Research Article - Der Pharma Chemica ( 2023) Volume 15, Issue 3
Arsenic Removal in Aqueous System to Synthesized, Characterized Thiol and Sulfonium Integrated Starch Biopolymer: An Innovative Approach
Vishal Priya*Vishal Priya, Department of Chemistry and Biochemistry, CSK Himachal Pradesh Krishi Vishvavidyalaya, Himachal Pradesh, India, Email: vishalpriyak@gmail.com
Received: 17-Oct-2019, Manuscript No. DPC-23-103883; Editor assigned: 22-Oct-2019, Pre QC No. DPC-23-103883 (PQ); Reviewed: 05-Nov-2019, QC No. DPC-23-103883; Revised: 30-Jun-2023, Manuscript No. DPC-23-103883 (R); Published: 28-Jul-2023, DOI: 10.4172/0975-413X.15.5.1-13
Abstract
The contamination of arsenic groundwater is the most serious global concern and responsibility for the death rate per year. Complete removal of arsenic through the conventional method is very difficult and cost consuming. We have synthesized the starch thiomer, sulfonium starch and iron immobilized starch derivatives for the removal of As (III) and As (V) from the aqueous system. The batch experiments were performed as a function of initial As (III) and As (V) concentrations, contact time and pH values. The results supported a little preferential sorption for As (V) (95%) in comparison to As (III) (92%) via thiomer, while sulfonium structure showed similar affinity for As (III) and As (V) sorption (92.7% for As (III) and 93% for As (V)) at pH 5.0. The present study also supported that synthesized thiomer and sulfonium structure are selective to arsenic and only marginal effect has been observed with competitive anions except for phosphate. The SEM-EDX studies confirmed the arsenic sorption mechanism of synthesized starch derivatives. Isotherm studies indicated that the Langmuir model is preferential, while in kinetics pseudo-second-order kinetic model is superior in describing the adsorption process for As (III) and As (V) adsorption through synthesized starch derivatives. The results of arsenic equilibrium adsorption at 35°C showed the endothermic nature of As (III) adsorption indicated by the positive ΔH° value support the result that As (III) adsorption decreased with temperature. The temperature effect on the intrinsic kinetics of As (V) adsorption for the present adsorbent is likely to surpass the endothermic effect on the adsorption reaction. Therefore, it is recommended that the excellent adsorption for As (III) and As (V) by thiomer and sulfonium structure would be a better solution and can be explored for commercialization in arsenic contaminated water for arsenic removal.
Keywords
Water contamination; Arsenic; Starch; Immobilization; Adsorption; Biopolymer
Introduction
Arsenic is present ubiquitously in the earth’s crust and is a very noxious element even at very low concentrations. Arsenic exists in two predominate speciation As (III) and As (V), but the As (III) is more toxic than As (V). As (III) is a neutral species and is very difficult to remove from the aqueous system. Arsenic rarely exists in the free form of mostly occurs as sulfide or oxide ores and introduced in surface water and groundwater as a result of several natural and anthropogenic sources. Arsenic poisoning through contaminated drinking water causes the number of adverse effects on human health [1]. The global scenario is highlighted in Mexico, arsenic is present within the volcanic belt where arsenic-rich soils contaminate the groundwater. In Chile, arsenic is present in all ecosystems in the north of the country because of the predominance of quaternary volcanism in the zone. Between 1955 and 1970 in Antofagasta, the average arsenic level in the water was 0.598 mg/l. In India, most of the rural populations are exposed to arsenic by arsenic contaminated groundwater which is used for drinking purposes. World Health Organization (WHO) has recommended 10 ppb as the permitted level for arsenic. But the arsenic concentration has been exceeded to 10 ppb in Australia, China, USA, Bangladesh and India [2].
Several methods have been established for removal of arsenic from the aqueous system and among these adsorption is one of the best method for arsenic removal from aqueous system. This method stands due to a safe and easy process with high efficiency and regeneration capacity, least sludge making and low cost. Therefore, a number of adsorbents have been used for arsenic removal. The conventional adsorbents have some drawbacks like incomplete metal removal, high resources and operational cost, disposal of the residual metal sludge and less competence for smallscale industries. To overcome these problems, the development of effective and sustainable sorbent for complete removal of arsenic in both of the speciation from the water is a research priority [3,4].
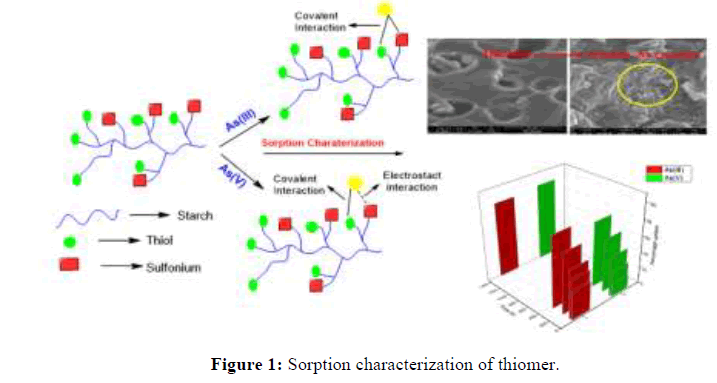
In recent years, the development of biopolymer based adsorbent for arsenic removal is attaining interest in research and -SH based materials are used as the most effective sorbent for heavy metal ions. Additionally, the iron based material shows a high affinity towards arsenic. Therefore, the immobilization of iron would be proved helpful to modulate the surface charge and to improve the extraction capability for arsenic [4]. Isotherms such as Langmuir, Freundlich models and kinetic models like pseudo-first-order, pseudo-second-order have been performed. Besides, the interference of different anions on adsorption of As (V) and As (III) has also been examined. Because of the above facts, the present effort is directed to synthesize green thiomers, sulfides and sulfonium adsorbents to remove arsenic in both of the speciation i.e. As (III) and As (V) from aqueous solutions. Sulfur fictionalization is targeted as the arsenic has a strong affinity for sulfur. To the best of our knowledge, there is no report to explore the possibility of sulfonium structure as an effective sorbent for arsenic removal. However, some reports for arsenic removal via anion exchanger have been reported, but they are only effective for As (V). The extensive experimental work has been carried out to estimate the performance of starch based sulfur derivatives for removal of As (III) and As (V). Therefore, a prospective benefit of this study is to characterize a new low-cost sorbent for the complete removal of As (V) and As (III) from aqueous solution (Figure 1) [5,6].
Materials and Methods
Starch, thiourea, sodium arsenite and sodium arsenate were purchased from Hi-media and used as received. All other chemicals used were of analytical grade. The stock solutions of As (III) and As (V) were prepared by dissolving a known amount in double-distilled water. All glassware was cleaned with aqua-regia and rinsed with double-distilled water before use [7].
Preparation of starch derivatives
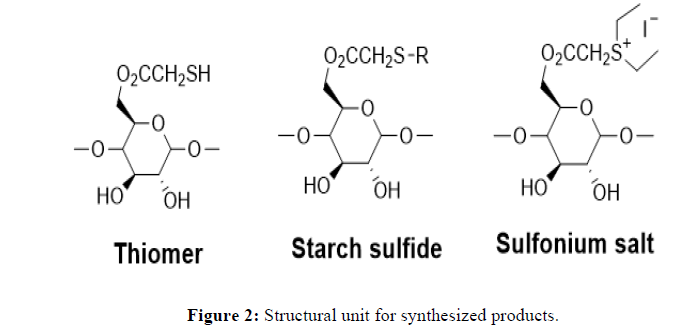
Thiomer, sulfide and sulfonium functionalized starch were processed as per earlier reported procedure. In brief, initially the starch was functionalized with chloroacetylation and then the chloroacetylated starch was further modified to obtain the thiolated and sulfide starch. After that thiolated starch was functionalized by exhaustive alkylation in sulfonium structure. The synthesized products were extensively characterized for the reproducibility of results. Henceforth the synthesized products are referred to abbreviations i.e. thiomerm, sulfoniumm, sulfidem, sulfidec and sulfoniumc. In the nomenclature of products, m stands for microwave and c for the conventional method [8].
