Research Article - Der Pharma Chemica ( 2018) Volume 10, Issue 3
Assessment of the Biodegradation Potentiality of Mono and Polynuclear Aromatic Hydrocarbons Using Aspergillus flavus
Dina F Abd Eltawab1, Nashwa A Ahmed2*, Yousseria M Shetaia3 and Ashraf Y El-Naggar4
1Petrotrade Company, Cairo, Egypt
2Microbiology Department, Faculty of Applied Medical Sciences, October 6 University, Egypt
3Microbiology Department, Faculty of Science, Ain Shams University, Egypt
4Chemisty department,Faculty of Science, Taif University, Kingdom of Saudi Arabia
- *Corresponding Author:
- Nashwa A Ahmed
Microbiology Department
Faculty of Applied Medical Sciences
October 6 University, Egypt
Abstract
In a way to find a new fungal isolate capable of degrading Polyneuclear Aromatic Hydrocarbons (PAHs) present in water, fourteen fungal isolates were recovered from two sites along the river Nile main stream. Screening of the fungal isolates revealed the selection of FX8 which was identified as Aspergillus flavus as the most potent one, further studies optimization studies indicated that maximum degradation was attained at pH 4 and incubation temperature 30°C at 150 rpm. Gas Chromatography-Mass Spectroscopy (GC-MS) analysis of mono aromatic mixture showed that the fungus can degrade efficiently all compounds as rhe share the same chemical structure. High Performance Liquid Chromatography (HPLC) profiles of the residuals of the sixteen most hazardous polyneuclear aromatic compounds revealed the ability of A. flavus to degrade nearly 85.4% of the mixture only after 4 days of incubation under optimum culture conditions. This supports its application in bioremediation programs of hazardous aromatic pollutants.
Keywords
Mononuclear aromatic compounds, Polyneuclear aromatic hydrocarbons, Biodegradation, Aspergillus flavus
Introduction
Polyneuclear Aromatic Hydrocarbons (PAHs) are the most widely distributed pollutants in the environment because of their association with anthropogenic activities [1]. PAHs have been divided into two categories, low molecular weight compounds (two or three rings) which are relatively volatile, soluble and more degradable and high molecular weight compounds (four or more rings) which are relatively less volatile and more resistant to microbial degradation [2]. PAHs are hydrophobic and highly lipophilic compounds [3], so when they reached soil and water they cause severe immediate and long term influence since they accumulate in food chains. They disrupt the biochemical and physiological processes of many organisms. Thus causing carcinogenesis or mutagenesis in the genetic material [4-6].
Many physical, chemical and biological methods have been used to clean up the PAHs contaminated sites but these methods are not economical in addition they may lead to the formation of other toxic compounds. Recently the application of biotechnology is the best choice for remediating such sites Kumar et al. Microorganisms of different origins are capable of converting the organic pollutants into less harmful compounds by aerobic and anaerobic respiratory reactions using them as the only source of carbon and energy [7-9]. Fungi are potential candidates for removal of PAHs from soil and water by the action of the fungal hyphae growth on toxic and undesirable substrates by their potential enzyme system [10,11]. Particular attention is devoted to indigenous ones improving their in situ degradation capability through determination of the optimal physico-chemical conditions as temperature, pH, nutrients and aeration [12,13].
The present study concerned with the isolation and consequently screening the PAHs degrading fungal isolates, and to optimize the cultural conditions influencing the biodegradation process.
Materials and Methods
Sample collection
For the isolation of the biodegrading fungal isolates, surface water samples were collected from two different sites (El-Giza and Helwan) along the river Nile main stream. The water samples were collected in pre-sterilized sample bottles and stored at 4°C.
Isolation and purification of the biodegrading fungal isolates
The fungal isolates were obtained by plating 100 μl of water samples on the surface of basal mineral salts agar medium (BMS) with the following composition (g/l): NaNO3; 3, KH2PO4; 1, MgSO4.7H2O; 0.5, KCl; 0.5, yeast extract; 1 and agar 20.
