Research Article - Der Pharma Chemica ( 2017) Volume 9, Issue 3
Anti-Protozoal Effect of Some Medicinal Oils on Potentially Pathogenic Naegleria Species
Mahmoud Afw Gad1*, Ahmad Zakaria Al-Herrawy1 and Sabry Abou-Elfath Mahfouz22Research of Medicinal and Aromatic Plants Department, National Research Centre, Dokki 12622 Giza, Egypt
Mahmoud Afw Gad, Water Pollution Research Department, National Research Centre, Dokki 12622, Giza, Egypt, Email: MahmoudAfwGad@gmal.com
Abstract
About 35,000 to 70,000 plants species has been used as medicaments. Nearly 70 percent of the world’s population relies on plants for their primary health care. Different concentrations from some medicinal plant oils (Eucalyptus citratus, Rosmarinus officinalis and Allium sativum) at different contact times were separately applied to cysts and trophozoites of Naegleria sp. (accession Number: KU312284) isolated from Egyptian swimming pool. Naegleria sp. viability was tested by culturing on non-nutrient agar and incubation at 45ºC and by staining with trypan blue vital stain. The results of the present study showed that the minimum amoebicidal concentrations of essential oils from Eucalyptus citratus, Rosmarinus officinalis and Allium sativum that produced complete death of Naegleria sp. trophozoites reached 15% with contact time 90 min, 25% with contact time 60 min, 15% with contact time 60 min, respectively. The minimum amoebicidal concentrations of oils from Eucalyptus citratus, Rosmarinus officinalis and Allium sativum that produced complete death of Naegleria sp. cysts reached 50% with contact time 6 days, 100% with contact time 3 days, 75% with contact time 3 days, respectively. In conclusion, Eucalyptus citratus showed the best concentration for inactivation of Naegleria sp. cysts and trophozoites.
Keywords
Naegleria sp., Amoebicidal effect, Medicinal plant oils
Introduction
Genus Naegleria taxonomically belongs to the family Vahlkampfiidae which contains 13 genera [1]. Forty seven species of Naegleria have been recognized based upon sequencing data. Naegleria fowleri is the only one that has been isolated from cases of amoebic meningoencephalitis. In addition, other species Naegleria australiensis and Naegleria italica may be pathogenic in the mouse model of primary amoebic meningoencephalitis (PAM), but have not been identified from any human cases [2,3].
Naegleria species were isolated from organs of freshwater fishes [4,5], dental treatment units [6,7], bottled mineral water [8], the atmosphere [9,10] soil [11]. In Egypt, Naegleria species were isolated from Nile River, tap and ground water [12]. Essential oils have gained a special consideration in the late years as a result of expanding the phenomena of acquiring antibiotic resistance by various bacterial species [13]. The utilization of plant essential oils in both the nourishment and the pharmaceutical industries has been created interestingly; a plant extracts systematic examination for these properties has become progressively important. The utilization of natural antimicrobial compounds is important not only in the control of microbial growth in the diseases condition but also in the preservation of food [14,15]. Some researchers revealed the antimicrobial activity of essential oils from rosemary, thyme, oregano, sage, clove, garlic, coriander, and onion against both molds and bacteria. The structure, composition as well as functional groups of the essential oils play an important role in detecting their antimicrobial activity [16]. Rosemary thyme essential oils were shown to increase bone density in vitro and inhibit osteoclast activity [17]. The essential oil extracted from the dried flower buds of cloves is useful for parasites, warts, scars and acne. The present study was therefore conducted to evaluate the antiprotozoal effect of essential oils extracted from Eucalyptus citratus, Rosmarinus officinalis and Allium sativum with different dilution concentrations against potentially pathogenic Naegleria sp.
Materials and Methods
Extraction of the essential oils was performed with a hydrodistillation method. The hydrodistillation lasted 3 hours. The essential oils were dehydrated with anhydrous sodium sulphate weighed and stored in a refrigerator at 4ºC. The chemical analysis of essential oils was done with a GC (gas chromatograph) MS.