Preparation of iron coated starch derivatives
Iron immobilized starch derivatives were prepared by placing 0.1 g of starch derivative in 0.01M FeCl3 solution and the solution was kept overnight. After that, the iron coated starch derivatives were collected and washed with distilled water. The product obtained was air dried for 24 h at 25°C and used for further experiments [9].
Characterization and instruments
FTIR spectrums were recorded on Perkin-Elmer 4100. SEM (Jeol JSM 6100), Energy-dispersive X-ray analysis (EDX) of the synthesized products were recorded by Joel JSM 6100 instrument and UV-spectrophotometer systronics. The pH of solutions was adjusted with 0.1M HCl and 0.1M NaOH solutions and were monitored by using a pH meter [10].
Batch adsorption experiment
Batch sorption experiments were performed with 0.1 g of synthesized sulfur derivative in 20 mL As (III) and As (V) solution at room temperature till equilibrium. The solution pH was adjusted for each experiment using 0.1M NaOH and 0.1M HCl. The residual concentration was determined from the test sets by removing 1.0 mL of aliquots at different contact times (i.e. 30 min, 1 h, 3 h, 6 h, 12 h and 24 h). The effect of various parameters such as pH (4.0 to 9.0) initial arsenic concentration (1.0 ppm, 6.0 ppm, 18 ppm, 30 ppm and 60 ppm) and presence of competitive ions (sulfate, nitrate, chloride and phosphate at 100 ppm) was investigated with contact time. All the experiments were performed in triplicate average of three observations was reported. The colorimetric technique has been carried out to quantify the As (III) and As (V) removal capacity of the synthesized starch derivatives at 220 nm and compare with a standard curve of unknown concentrations from the test-sets. The residual solids in test-sets were washed with deionized water and dried at 50°C. The dried sample was characterized for sorption by SEM-EDX. The adsorption isotherms were calculated by plotting the equilibrium adsorption capacity (qe) versus the equilibrium concentration of residual As (III) and As (V) in the solution (Ce). The qe was calculated by the following formula [11].
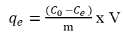
Where qe is the equilibrium adsorption capacity (mg g-1), C0 and Ce are the initial and equilibrium concentration (ppm) of As (III) and As (V) respectively, V is the volume of aqueous phase (L) and m is the weight of polymer (g). The % uptake was calculated by the following relationship [12].
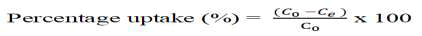
Results and Discussion
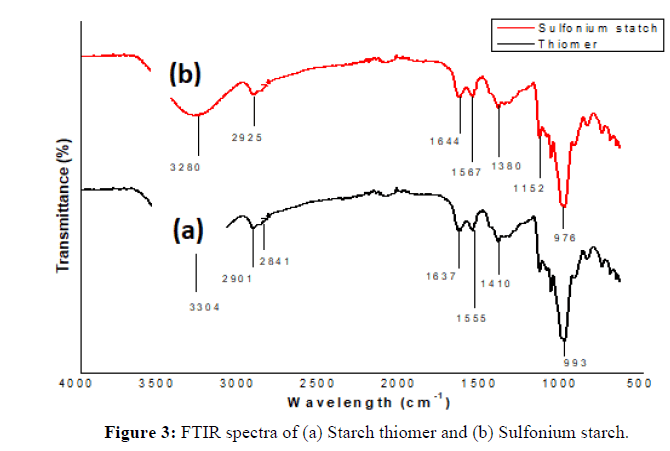
Successfully synthesized and characterized starch-based derivatives as per our earlier reported method. The detailed structure for the derivatives is depicted in Figure 1. The FTIR spectrum of thiomerm, sulfoniumm and iron immobilized adsorbent showed (4000 cm-1-500 cm-1). The characteristic peak due to S-H stretching vibration at 2841 cm-1 which indicates the modification of starch to the thiol structure. Peak at 1380 cm-1 confirms the presence of an ethyl group and hence displays successful derivatization to sulfonium ion structure (Figure 2).
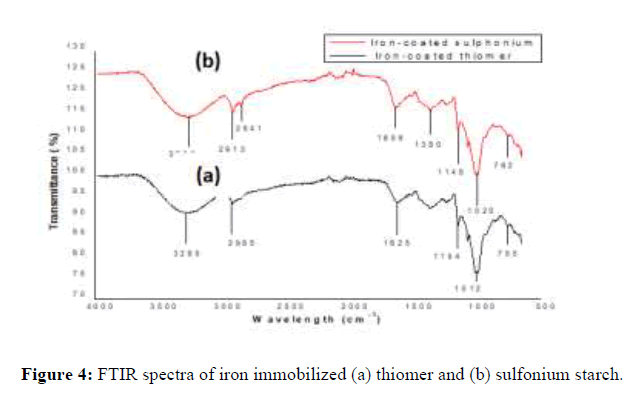
The adsorption peaks at 755 cm-1 and 762 cm-1 assigned to the Fe-OH stretching vibrations, which revealed the incorporation of iron in thiomer and sulfonium structure (Figure 3).
Effect of structure and concentration
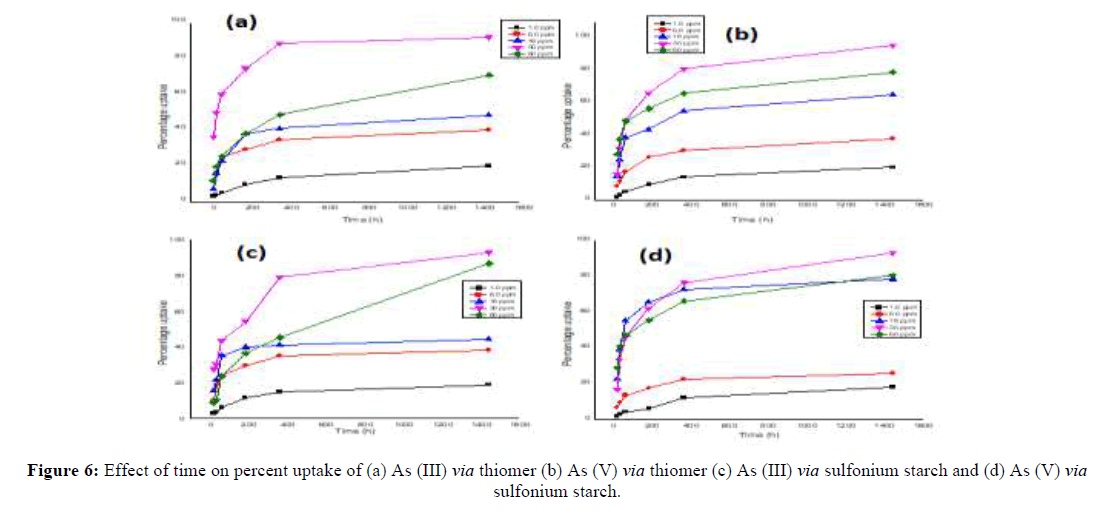
The synthesized starch derivatives were evaluated for effective removal of As (III) and As (V) removal from aqueous solution at 30 ppm and found that maximum percent uptake was 92%, 92.7%, 57% for As (III) and 95%, 93%, 37% for As (V) in thiomerm, sulfoniumm and sulfidem adsorbents after 24 h at 30 ppm. Conversely sulfoniumc and sulfidec adsorbents (prepared via conventional method) confirm low uptake i.e. 45% and 48% for As (III) and 55% and 34% for As (V) (Table 1). Thus it is concluded that starch derivatives i.e. thiomerm and sulfoniumm are preferential for sorption application in comparison to conventional products [13]. Therefore the further sorption studies for As (III) and As (V) were performed with these derivatives and hereafter they are named as thiomer and sulfonium starch. The results obtained with initial concentration varied from 1.0 ppm to 60 ppm, which depicts the effect of initial feed concentration of arsenic species on the removal capacity of thiomer and sulfonium with time (Figure 4 and Table 1).
| Polymer | Percentage uptake | Polymer | Percentage uptake | ||
|---|---|---|---|---|---|
| As (III) | As (V) | As (III) | As (V) | ||
| Thiomerm | 92 | 95 | Thiomerc | - | - |
| Sulfoniumm | 92.7 | 93 | Sulfoniumc | 45 | 55 |
| Sulfidem | 57 | 37 | Sulfidec | 48 | 34 |
Note: m=microwave; c=conventional
Table 1: Comparison of percentage uptake with structural effect.