The pH was adjusted at 6 before autoclaving [10]. The medium was enriched with xylene (1% v/v), naphthalene (0.3% v/v) and anthracene (0.2% v/v) individually as sole carbon sources. At the end of the incubation period (3 days at 30 ± 2°C), pure colonies were picked up and re cultivated on Sabouraud's dextrose agar medium for further studies.
Selection of the most potent fungal isolate
Selection of the most potent fungal isolate was assessed by inoculation of all the purified fungal isolates (2 discs) into 250 ml Erlenmayer flasks containing 100 ml BMS broth media amended with 1% v/v of an equivalent mixture of xylene, naphthalene and anthracene. The flasks were incubated with shaking (150 rpm) at 30 ± 2°C. After 3 days the fungal biomass was obtained by filtering the culture broth through Watmann No. 1 filter paper. The recovered biomass samples were dried at 80°C to a constant weight and were weighed [14].
Chromatographic assessment of the degrading efficiency of the most potent fungal isolate
One hundred ml of BMS broth medium was supplemented with 1% v/v of the mixture (Xylene, naphthalene and anthracene) was inoculated with the fungal isolate (2 discs) and incubated for three days at 150 rpm and 30 ± 2°C. The residual mixture was extracted with equal volume of ethyl acetate; the organic layer was allowed to separate for 10 min and dried by passing through a funnel containing anhydrous sodium sulphate. For analysis, the used column was HP-5MS 5% Phenyl Methyl Silox (30 m × 250 μm × 0.25 μm) mounted in an Agilent 19091S-433: 3 gas chromatography mass spectrometer. Helium was used as carrier gas at flow rate 1 ml/min. The oven program was set at 60°C for 1 min then was raised by 20°C/min till it reached 160°C for 3 min followed by another raise by 3°C/min till it was reached to 250°C, then it was raised again by 20°C/min till the end at 300°C. The solvent delay time was set to 2.2 min. The run time was 41.5 min, the injector and transfer line temperatures were 250°C and 280°C respectively [15].
Identification of the most potent fungal isolate
The macroscopic morphology of the fungal isolate was studied on Sabouraud's dextrose agar medium [16]. The microscopic morphology of the fungal culture was also examined by preparing slide culture for the fungus on Sabouraud's dextrose agar medium [17]. The identification of the fungal isolate was determined on the basis of growth and microscopic morphology using the following universal manuals [18-20].
Optimization study
Effect of pH
Duplicate 250 ml Erlenmeyer flasks containing 100 ml of BMS broth medium were adjusted at different pH values (4, 5, 6, 7) and were inoculated with 1% (v/v) of the mixture (Xylene, naphthalene and anthracene) and a standard fungal inoculum (2 discs). The fungal was measured after 3 days of incubation with shaking (150 rpm) at 30 ± 2°C.
Effect of incubation temperature
Similarly for the incubation temperature, duplicate flasks containing the previously described broth medium were adjusted at the optimum pH, after inoculation the maximum dry weight yield was detected after 3 days of incubation under shaking at the following temperatures 25, 30, 35 and 40°C.
Effect of agitation speed
Also the effect of varying the speed of agitation (150, 200 and 250 rpm) was detected in a medium adjusted at the optimum pH and incubation temperature. For each treatment the corresponding control was recorded and the difference between them was considered to be due to the fungal degradation activity.
Assessment of the degradation efficiency of monoaromatic hydrocarbon mixture
BMS broth medium flasks were inoculated with 0.4% (v/v) of the mono aromatic mixture (Benzene, toluene, ethyl benzene and xylene) and were inoculated with the fungal isolate, at the end of the incubation period (4 days at 30 ± 2°C with intermittent shaking at 150 rpm), the residual monoaromatic mixture in both the control and the sample were chemically analyzed via GC-MS Purge Trap (7890A-5975C) (model 4660). The column used is HP-5MS 5% Phenyl Methyl Silox 325°C (30 m × 250 μm × 0.25 μm) mounted in an Agilent 19091S-433. Helium gas was used as a carrier gas with flow rate 1 ml/min. The purge trap used model is 4660. The thermal programming of the GC-Oven was started at 30°C and held for 5 min then raised to 100°C at the rate of 3°C/min and held for 1 min. The run time was 29.33 min. The inlet heater and pressure were adjusted at 250°C and 6.4845 psi respectively using a split mode with split ratio 1:40. An injection volume of 40 ml was used with Purge Flow to Split Vent of 40 ml/min at 0.75 min. After that, only 5 ml was taken by the trap to finally pass a volume of 1 ml to the mass spectrometer, where its transfer line was adjusted at 280°C at t˳ (min). MS 5975C was adjusted at the following parameters: solvent delay is 1.70 min and its EMV Mode is with relative voltage 200. The Scan Parameters ranged from Low Mass at 40.0 amu to 260 amu with EM volt equal 1400 and electron impact ionization 70 ev [21].