The molecularly identified Naegleria sp (accession Number: KU312284) was subcultured on non-nutrient agar (NNA) plate seeded with heat-killed E. coli and incubated at 45oC. The plate was used for collection of Naegleria cysts after one week incubation. After incubation period, the agar surfaces of the plate were flooded with 5 ml of Page’s amoebic saline and amoebae cysts were gently scraped with an inoculating loop. Naegleria cysts were harvested from the suspension by centrifugation at 350 g for 10 min. The supernatant was aspirated, and the sediments were washed twice with Page’s amoebic saline in order to eliminate most of the remaining bacteria. The subjected Naegleria cysts were adjusted at concentration 104 using Sedgewick Rafter counting cell slide. The essential oils were separately dissolved in 3% Tween 80. Control samples (trophozoites and/or cysts) were comparatively used without any treatment to assess the effect of some medicinal oils (Eucalyptus citratus, Rosmarinus officinalis and Allium sativum). Dissolved oils of Eucalyptus citratus, Rosmarinus officinalis and Allium sativum were separately applied to Naegleria trophozoites in concentrations 5, 10, 15, 20 and 25% with contact time 15, 30, 45, 60, 75 and 90 min. Again the previously mentioned dissolved essential oils were separately applied to Naegleria cysts in concentrations 15, 25, 50, 75 and 100% with contact time 1, 2, 3, 4, 5, 6 and 7 days. The application of different essential oils on Naegleria sp. cysts/trophozoites were performed in tissue culture plates. All the inoculated tissue culture plates were sealed with parafilm to prevent evaporation during the incubation. Incubated plates containing trophozoites were examined every 15 min. till 90 min, while those containing cysts were examined daily for 7 days. Amoebicidal effects of the tested plant extracts were evaluated by checking the viability of trophozoites/cysts through cultivation on fresh NNA plates and use of trypan blue stain [18-20].
Results
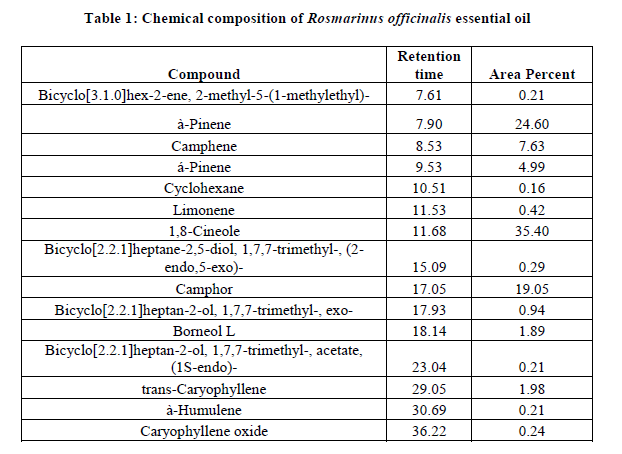
Chemical composition of Rosmarinus officinalis essential oil
The major components of Rosmarinus officinalis essential oils are 35.4% 1,8-cineole, 21.6% α-pinene and 19.05% Camphor. Other compounds as à-pinene, Camphene, trans-Caryophyllene and BORNEOLL represent 7.63, 4.99, 1.98 and 1.89% from the essential oil, respectively (Table 1).
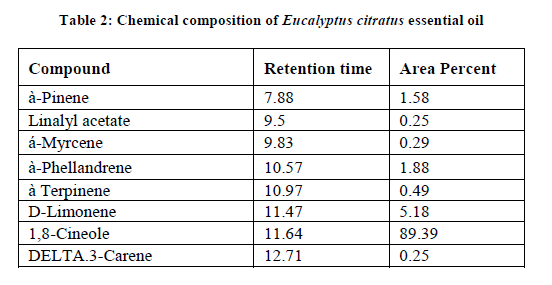
Chemical composition of Eucalyptus citratus essential oil
The major chemical composition percentages of Eucalyptus citratus essential oil are 89.39% followed by 5.18% D-Limonene. The minor compounds are à-pinene (1.88%), à-Phellandrene (1.58%), à terpinene (0.49%), á-Myrcene (0.29%), linalyl acetate (0.25%) and delta.3-Carene (0.25%) (Table 2).