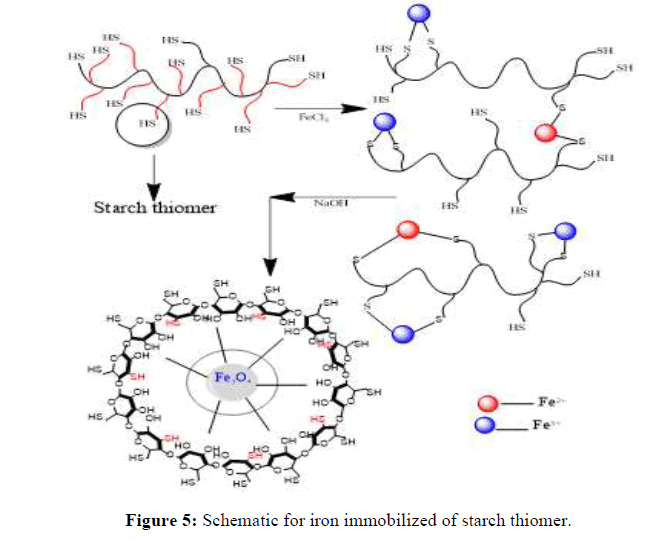
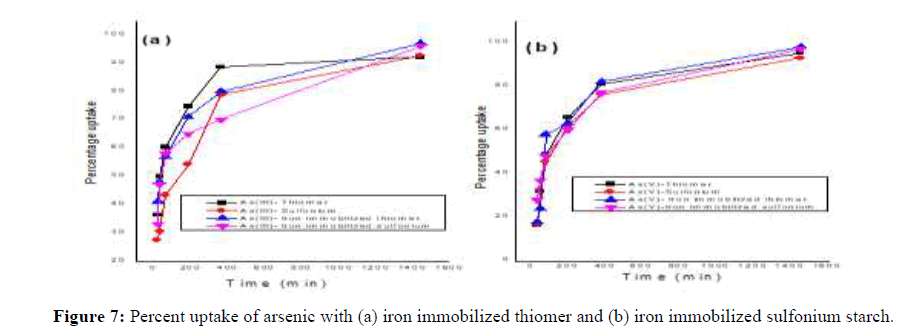
In general, the arsenic removal efficiency increases with an increase in the initial arsenic concentration and equilibrium is attained within 6 hrs [14]. In iron immobilized adsorbent the percentage uptake is increased by the magnitude of ~5%. The percentage uptake of As (III) and As (V) with iron immobilized thiol starch is 97.6% and 98.4% respectively (Figure 5). Similarly, in iron immobilized sulfonium starch the percentage uptake is 96.8% and 97.7% respectively for As (III) and As (V). The marginal efficiency for arsenic removal in ionic state As (V) from aqueous solutions could be ascribed to the successful iron immobilization throughout the starch backbone (Figure 5).
Arsenic speciation and surface charge of the thiolated and sulfonium starch
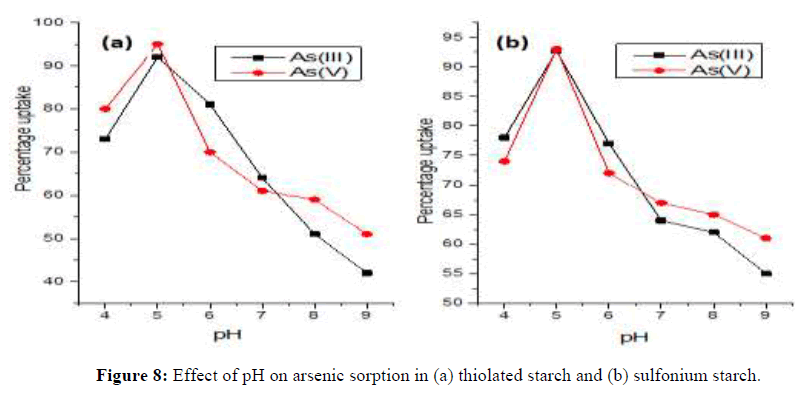
The arsenic speciation is dependent on the pH and redox potential of the natural environment of the forms of water. Hence, pH value of water could significantly influence the adsorption performance of arsenic. Therefore, the effect of initial pH of arsenic solutions on the adsorption capabilities of thiomer was investigated for % removal of As (III) and As (V) by varying pH from 4.0 to 9.0 with (0.1 M HCl and 0.1 M NaOH) with 30 ppm concentration which was optimized concentration. The pH of the solution has significance and a restrictive outcome in the adsorption thiomerm and sulfoniumm adsorbents. It is perceptible that the As (III) and As (V) sorption capacity increases as the pH increases from 4.0 to 5.0, and then the sorption capacity decreases in alkaline medium from 6.0 to 9.0 pH. In the present study, As (III) and As (V) removal was observed maximum at 5.0 pH. The percentage removal of As (III) and As (V) by thiomer adsorbent was found 94% and 97% at 5.0 pH which is the maximum percentage uptake through all over the pH values. In the case of sulphonium adsorbent, the uptake revealed the lowest removal viz. 93% and 92%. Conversely, the conventional heating sulfidec sample had less uptake i.e. 57% and 37% for As (III) and As (V) respectively as compared to 48% and 31% in the case of sulfoniumc [15]. The decrease in sorption was evaluated at 6.0, 7.0, 8.0 and 9.0 pH with less percentage uptake. At acidic pH, the results are comparable and can be accounted for by a highly protonated adsorbent surface, which is capable of attracting arsenic species by electrostatic forces. Conversely, in higher pH values, the solution donates more hydroxide (OH-) groups and the presence of a large amount of OH-ions in the aqueous solution starts competing with arsenic for binding with the thiomerm and quaternary sulfoniumm groups. Similar results were also reported by the maximum removal rate for these adsorbents with positive surface charges at the 5.0 pH range and thereafter a decrease in the arsenic adsorption rates during increased pH values for biopolymeric quaternary and thiomerm products [16]. Thus, maximum sorption capacity on As (III) and As (V) by thiomerm and sulphoniumm adsorbents has been observed through microwave heating method at 5.0 pH which indicates performances of adsorbent was effective. However, at acidic pH starch thiomer and sulfonium are positively charged and leads to interaction with As (III) and As (V). Marginally favored domino effect of sorption for As (V) in acidic pH in comparison to As (III) can be explained by the strong electrostatic interaction between the protonated starch thiomer and anionic H2AsO4-, which is predominant species in this pH range. This also confirms the electrostatic nature of the arsenic sorption. Therefore, pH 5.0 was selected as optimum for solidphase extraction of arsenic for subsequent experiments. Comparison of arsenic adsorption by that the adsorption capacity of iron oxide decreases with an increase in pH. An increase in pH causes the decrease of surface positive charge, which results in a decreased attraction between thiomerm and sulphonium surfaces and arsenite and arsenate species. The difference in percentage uptake as a function of solution pH could be explained by the change of arsenic speciation and surface charge of the thiolated starch and sulfonium starch [17]. Redox potential (Eh) and pH are the most important factors to control the speciation of arsenic. Therefore, pH 5.0 was selected as optimum for solid-phase extraction of arsenic form subsequent experiments (Figure 6).