Assessment of the degradation efficiency of PAHs mixture
The experiment was performed in 100 ml of BMS broth medium inoculated with 1% (v/v) of PAHs mixture under optimum conditions, after two periods of incubation (24 h and 4 days) at 30 ± 2°C and 150 rpm the residual PAHs for the samples and control were extracted using chloroform in the ratio 3 sample: 1 chloroform and assessed using High Performance Liquid Chromatography (HPLC) of model Waters 600E equipped with auto sampler Waters 717 plus and dual wavelength absorbance detector Waters 2487 set at 254 nm. An Agilent column (LCPAH) of dimensions: 15 cm length, 4.6 mm internal diameter, 5 mm particle size was used with its guard. One micron was injected at flow rate of 1 ml/min and the run time was 50 min. Mobile phase; Acetonitrile: Water HPLC grades, gradient was 40% ACN at 0.66 min to 100% ACN at 22 min up to 38 min then return to 40% ACN at 40 min.
Concentrations of the sixteen most hazardous PAHs were calculated by comparing the peak areas of sample chromatogram with that of the peak area of the control [22].
Results and Discussion
Isolation and screening of the most potent biodegrading fungal isolate
A total of fourteen biodegrading fungal isolates were recovered from the collected water samples (9 isolates on xylene, 2 isolates on naphthalene and 3 isolates on anthracene), all the fungal isolates were recultivated on BMS medium supplemented with an equivalent mixture of xylene, naphthalene and anthracene. The results in Table 1 indicated that only 3 of the fungal isolates were capable of growing on the mixture, which may reflect their potentialities for biodegradation of the mixture components. This probably means that these isolates break down hydrocarbons and use the released energy to synthesize cellular biomass as reported by [23,24].
| Fungal code | Dry weight (g/l) |
|---|---|
| FX1 | - |
| FX2 | - |
| FX3 | - |
| FX4 | - |
| FX5 | - |
| FX6 | - |
| FX7 | 0.328 |
| FX8 | 1.028 |
| FX9 | - |
| FN10 | - |
| FN11 | 0.722 |
| FA12 | - |
| FA13 | - |
| FA14 | - |