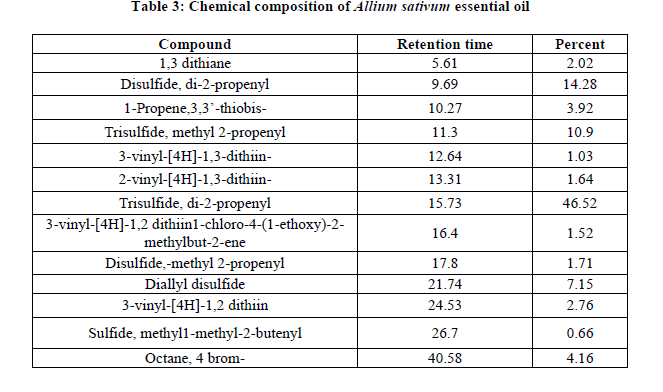
Chemical composition of Allium sativum (garlic) essential oils
The major principal components of garlic represented mainly by Trisulfide, di-2-propenyl (46.52%) and Disulfide, di-2-propenyl (14.28%). Moreover, the minor chemical compounds are 1-Propene,3,3’-thiobis-(3.92%), Disulfide,-methyl 2-propenyl (2.76%), 1,3 dithiane (2.02%), 2-vinyl-[4H]-1,3-dithiin-(1.64%), 3-vinyl-[4H]-1,2dithiin1-chloro-4-(1-ethoxy)-2-methylbut-2-ene (1.52), 3-vinyl-[4H]-1,3-dithiin-(1.03%), Sulfide, methyl1-methyl-2-butenyl (0.66%) (Table 3).
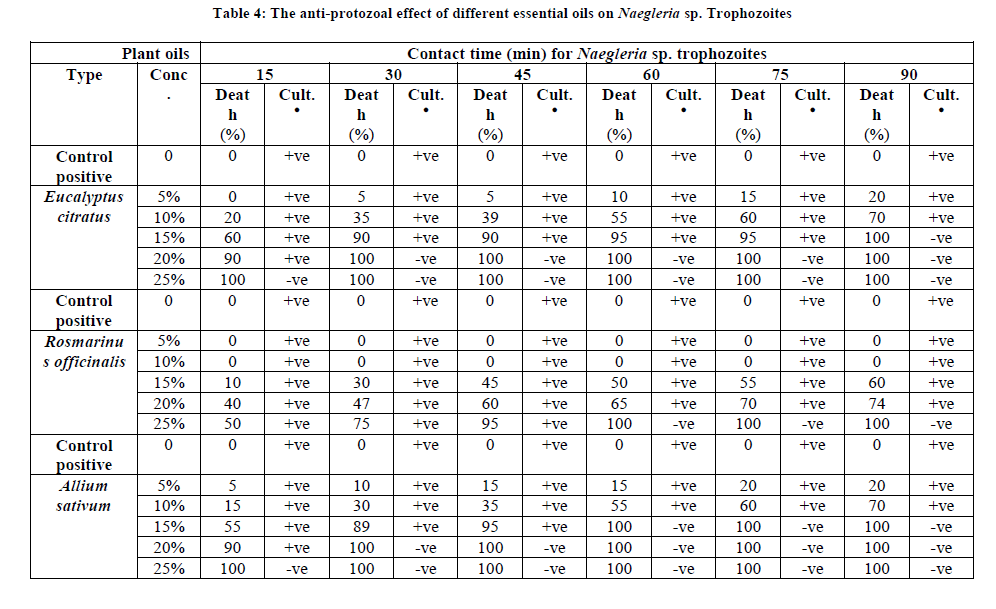
The anti-protozoal effect of different medicinal oils on Naegleria sp. Trophozoites
It was found that oil of Rosmarinus officinalis in a concentration 5% and 10% had no amoebicidal effect on Naegleria sp. trophozoites after exposure time from 15 to 90 min. While, Eucalyptus citratus in a concentration 5% had no amoebicidal effect on Naegleria sp. trophozoites after exposure time 15 min.
On the other hand, oils of Eucalyptus citratus and Allium sativum began to be effective against 5% of Naegleria sp. trophozoites at the same concentration 5% and contact time 30 and 15 min, respectively. While, Rosmarinus officinalis began to be effective against 10% of Naegleria sp. trophozoites at a concentration 15% and contact time 15 min. Consequently, the inhibitory effect of plant oils increased with the increase in concentration and contact time of them against Naegleria sp. trophozoites. The minimum amoebicidal concentrations of essential oils from Eucalyptus citratus, Rosmarinus officinalis and Allium sativum that produced complete death of Naegleria sp. trophozoites reached 15% with contact time 90 min, 25% with contact time 60 min, 15% with contact time 60 min, respectively (Table 4 and Figure 1).