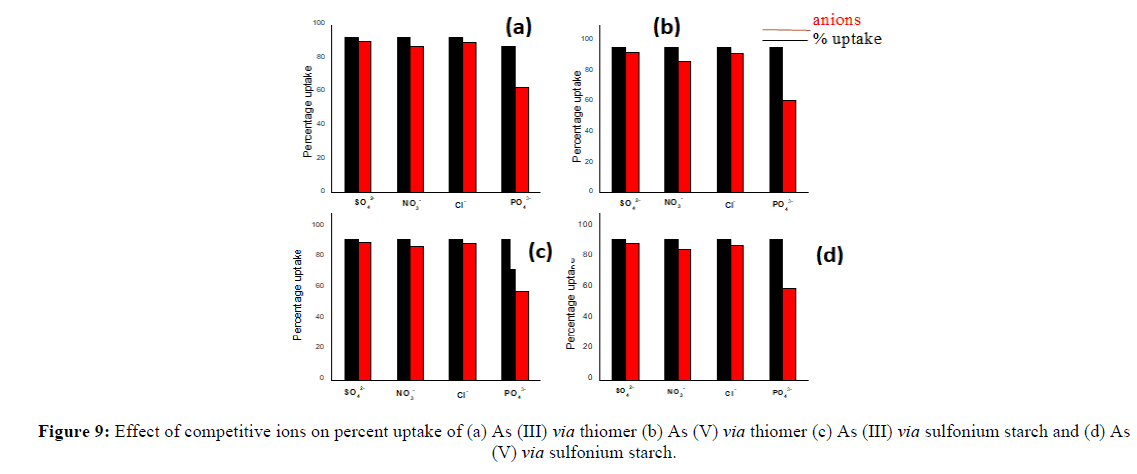
Effect of competitive anions on As (III) and As (V) removal efficiency
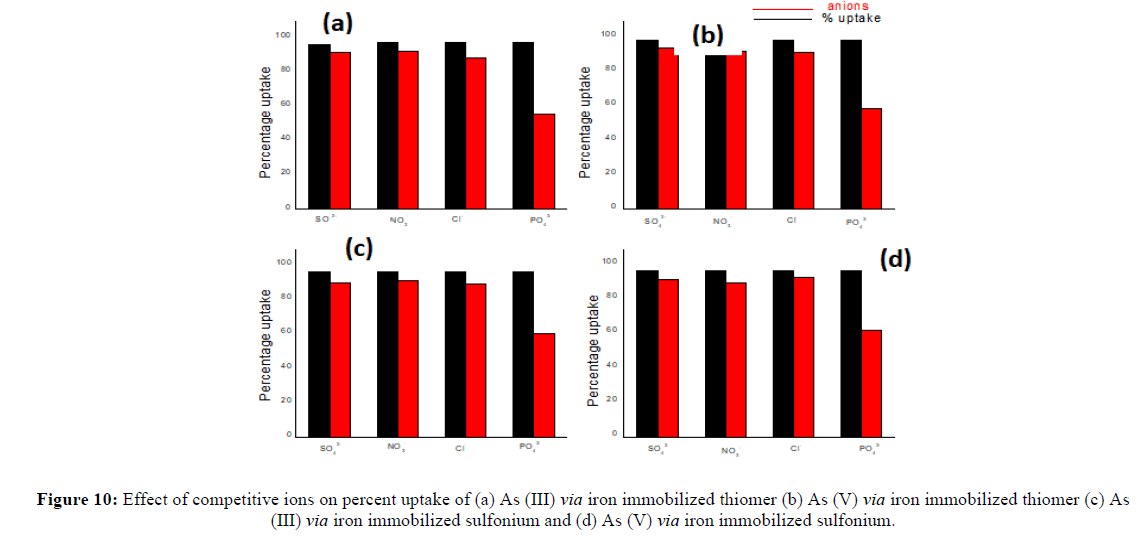
As well-known that various cations and anions may coexist with arsenic ions in natural waters. The interference of various coexisting ions such asCl-, PO42-, SO42- and NO3- could influence aqueous phase equilibrium during As (III) and As (V) sorption. Subsequently, it is a necessity to test theadsorbent capability in such an environment. Cl-, SO42- and NO3- show negligible influence (2% to 8%) on As (III) and As(V) removal efficiency(Figure 7). The presence of PO42- depicts comparatively highest decrease in As (III) and As (V) removal. In the presence of PO42- the maximum removal efficiency decreases from 92% to 62% for As (III) and 95% to 60% for As (V) in thiomers. While in sulfoniumm the results support decreases from 92.7% to 58% for As (III) and 93% to 60% for As (V). The iron immobilized thiomer and iron immobilized sulfoniumm starch also follow the same trend and depict only negligible effect with Cl-, SO42- and NO3- on removal efficiency of arsenic (2% to 10%) (Figure 8). Similarly, in the presence of PO42- the percent uptake decreases from 97% to 55% for As (III) and 98% to 58% for As (V) [18]. In the case of iron immobilized sulfoniumm starch the percentage uptake decrease from 96.8% to 60% for As (III) and 97.7% to 62% for As (V). The presence of co-existing ions affects the arsenic removal efficiency by thiomer and sulfonium adsorbent in the order of PO43->NO32->Cl->SO42-. The decrease in the adsorption efficiency is inferred due to competition for the binding sites of the adsorbent between phosphate and arsenic as phosphate and arsenic both havesimilar molecular structures (Figures 7 and 8).
Adsorption isotherms
Adsorption isotherms are used to ascertain the mechanism and distribution of the adsorbate between liquid and solid adsorbent phase at equilibrium during the process of removal. The experimental data of As (III) and As (V) removal by starch derivatives for different initial concentrations were plotted in linearized forms of Langmuir and Freundlich model [19].
Langmuir model is valid for monolayer adsorption over a homogenous surface with a finite number of active sites and identical adsorption energy. The linear equation that describes the Langmuir model is given as:
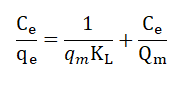
Where qe is the amount of adsorbed arsenic per gram of sorbent (mg/g); Ce is the equilibrium concentration of adsorbate in the solution (mg/L); qm is the maximum adsorption capacity at monolayer coverage in mg/g; KL is the Langmuir equilibrium constant (L/mg).
For further analysis of the adsorption process, a dimensionless constant separation factor RL is also calculated which reflects the essential characteristics of Langmuir adsorption.
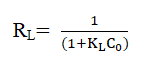
Where KL is the Langmuir constant and Co is initial arsenic concentration. The RL value has importance when it is between 0 and 1, where it indicates an effective interaction between the adsorbent and the adsorbate. Values greater than one indicates an unfavorable isotherm and RL equal to zero is accounted for an irreversible isotherm [20].
The Freundlich model is widely used to describe the adsorption of an analyte on a heterogeneous surface and is applies to monolayer chemisorptions and multilayer adsorption physisorption. The Freundlich isotherm is described as.
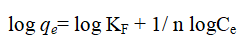
Where qe is the amount adsorbed per unit mass of adsorbent (mg/g), Ce is the equilibrium adsorbate concentration in solution; Kf and n are the Freundlich constants related to the adsorption capacity and adsorption intensity respectively.