Table 1: Assessment of the growth of the fourteen biodegrading fungal isolates on a mixture of xylene, naphthalene and anthracene
Assessment of the degradation efficiency of the most potent fungal isolate on a mixture of xylene, naphthalene and anthracene
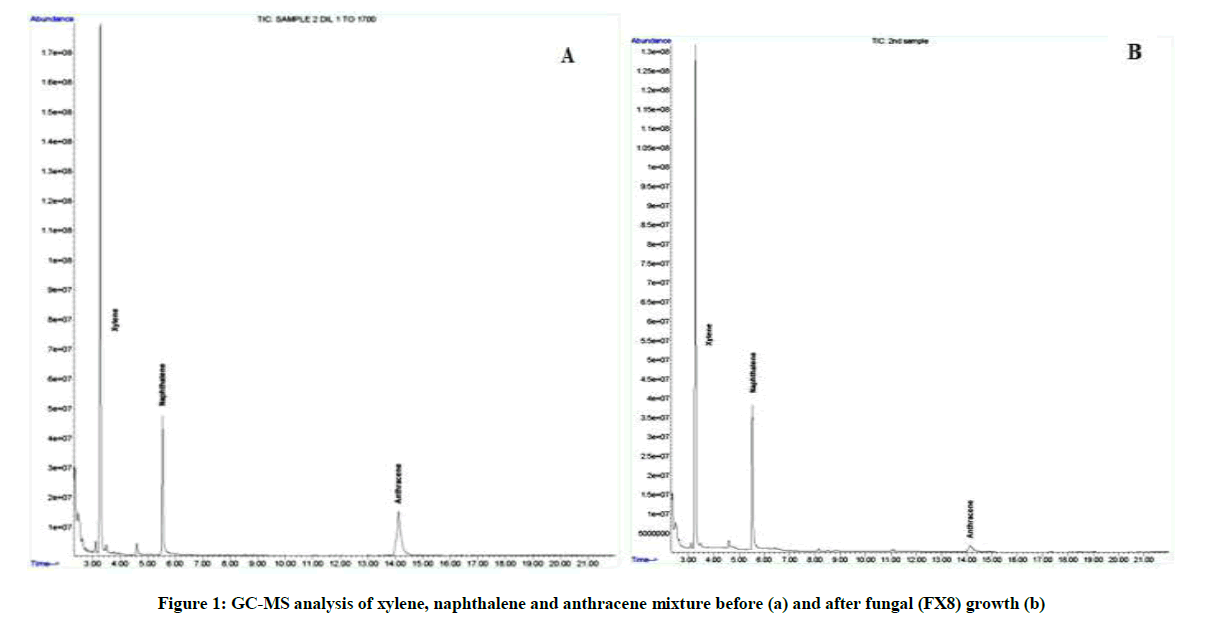
The most potent fungal isolate was subjected to quantitative assessment using GC-MS analysis to quantify its degradation potentiality toward the mixture of xylene, naphthalene and anthracene. The results obtained in Table 2 and Figure 1 showed the greater degradation capacity of the fungal isolate to the mixture components indicating that the increase in the fungal biomass is directly proportional to the degradation capacity as the fungus produce extracellular enzymes which metabolized the hydrocarbons and helped in biomass production and cell growth [25-27].
| Substrate | Concentration (ppm) | |||
|---|---|---|---|---|
| Control | Residue | Degraded | Degradation percentage (%) | |
| Xylene | 3169 | 588 | 2581.07 | 81.4 |
| Naphthalene | 38026 | 10292 | 27734 | 72.9 |
| Anthracene | 42464 | 2640 | 39825 | 93.7 |
| Total mixture | 83659 | 13519 | 70140 | 83.8 |
Table 2: Degradation percentage of the mixture of xylene, naphthalene and anthracene
In contrast to the fact; that low molecular weight PAHs are more susceptible to microbial attack than high molecular weight PAHs, it is obvious from the above data that the three ring hydrocarbon, anthracene, was the most degraded one (93.7%) among the other two compounds although its molecular weight is higher.
This can be referred to the fact that co-metabolism of one PAH could have a synergistic effect on the degradation of other PAHs, specifically in case of high molecular weight poly aromatics [28,29].
Identification of the most potent fungal isolate
Macroscopic characteristics
Colonies are lime green with wooly to cottony texture.
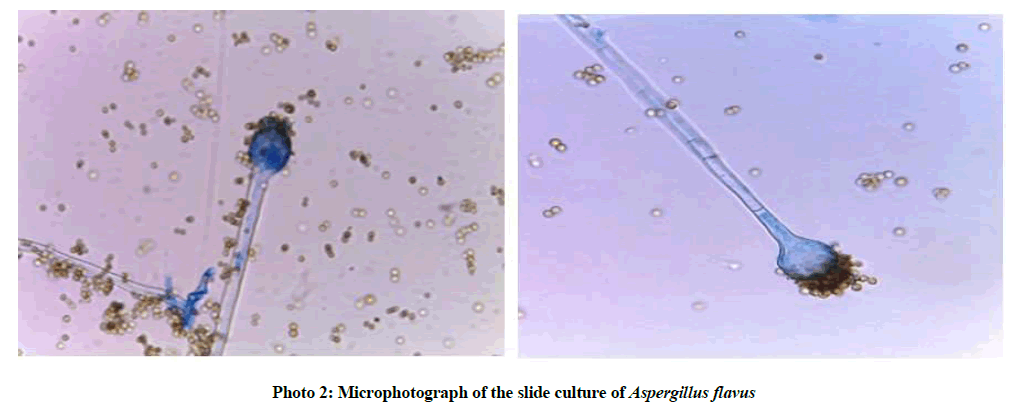
Microscopic characteristics
Hyphae are septated hyaline. Conidial heads radiate to loosely columnar conidiophores end with subglobose vesicle, matulae cover nearly the entire surface in biseriate form. Conidia are globose to ellipsoidal.