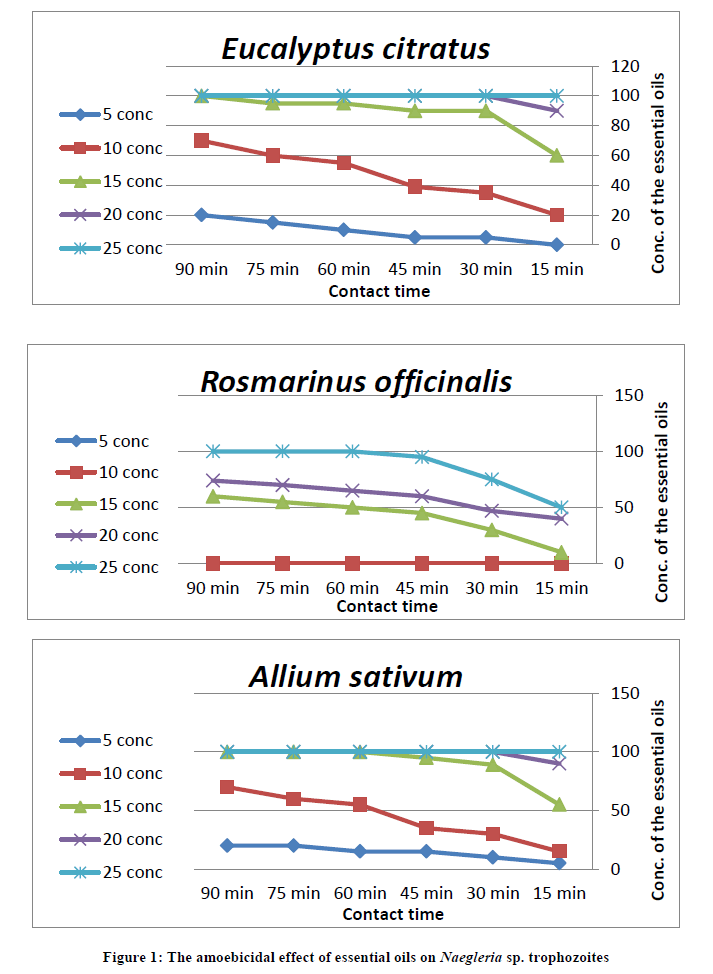
The anti-protozoal effect of different essential oils on Naegleria sp. cysts
The use of Rosmarinus officinalis in concentrations 15% had no amoebicidal effect on Naegleria sp. cysts after 1 to 7 days exposure time. On the other hand, oils of Eucalyptus citratus and Allium sativum began to be effective against 5% of Naegleria sp. cysts at the same concentration 15% and contact time 6 and 1 days, respectively. While, Rosmarinus officinalis began to be effective against 5% of Naegleria sp. cysts at a concentration 25% and contact time 6 days. Consequently, the inhibitory effect of plant oils increased with the increase in concentration and contact time against Naegleria sp. cysts. The minimum amoebicidal concentrations of plant oils from Eucalyptus citratus, Rosmarinus officinalis and Allium sativum that produced complete death of Naegleria sp. cysts reached 50% with contact time 6 days, 100% with contact time 3 days, 75% with contact time 3 days, respectively (Table 5 and Figure 2).
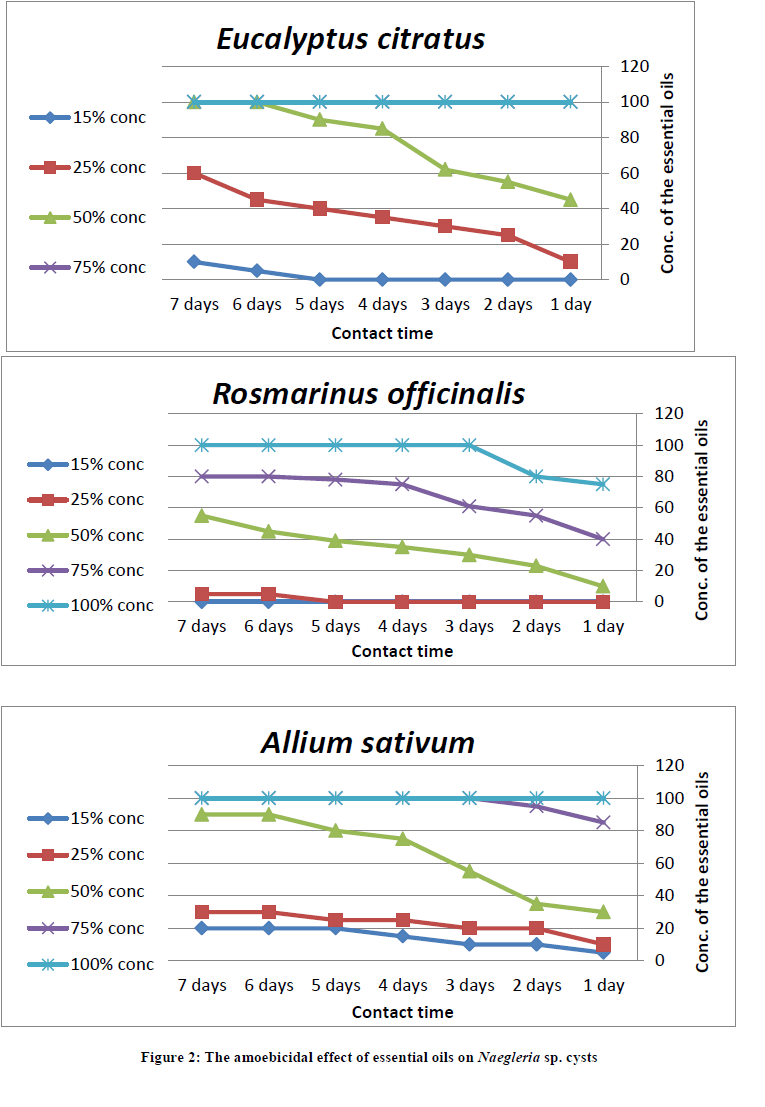
Discussion
Till now there is no study investigated the interactions of the antiprotozoal effect of essential oils Eucalyptus citratus, Rosmarinus officinalis and Allium sativum against potentially pathogenic Naegleria sp. The minimum amoebicidal concentrations of plant oils from Eucalyptus citratus, Rosmarinus officinalis and Allium sativum that produced complete death of Naegleria sp. trophozoites reached 15% with contact time 90 min, 25% with contact time 60 min, 15% with contact time 60 min, respectively. To our knowledge, no data concerning the amoebicidal effect of the tested plant oils on Naegleria was available. Other researchers reported that essential oils have been shown to exhibit antibacterial [21], antiviral [22], antimitotic [23] and antiparasitic activities [24]. Croton cajucara and Cochlospermum planchonii oils specifically inhibit Leishmania amazonensis and Plasmodium falciparum, respectively. Garlic oil has broad-spectrum activity against Plasmodium, Trypanosoma, Leishmania and Giardia [24].