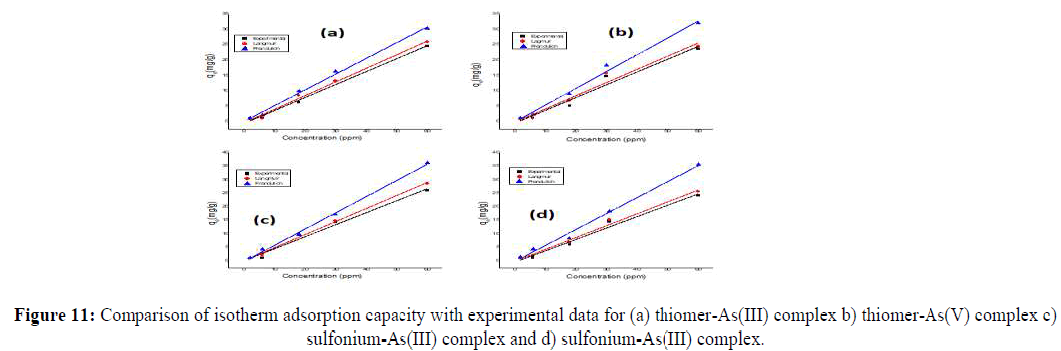
The adsorption isotherms were regressively simulated with the Langmuir and Freundlich models. The values of the relative parameters calculated from the two isotherm models (Tables 2 and 3). The R2 values of the Langmuir model are higher than those of the Freundlich model, indicating that the Langmuir model fits the adsorption isotherms better than the Freundlich model. This suggests that the adsorption of As (III) and As (V) on thiolated starch and sulfonium starch is monolayer adsorption. The RL values are also calculated between 0 and 1 which indicates favorable adsorption. Both the models are then compared for experimental and theoretical adsorption capacity. It is indicated, that the Langmuir model confirms a slightly preferential match with the value of sorption capacities obtained from the experimental (Figure 9).
| Langmuir adsorption isotherm parameters for As (III) and As (V) with thiomer | ||||||||
|---|---|---|---|---|---|---|---|---|
| Initial concentration (ppm) | As (III) | As (V) | ||||||
| qm (mg/g) | KL | R2 | RL | qm (mg/g) | KL | R2 | RL | |
| 1.0 | 0.89 | 0.008 | 0.998 | 0.98 | 0.93 | 0.0396 | 0.999 | 0.93 |
| 6 | 0.94 | 0.003 | 0.999 | 0.98 | 2.44 | 0.0039 | 0.999 | 0.97 |
| 18 | 8.4 | 0.012 | 0.999 | 0.81 | 4.87 | 0.0192 | 0.996 | 0.7 |
| 30 | 13.02 | 0.007 | 0.999 | 0.77 | 11.75 | 0.003 | 0.999 | 0.9 |
| 60 | 25.96 | 0.008 | 0.999 | 0.51 | 25.45 | 0.015 | 0.999 | 0.09 |
| 1.0 | 0.75 | 0.36 | 0.999 | 0.27 | 0.9 | 0.0024 | 0.998 | 0.99 |
| 6 | 2.1 | 0.009 | 0.999 | 0.95 | 2.12 | 0.0065 | 0.999 | 0.97 |
| 18 | 6.85 | 0.005 | 0.998 | 0.89 | 6.82 | 0.025 | 0.999 | 0.85 |
| 30 | 9.5 | 0.013 | 0.999 | 0.95 | 14.22 | 0.031 | 0.999 | 0.90 |
| 60 | 28.64 | 0.007 | 0.987 | 0.58 | 25.62 | 0.004 | 0.998 | 0.64 |
Table 2: Langmuir adsorption isotherm parameters.
| Freundlich adsorption isotherm parameters for As (III) and As (V) with thiomer | ||||||
|---|---|---|---|---|---|---|
| Initial concentration (ppm) | As (III) | As (V) | ||||
| kf (mg/g) | 1/n | R2 | kf (mg/g) | 1/n | R2 | |
| 1 | 0.7 | 0.87 | 0.998 | 0.73 | 0.99 | 0.998 |
| 6 | 1.08 | 0.38 | 0.969 | 1.36 | 0.39 | 0.969 |
| 18 | 0.8 | 0.87 | 0.998 | 0.93 | 0.99 | 0.998 |
| 30 | 0.74 | 0.92 | 0.997 | 0.74 | 0.81 | 0.997 |
| 60 | 0.69 | 0.94 | 0.991 | 0.73 | 0.73 | 0.998 |
| 1 | 0.7 | 0.7 | 0.998 | 0.73 | 0.99 | 0.997 |
| 6 | 0.74 | 0.98 | 0.997 | 0.75 | 0.98 | 0.996 |
| 18 | 0.66 | 0.93 | 0.972 | 1.08 | 0.99 | 0.998 |
| 30 | 0.68 | 0.95 | 0.996 | 0.76 | 0.98 | 0.997 |
| 60 | 0.74 | 0.97 | 0.998 | 0.93 | 0.99 | 0.998 |
Table 3: Freundlich adsorption isotherm parameters.
Kinetic studies
To understand the process of adsorption of As (III) and As (V) onto starch derivatives, the pseudo-first and pseudo-second order were analyzed. The kinetic models help to understand the mechanism of sorption on the surface of the adsorbent. The pseudo first order model is expressed as.
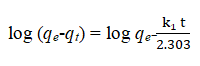
Where qe (mg g-1) and qt (mg g-1) are the adsorption amount at equilibrium and at time t, respectively and k1 (min-1) is the pseudo first order rate constant. The values of the adsorption rate constant (k1) for the sorbents at different initial arsenic concentrations were obtained from slopes of the plots of log (qe-qt) versus time. The pseudo second order model is expressed as.
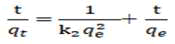
Where, k2 (g (mg min-1)) is the pseudo-second-order rate constant.
The kinetic results depict that pseudo-first-order for arsenic sorption diverges from linearity with a lower correlation coefficient (R2) value in comparison to the pseudo second order model. Therefore, the best suitability of the kinetic data corresponds to the pseudo-second-order model for the entire adsorption period. The suitability of pseudo-second order model, which is based on the possibility of chemisorption confirms the electrostatic attraction between the adsorbents and adsorbate. Thus, the correlation coefficient (R2) has the highest value for the pseudo-secondorder model (Table 4).
| Pseudo-first-order kinetic parameters | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CO (ppm) | As (III) with thiomer | As (V) with thiomer | As (III) with sulfonium | As (V) with sulfonium | ||||||||
| qe | K2 | R2 | qe | K2 | R2 | qe | K2 | R2 | qe | K2 | R2 | |
| 1 | 0.51 | 0.01 | 0.7062 | 0.38 | 0.004 | 0.7146 | 0.45 | 0.006 | 0.8476 | 0.52 | 0.0059 | 0.8476 |
| 6 | 0.93 | 0.004 | 0.9405 | 0.92 | 0.005 | 0.8527 | 0.78 | 0.005 | 0.9811 | 0.85 | 0.0057 | 0.9372 |
| 18 | 1.65 | 0.003 | 0.7825 | 2 | 0.005 | 0.9082 | 1.62 | 0.006 | 0.8995 | 4 | 0.0059 | 0.9013 |
| 30 | 2.8 | 0.465 | 0.9574 | 2.57 | 0.778 | 0.9832 | 2.75 | 0.004 | 0.9531 | 2.8 | 0.0043 | 0.9636 |
| 60 | 3.21 | 0.001 | 0.9355 | 3.38 | 0.003 | 0.968 | 3.05 | 0.003 | 0.9618 | 4 | 0.0069 | 0.9013 |
| 1 | 0.17 | 0.21 | 0.9996 | 0.344 | 0.41 | 0.9997 | 0.262 | 0.338 | 0.9979 | 0.111 | 0.14 | 0.9976 |
| 6 | 1.13 | 0.024 | 0.995 | 0.016 | 0.015 | 0.9984 | 0.029 | 0.003 | 0.9951 | 0.012 | 0.023 | 0.9954 |
| 18 | 0.23 | 0.006 | 0.9987 | 0.228 | 0.027 | 0.999 | 0.233 | 0.009 | 0.9985 | 0.249 | 0.075 | 0.9994 |
| 30 | 0.49 | 0.002 | 0.9939 | 2.37 | 0.001 | 0.9956 | 0.242 | 0.001 | 0.9471 | 0.199 | 0.001 | 0.9946 |
| 60 | 0.26 | 0.001 | 0.988 | 1.01 | 0.002 | 0.9915 | 0.136 | 0.002 | 0.9724 | 0.81 | 0.002 | 0.9925 |
Table 4: Pseudo-first-order and second-order kinetic parameters for As (III) and as (V) sorption via thiomer and sulfonium starch.
