control; much less the sample training completed time [9].
Auto sampler stability (Table 10): Auto sampler stability of the analyte and inner widespread is done at a selected temperature for the time frame relying at the anticipated run time for the whole evaluation of a batch length. Number of low and excessive quality controls samples (6 replicates) is facilitated to inject at proposed Stability durations, hold the processed samples at room temperature. On the day of stability, inject qc samples in replicates (6 samples every) along with the freshly spiked calibration requirements of concentration range equal to that used for the calculation of precision and accuracy [10].
Results and Discussion
Carry over test
Precision and accuracy
| Nominal concentration ng/ml | Within batch | ||
|---|---|---|---|
| Mean ± SD | Accuracy (%) | CV (%) | |
| LQC (0.28 ng/ml) | 0.26 ± 0.01 | 91.35 | 7.61 |
| MQC1 (1.1 ng/ml) | 1.11 ± 0.007 | 93.36 | 0.69 |
| MQC2 (4.9 ng/ml) | 4.97 ± 0.05 | 99.65 | 1.01 |
| HQC (7.7 ng/ml) | 7.37 ± 0.11 | 94.62 | 1.55 |
Table 2: With-in, batch precision and accuracy for analysis of amlodipine in human plasma
| Nominal concentration ng/ml | Intra batch | ||
|---|---|---|---|
| Mean ± SD | Accuracy (%) | CV (%) | |
| LQC (0.28 ng/ml) | 0.28 ± 0.02 | 100.03 | 10.35 |
| MQC1 (1.1 ng/ml) | 1.11 ± 0.01 | 93.12 | 1 |
| MQC2 (4.9 ng/ml) | 4.96 ± 0.04 | 99.39 | 0.86 |
| HQC (7.7 ng/ml) | 7.36 ± 0.08 | 94.44 | 1.21 |
Table 3: Intraday precision and accuracy for analysis of amlodipine in human plasma
| Nominal concentration ng/ml | Inter batch | ||
|---|---|---|---|
| Mean ± SD | Accuracy (%) | CV (%) | |
| LQC (0.28 ng/ml) | 0.29 ± 0.02 | 103.79 | 6.95 |
| MQC1 (1.1 ng/ml) | 1.11 ± 0.01 | 93.03 | 1.16 |
| MQC2 (4.9 ng/ml) | 4.95 ± 0.04 | 99.19 | 0.86 |
| HQC (7.7 ng/ml) | 7.35 ± 0.08 | 94.26 | 1.12 |
Table 4: Interday precision and accuracy for analysis of amlodipine in human plasma
Linearity
Sensitivity
| QC level | Mean ± SD | % CV | % Accuracy |
|---|---|---|---|
| LLOQ | 0.099 ± 0.004 | 4.13 | 99.83 |
Table 5: Within Batch Precision and Accuracy for Sensitivity of Amlodipine
Dilution integrity
| Dilution factor | Mean ± SD | % CV | % Accuracy |
|---|---|---|---|
| ½ | 17.0 ± 0.06 | 0.04 | 105.67 |
| ¼ | 34.1 ± 0.19 | 0.57 | 105.81 |
Table 6: Dilution Integrity Data for Amlodipine (Two Times and Four Times)
Stabilities
| ANALYTE (Amlodipine) | LQC | HQC | ||
|---|---|---|---|---|
| % Nominal | C.V (%) | % Nominal | C.V (%) | |
| Freeze-thaw stability | 107.74% | 1.48% | 96.14% | 0.86% |
Table 7: stability data for free-thaw stability
| ANALYTE (Amlodipine) | LQC | HQC | ||
|---|---|---|---|---|
| % Nominal | C.V (%) | % Nominal | C.V (%) | |
| Re-injection stability(46 h-30 min) | 107.61% | 2.29% | 94.46% | 0.46% |
Table 8: stability data for re-injection stability
| ANALYTE (Amlodipine) | LQC | HQC | ||
|---|---|---|---|---|
| % Nominal | C.V (%) | % Nominal | C.V (%) | |
| Wet extract stability(69 h-50 min) | 105.92% | 2.12% | 98.87% | 1.02% |
Table 9: stability data for wet extract stability
| ANALYTE (Amlodipine) | LQC | HQC | ||
|---|---|---|---|---|
| % Nominal | C.V (%) | % Nominal | C.V (%) | |
| Auto sampler stability (74 h-25 min) | 105.05% | 1.25% | 94.44% | 2.70% |
Table 10: stability data for auto sampler stability
Conclusion
In summary, an enormously, unique, reproducible and high-throughput LC-MS/MS approach become advanced and tested based on the procedure of SPE for quantification of Amlodipine pharmacokinetic and bioequivalence research. The extraction manner and LC-MS/MS conditions have been optimized to be able to enhance the sensitivity and robustness of the technique. In the end, the technique turned into completely tested to fulfill the necessities for sensitivity, accuracy and precision defined via kingdom food and drug administration and glp guidelines.
From the optical characteristics, of the proposed method, it changed into determined that the results of sensitivity, carry over test, linearity, precision accuracy and stabilities provided in this article are in the recognition range,and the analytical approach which is defined above is legitimate for the estimation of amlodipine, in human plasma over various 0.100 ηg/ml to 9.990 ηg/ml with the detection of amlodipine m/z-409.10 (parent) and 238.00 (product) And Internal Amlodipine Maleate d4 m/z-413.20 (parent) and 238.00 (product) in positive ion mode. A summary of the validation parameters and the effects is provided above.
Acknowledgement
Authors had been grateful to the Anacipher Clinical Research Organisation and Anurag Group of Institutions for presenting all the vital centers to carry out this studies work.
References
- www.wikipedia.org/wiki/Amlodipine
- K. Gohil, Indian J. Pharm. Sci., 2005, 67(3), 376.
- J. Bhatt, S. Singh, G. Subbaiah, Biomed. Chromatogr., 2007, 21, 169-175.
- J. Macek, J. Klima, P. Ptacek, J. Chromatogr. B Analyt. Technol.Biomed. Life Sci., 2006, 832(1), 169-172.
- N. Rahman, S.N.H. Azmi, Farmaco., 2001, 56, 731-735.
- A. Zarghi, S.M. Foroutan, A. Shafaati, A. Khoddam, Farmaco., 2005, 60, 789-792.
- J.R. Rao, Indian drugs., 2002, 39(7), 378-381.
- G.L. Lopez, J.R. Alonso, R. Jimenez, J. Chromatogr. A, 2002, 9499, 49-60.
- T. Philipp, T.R. Smith, R. Glazer, M. Wernsing, J. Yen, J. Jin, H. Schneider, R.Pospiech, Clin. Ther., 2007, 29(4), 563-580.