On the basis of the previously mentioned macroscopic and microscopic morphology, the most potent fungal isolate FX8 was identified as Aspergillus flavus (photo 1, 2) by the aid of the following universal manuals [18,20].
Optimization study
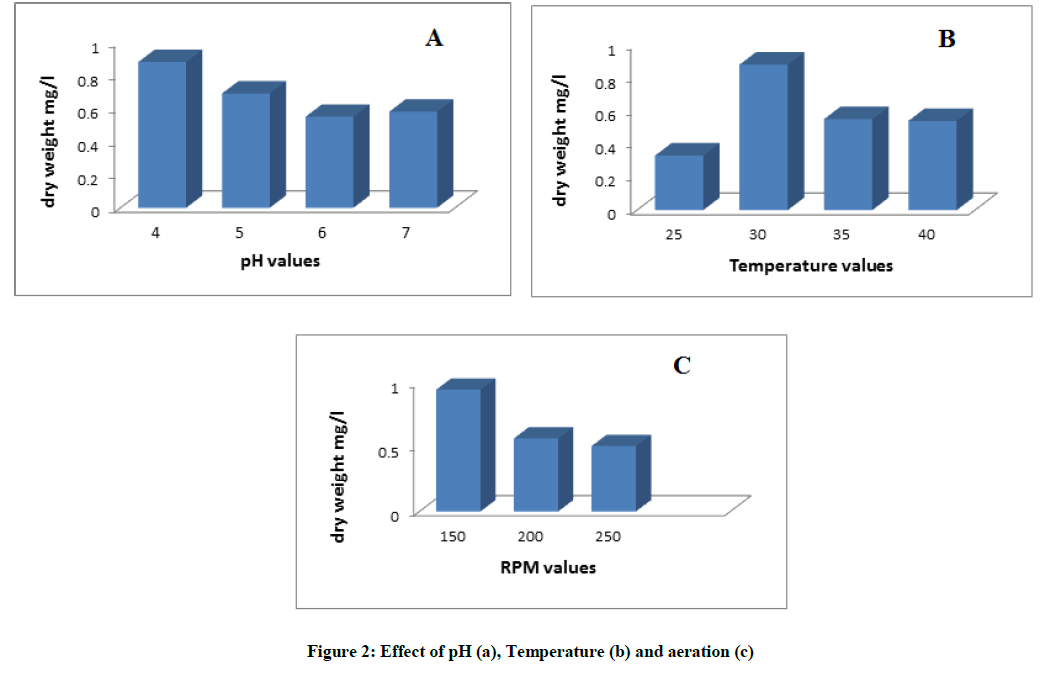
The pH value is considered as one of the main factors affecting the progress of the bioremediation process. Othman et al. [30] stated that, changes in pH can alter the electrical charges on various chemical groups in enzyme molecules. This can probably alter the enzyme's ability to bind to its substrate and catalyze a reaction. Imbalance of the electrical charges in very acidic and alkali conditions can disrupt hydrogen bonds and other weak forces that maintain enzyme structure. This disruption of the enzyme structure is called denaturation which can result in poor biodegradation process. The results obtained in Figure 2a indicated that pH 4 is the optimum one for fungal growth and consequently the biodegradation efficiency increasing the pH value led to decrease in the biomass yield. Similar results were obtained by Ali et al. [25] who found that the optimum pH for degradation of PAHs by A. terreus was 4.1.