Sorption characterization by SEM-EDX
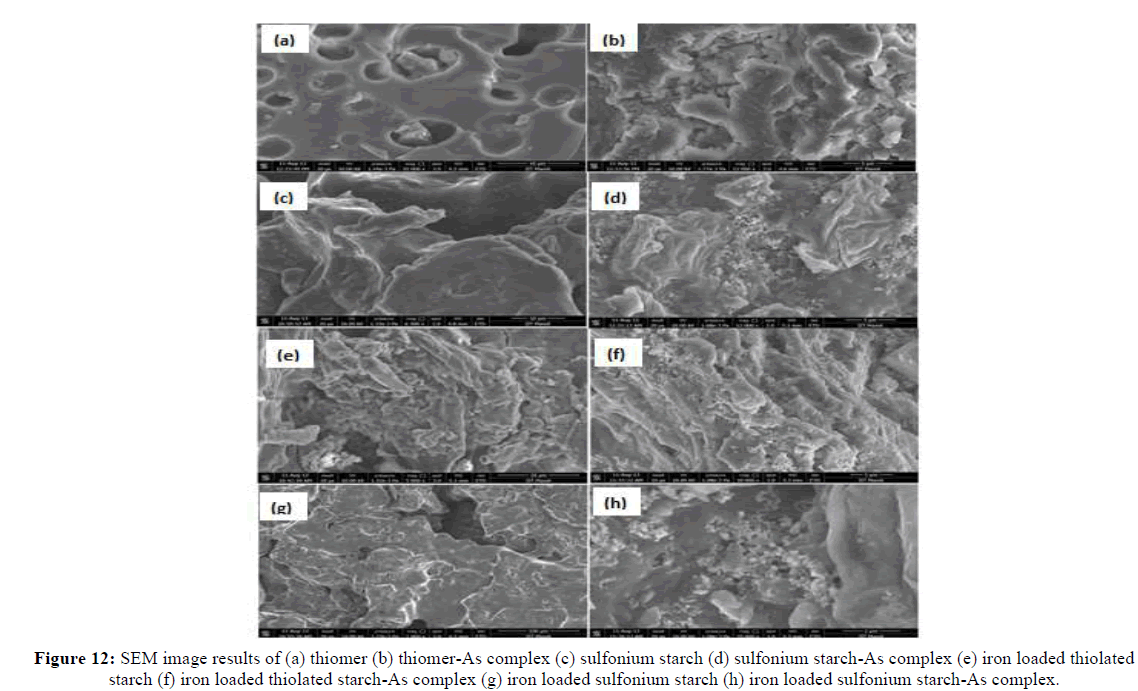
Microphotographs of modified thiol starch, sulfonium starch, iron immobilized thiomer and iron immobilized sulfonium starch were obtained in support of the sorption study (Figure 10). From the SEM images, it is evident that thiol starch and sulfonium starch possess heterogeneous surfaces due to small out-growths. This surface roughness in starch derivatives may probably be useful in arsenic sorption from aqueous solutions. After arsenic sorption thiol starch and sulfonium starch adsorbents surface is attaining smoothness. It is clear from the increased smoothness of the matrix surface and decrease in the porosity distance that the absorbed arsenic species are aggregated on the surface of sulfonium and thiomer products. The significant deformation in the structural organization of the starch derivative safer sorption is an attribute towards the high adsorption of arsenic on the adsorbent surface (Figure 10).
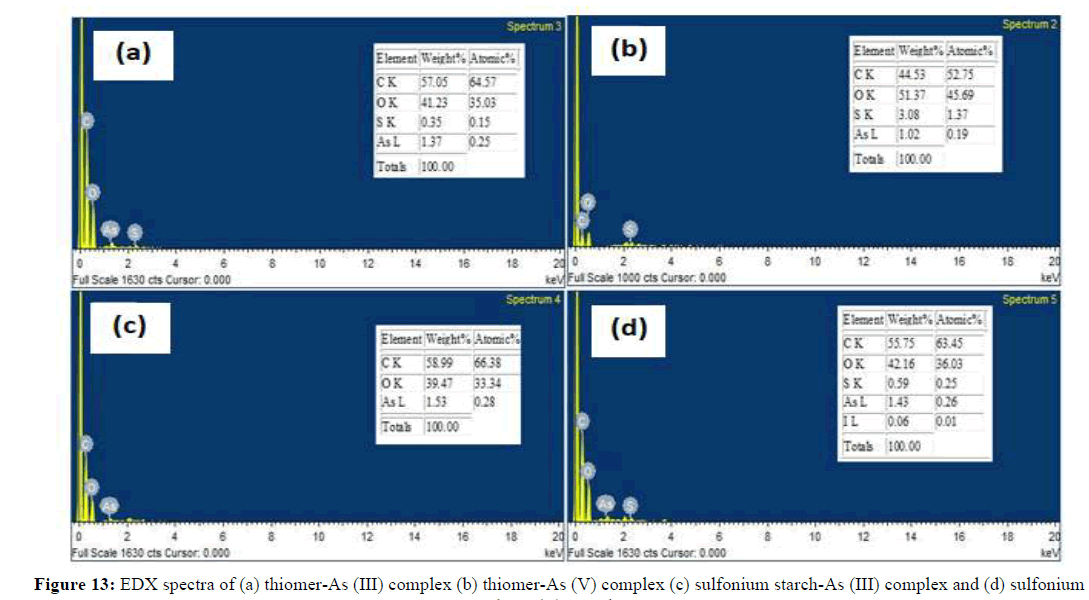
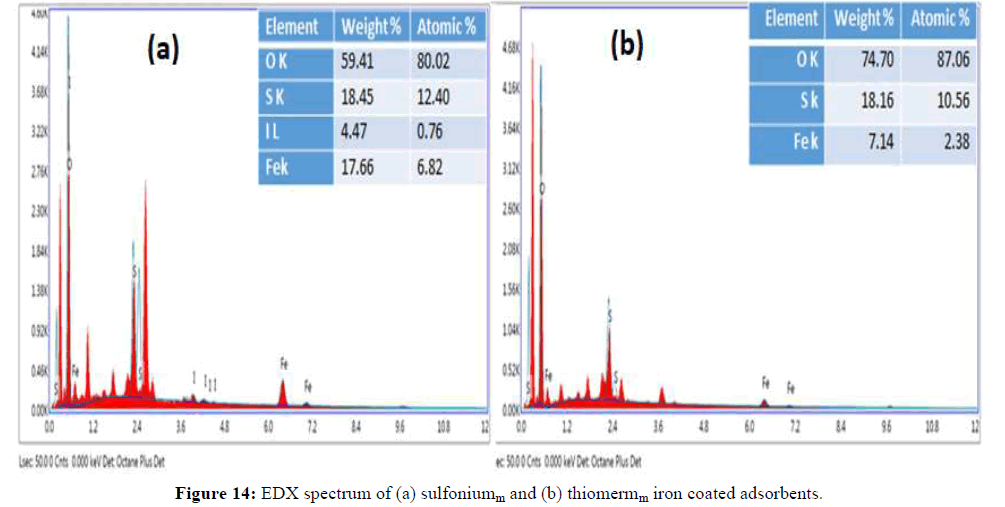
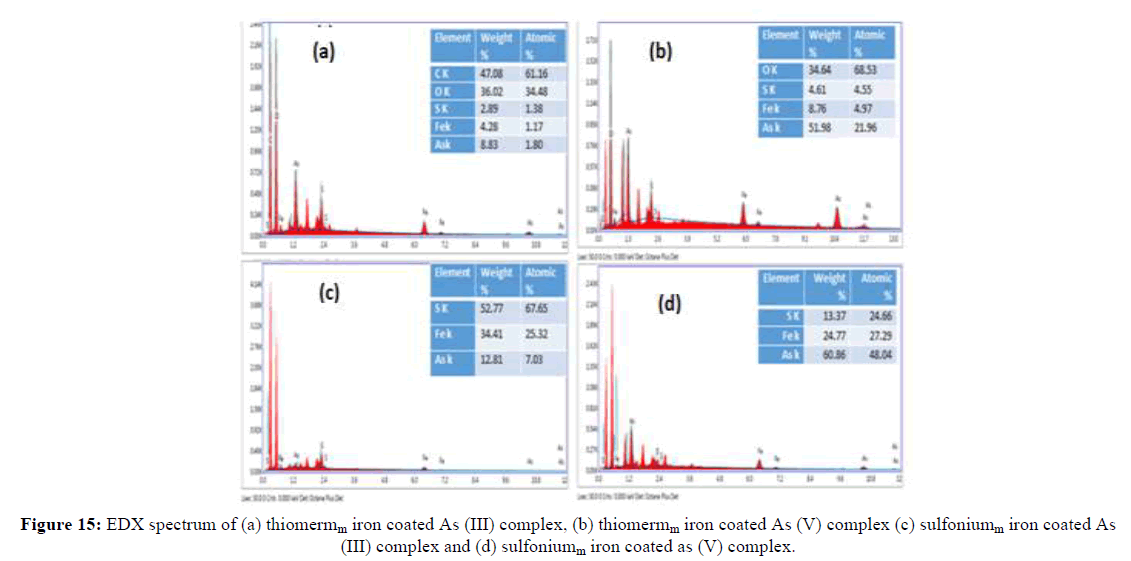
On other hand, there is remarkable change in the morphology and structure when the iron is incorporated on thiol starch and sulfonium starch and this accounts the binding of iron with thiolated starch and sulfonium starch. The SEM images show some white spots and out-growths on the surface of iron loaded thiolated starch and iron loaded sulfonium starch which indicate the sorption of arsenic. Furthermore, EDS analysis also supports the sorption. The results clearly show the presence of arsenic on the surfaces of thiol starch and sulfonium starch (Figure 11). The EDS analysis of sulfoniumm and thiomerm have major component Fe (17.86% and 7.14%) iron content was also observed. Upon immobilization of Fe on starch derivatives surface, there is an increase in iron percentage and adsorption of arsenic onto the surface of iron coated thiomerm and sulfoniumm showed the presence of As (III) and As (V) upto a maximum concentration of iron increased upto 8.83%, 51.98%, 12.81% and 60.86% (Figure 13) compared to precursor (Figure 12), which indicates significant adsorption of arsenic on to the surface of polymer (Figures 11-13).
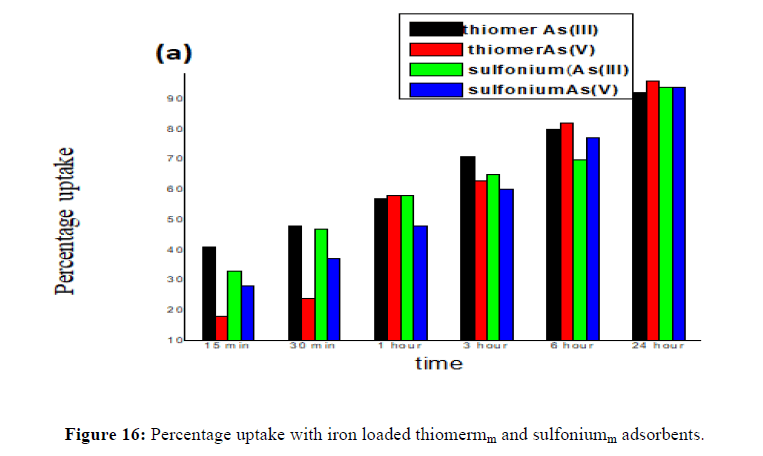
Effect of contact time iron loaded starch derivatives
Previously prepared iron loaded starch derivatives were further used for this experiment. Place 100 mg iron coated thiomer adsorbents in 30 ppm. concentration of As (III) and As (V). It is clear that adsorption efficiency increases with increasing the contact time, reaching nearly maximum removal at contact time of 24 hrs with 96% and 98% arsenic removal in thiomer and sulfonium adsorbents which is comparatively more than the removal percentage of