Essential oils and/or active components can be used as adjuncts or alternatives to current antiparasitic durgs [24]. Also, diseases caused by protozoa have been successfully treated by essential oils [25]. Eucalyptus robusta and Rosmarinus officinalis were reported to have cytotoxic effects on Staphylococcus aureus. In addition, Eucalyptus globulus was used as effective drugs for healing the cutaneous lesions of leishmaniasis [26]. In other study, the Eucalyptus oils needing higher doses to obtain a better effect on the most resistant egg phase of L. longipalpis. One theory to clarify the greater resistance of the eggs is that the neurotoxic activity of this compound acts only after nervous system of the embryo begins to grow. Another conceivable clarification is the lesser porousness of the egg surface toward the start of embryogenesis [27]. There is no standard treatment, effective treatments for PAM can be administered if the patient is known to have engaged in a recreational activity and exhibits the symptoms of a Naegleria fowleri infection [2]. The emergence of parasites resistant to current chemotherapies highlights the importance of plant essential oils as novel antiparasitic agents [24]. The minimum amoebicidal concentrations of plant oils from Eucalyptus citratus, Rosmarinus officinalis and Allium sativum that produced complete death of Naegleria sp. cysts reached 50% with contact time 6 days, 100% with contact time 3 days, 75% with contact time 3 days, respectively. Other Egyptian researcher, Gaafar [28] concluded that garlic (Allium sativum) has a good efficacy as a prophylactic and a promising therapeutic agent against Cryptosporidium and therefore the traditional use of this plant in parasitic infections was validated. Also, Allium sativum had a cytotoxic effect on the fungus Trichophyton rubrum [25,29]. Garlic has parasiticidal activity against Giardia duodenalis and Cryptosporidium spp. [30,31]. Pourjafar et al. [32] reported that Mentha piperita have antimicrobial and pharmacological effects in monogastric animals in vivo and in vitro. Also, Mentha piperita showed higher toxicity against the house fly (Musca domestica) larvae [29]. Other researchers showed that the extract of Camellia sinensis had a strong inhibitory activity against Trypanosoma cruzi arginine kinase [33,34].
Conflict of Interest
Conflict of interest declared none.
References
[1] N.A. Khan, FEMS Microbial. Rev., 2006, 30(4), 564-595.