arsenic with precursor thiomerm and sulfoniumm 95%, 94% for As (III) and 98%, 96% for As (V) and thereafter, removal becomes nearly constant for both the cases with iron loaded sulfoniumm and thiomerm. This can be because initially all adsorbent sites were empty and the arsenic concentration was high. Afterward, the As (III) and As (V) adsorption capacity of the adsorbents were decreased significantly due to the decrease in available adsorption sites as well as arsenic concentration. The adsorption capacity for arsenic is strongly dependent on the species and content of the reactive components and the category and characteristics of the carriers. The content of iron is a crucial factor to impact the arsenic adsorption capacity for adsorbents loaded with iron. Therefore, the iron amount on thiomerm and sulfoniumm adsorbents increased significantly compared with uncoated starch derivatives. FeCl3 could be uniformly loaded into the carriers with a relatively high content of iron if the media were not only highly porous, but also presented with adsorption sites for Fe (III). This could be attributed to the carrier’s chemical and physical properties and the innovative method to realize the loading process. As for the chemical properties, the possible situation was that hydroxyls, which made the media highly hydrophilic, had a strong affinity toward Fe (III).
Temperature effect and thermodynamic parameters
The results of arsenic equilibrium adsorption at 35°C and pH 5 were satisfactorily fitted to the Langmuir isotherm model. The adsorption capacities for both As (V) and As (III) were lower at an elevated temperature. However, this decrease was more significant for As (V) than for As (III). The arsenic equilibrium adsorption capacity (mg As/g adsorbent) obtained from the Langmuir equation increased from 5.75 to 14.25 for As (III) and from 3 to 12.65 in 24 hrs for As (V) in thiomerm adsorbent and for sulfoniumm adsorbent it is 4.38 to 14.39 for As (III) 3 to 14.95 for As (V) when temperature was increased from 30°C to 35°C. The extents by which the arsenic adsorption capacity increased with temperature might be attributed to the change in surface properties of the adsorbent, solubility of the adsorbate species and endothermic or exothermic nature of the adsorption, but it is perceived that temperature increases only upto 35°C after that starts decreasing upto 45°C. The Langmuir constants can be used for the estimation of several process thermodynamic parameters based on the following equations.
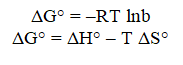
Where, b is a Langmuir isotherm constant (L/mol) at temperature T (K), R is an ideal gas constant (8.314 J/mol.K), ΔG° (J/mol), ΔH° (J/mol) and ΔS° (J/mol.K) are the Gibb’s free energy, enthalpy and entropy changes, respectively.
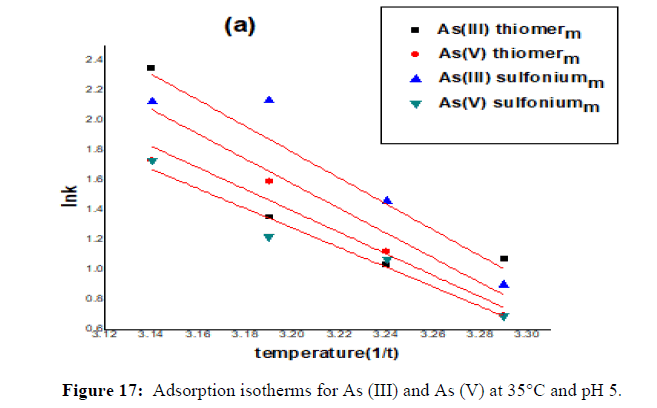
The regressed Langmuir constants (with R2 ≥ 0.98) and calculated thermodynamic parameters are given in (Table 5). The negative ΔG° values for both As (V) and As (III) confirm the spontaneous nature of the arsenic adsorption process. With temperature 35°C, ΔG° showed a slight decrease for As (V) and As (III). This indicates that better adsorption was obtained at this temperature, which is in agreement with the result that the maximum adsorption capacity qm increased with temperature for As (III) and As (V). The ΔH° value was positive for As (III) and for As (V), respectively, indicating the endothermic nature of As (III) and As (V) adsorption. The positive ΔS° values reflect the affinity of the adsorbent material for As (III) and As (V) and suggest some structural changes in the arsenic species and the adsorbent. Furthermore, the positive value of ΔS° show the increasing randomness at the solid/liquid interface during arsenic adsorption on the adsorbent. However, the endothermic nature of As (III) adsorption indicated by the positive ΔH° value support the result that As (III) adsorption decreased with temperature. The temperature effect on the intrinsic kinetics of As (V) adsorption for the present adsorbent is likely to surpass the endothermic effect on the adsorption reaction. The consequence of the difference in the thermodynamic parameters between As (III) and As (V) is that the surface adsorption of anionic As (V) species might be different from that of neutral As (III) species on this binary oxide adsorbent (Figures 14-17).
| As species | Temperature (°C) |
ΔG° (kJ/mol) |
ΔH° (kJ/mol) |
ΔS° (kJ/mol·K) |
|---|---|---|---|---|
| As (III) | 35 | -52.03 | 68.07 | 232.22 |
| As (III) | 35 | -45.23 | 71.99 | 245.17 |
| As (V) | 35 | -62.96 | 59.69 | 202.52 |
| As (V) | 35 | -67.96 | 54.37 | 184.57 |
Table 5: Calculated Langmuir constants and thermodynamic parameters at pH 5 with thiomermm and sulfoniumm adsorbents.
Conclusion
The present study involves the synthesis and characterization of starch based thiomer or starch derivative with -SH functionalities and recommends the more uniform functionalization is reported for the samples synthesized under microwave irradiation in comparison to the conventional method. In conclusion, the results revealed the potential of the applied new strategy to synthesize starch-based materials using a mild urea reagent and a green synthetic methodology thiolated and sulfonium starch in a high degree of substitution is highly proficient adsorbents for the removal of arsenic from an aqueous solution. However, iron immobilized starch derivatives have supported the sorption capability increase by the magnitude of ~5%. The results support the highest adsorption in the pH range from 4.0 to 5.0. The competitive ions i.e. nitrate, sulfate and chlorides have a marginal influence on the removal capacity of arsenic, whereas phosphate influences the removal in a greater amount. This can be accounted for the fact that arsenic and phosphate belong to the similar element group (main group V) and have chemical resemblance and analogous ionic structures. The results were evaluated for mechanistic details via different isotherms. The kinetic batch experiments indicated that more than 95% or 98% of arsenic is absorbed by the starch derivatives or iron-immobilized starch derivatives within 6 h of adsorption and confirmed the second-order kinetic model. The results supported Langmuir isotherm with high values of R2 which indicates that the removal of As (III) and As (V) in the thiomer and sulfonium starch follows the monolayer adsorption mechanism. The better fit to the pseudo-second order model confirms chemisorption via electrostatic attraction between adsorbents and arsenic. SEM-EDX data also supported the high adsorption results in the arsenic removal with thiol and sulfonium ion derivatives of starch. Therefore, the newly synthesized thiolated and sulfonium starch have hi-tech applications and can be applied more extensively in sorption or ion exchange processes of contaminated water resources for arsenic (As (III) and As (V)) removal in the real potential. The study concluded that the synthesis of thiomer and sulfonium structure via green protocols is successful and supports the high degree of modification by different analytical estimations. The products in a higher content of modification also support the exception application potential for arsenic sorption. Hence the synthesized materials have great technological potential and can be explored for commercialization in the removal application of arsenic from aqueous solution.
Acknowledgment
The author is thankful to the vice chancellor, Shoolini university, Solan, Himachal Pradesh, India for the facilities and support. Author also thank to the head, department of chemistry and biochemistry, CSK Himachal Pradesh Krishi Vishvavidyalaya, Palampur, Himachal Pradesh, India for his kind cooperation during the work.
References
- Ahmad M, Ahmed S, Swami BL, et al. Langmuir. 2015, 2(6): p. 280-289.
- Ahn JS, Chon CM, Moon HS, et al. Water Res. 2003, 37(10): p. 2478-2488.
[Crossref] [Google Scholar] [PubMed]
- Ali I. Chem Rev. 2012, 112(10): p. 5073-5091.
[Crossref] [Google Scholar] [PubMed]
- Ali I, Gupta VK. Nat Protoc. 2006, 1(6): p. 2661-2667.
[Crossref] [Google Scholar] [PubMed]
- Anirudhan TS, Jalajamony S. J Environ Manage. 2010, 91(11): p. 2201-2207.
[Crossref] [Google Scholar] [PubMed]
- Awual MR, Hossain MA, Shenashen MA, et al. Environ Sci Pollut Res Int. 2013, 20(1): p. 421-430.
[Crossref] [Google Scholar] [PubMed]
- Awual MR, Jyo A, El-Safty SA, et al. J Hazard Mater. 2011, 188(1-3): p. 164-71.
[Crossref] [Google Scholar] [PubMed]
- Bagla P, Kaiser J. Science. 1996; 274(5285): p. 174-175.
[Crossref] [Google Scholar] [PubMed]
- Bhattacharya AK, Mandal SN, Das SK. Chem Eng J. 2006, 123(1-2): p. 43-51.
- Bhattacharya P, Chatterjee D, Jacks G. Int J Water Resour Dev. 1997; 13(1): p. 79-92.
- Chauhan K, Kaur J, Singh P, et al. Ind Eng Chem Res. 2016, 55(9): p. 2507-2519.
- Chauhan K, Priya V, Singh P, et al. RSC Adv. 2015, 5(64): p. 51762-51772.
- Dhar RK, Zheng Y, Rubenstone J, et al. Anal Chim Acta. 2004, 526(2): p. 203-209.
- Dutta PK, Tripathi S, Mehrotra GK, et al. Food Chem. 2009, 114(4): p. 1173-1182.
- Fendorf S, Michael HA, van Geen A. Science. 2010, 328(5982): p.1123-1127.
- Genc-Fuhrman H, Tjell JC, McConchie D. Environ Sci Technol. 2004, 38(8): p. 2428-2434.
[Crossref] [Google Scholar] [PubMed]
- Guo X, Chen F. Environ Sci Techno. 2005, 39(17): p. 6808-6818.
[Crossref] [Google Scholar] [PubMed]
- Gupta A, Chauhan VS, Sankararamakrishnan N. Water Res. 2009, 43(15): p. 3862-3870.
[Crossref] [Google Scholar] [PubMed]
- Gupta VK, Ali I, Saleh TA, et al. Rsc Advances. 2012, 2(16): p. 6380-6388.
- Ho YS, McKay G. Process Biochem. 1999, 34(5): p. 451-465.