[2] G.S. Visvesvara, H. Moura, F.L. Schuster, FEMS Immunol. Med. Microbiol., 2007, 50(1), 1-26.
[3] J.F. De Jonckheere, Infec. Genet. Evol., 2005, 11(7), 1520-1528.
[4] I. Dykova, I. Kyselova, H. Peckova, M. Obornik, J. Lukes, Dis. Aqua. Org., 2001, 46(2), 115-21.
[5] H. Shin, K. Im, Korean. J. Parasitol., 2004, 42(3), 93-119.
[6] R. Michel, Hygiene., 1984, 179(1), 56-72.
[7] A. Leduc, S. Gravel, J.R.M. Abikhzer, Sp. Roy, J. Barbeau. 1983, Can. J. Microbial., 58(7), 884-886.
[8] F. Rivera, M. Galván, E. Robles, P. Leal, L. González, A.M. Lacy, J. Protozool., 1981, 28(1), 54-56.
[9] R.V. Lawande, Ann. Trop. Med. Parasitol., 1983, 77(1), 45-49.
[10] F. Rivera, G. Roy-Ocotla, I. Rosas, E, Ramirez, P. Bonilla, F. Lares, Environ. Res., 1987, 42(1), 149-154.
[11] L. Conza, S.C. Pagani, V. Gaia, PloS ONE., 2013, 8(7), e68244.
[12] M.A. Gad, A.Z. Al-Herrawy, Res. J. Pharm. Biol. Chem. Sci., 2016, 7(1), 1872-1832.
[13] E.S. Al-Sheddi, King Saud University, 2009.
[14] S. Prabuseenivasan, M. Jayakumar, S. Ignacimuthu, BMC. Compl. Alter. Med., 2006, 6(1), 1.
[15] N.M.A. Chaudhry, P. Tariq, Pak. J. Bot., 2006, 38(1), 169.
[16] M. Omidbeygi, M. Barzegar, Z. Hamidi, H. Naghdibadi, Food Control., 2007, 18(12), 1518-1523.
[17] S.E. Putnam, A.M. Scutt, K. Bicknell, C.M. Priestley, E.M. Williamson, Phytother. Res., 2007, 21(2), 99-112.
[18] APHA, 21th Edn., 2005, APHA, WEF and AWWA, Washington, DC.
[19] C. Coulon, A. Collignon, G. McDonnell, V. Thomas, J. Clin. Microbiol., 2010, 48(8), 2689-2697.
[20] M.A. Gad, G.A. Osman, Res. J. Pharm. Biol. Chem. Sci., 2016, 7(1), 961-970.
[21] S. Burt, Int. J. Food. Microbiol., 2004, 94(3), 223-253.
[22] A.E. Edris, Phytother. Res., 2007, 21(4), 308-323.
[23] F. Pisseri, A. Bertoli, L. Pistelli, Parassitologia., 2008, 50(1-2), 89-91.
[24] J.P. Anthony, L. Fyfe, H. Smith, Trends. Parasitol., 2005, 21(10), 462-468.
[25] G.S. Pérez, M.A. Lamos-lópez, E. Sánchez-miranda, M.C. Fresán-orozco, J. Pérez-ramos, J. Med. Plant. Res., 2012, 6(15), 2901-2908.
[26] L. Babaee, M. Khou Mohebali, N. Lahiji Mehrabi, A. Tavana, Hakim. Res. J., 2007, 10(2), 21-27.
[27] D.C. Stamopoulos, P. Damos, G. Karagianidou. J. Stored. Prod. Res., 2007, 43(4), 571-577.
[28] M.R. Gaafar, Alexandria J. Med., 2006, 48(1), 59-66.
[29] I. Kosalec, S. Pepeljnjak, D. Kustrak. Acta Pharmaceutica-Zagreb., 2005, 55(4), 377.
[30] T. Sréter, Z. Széll, I. Varga, J. Parasitol., 1999, 989-991.
[31] J.C. Harris, S. Plummer, M.P. Turner, D. Lloyd, Microbiology., 2000, 146(12), 3119-3127.
[32] M. Pourjafar, G. Kojouri, A. Mohammadnia, M. Hayatifard, Planta Medica., 2008, 74(09), PA76.
[33] A. Bosly, J. Entomol. Nematol., 2004, 5(4), 50-54.
[34] G.D. Alonso, C.A. Pereira, M.S. Remedi, M.C. Paveto, L. Cochella, M.S. Ivaldi, FEBS lett., 2001, 498(1), 22-25.