Also, temperature plays an important role due to its effect on the solubility of PAHs and the physiology of the microbial flora [31]. In this experiment, different incubation temperature values were tested in order to select the optimum one at which both maximum growth and degradation percentage were attained. The results in Figure 2b showed that, maximum dry mycelial weight was recorded at 30°C, on increasing the temperature the dry weight was decreased. Similarly Moody et al. [32] reported that the biodegradation efficiency was higher at temperatures above 20°C also Ali et al. [25] indicated that the optimum temperature for biodegradation of PAHs by A. terreus lie in the mesophilic range.
During aerobic PAHs metabolism, oxygenase enzymes are the primary enzymes needed for degradation to occur Gopinathan et al. For this reason the molecular oxygen is required for the function of oxygenase as it is integral to the action of these enzymes in the initial catabolism of PAHs by both bacteria and fungi. Aerobic conditions are more favorable than anaerobic ones, where degradation rates are higher in aerobic conditions as compared to those under anaerobic conditions [33]. The present results in Figure 2c showed that the maximum dry weight was obtained at a shaking speed 150 rpm.
Assessment of the degradation efficiency of monoaromatic (BTEX) mixture:
Fungal degradation of monoaromatic compounds has clear implications for bioremediation, and the role of fungi in the removal of these contaminants from the environment has been a subject of extensive study. An understanding of the mechanisms involved in the degradation of benzenoid compounds and elucidation of the catabolic pathways is also important for predicting the recalcitrance of new products in the environment. Furthermore, enzymes catalyzing key steps in a catabolic pathway could be used in the design and operation of biosensors for detecting environmental pollutants.
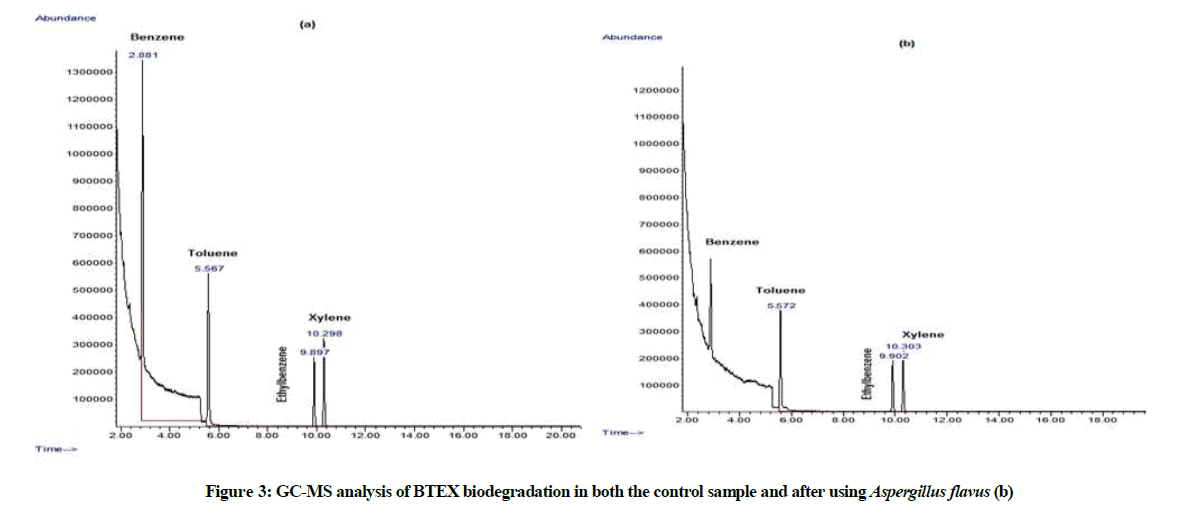
our results presented in Figure 3 and Table 3 indicated that GC-MS analysis of the biodegradation of the residual BTEX mixture, indicated the ability of A. flavus to utilize all mixture compounds efficiently as they share the same chemical structure, benzene was the most degraded one followed by toluene, xylene and finally ethyl benzene with degradation percentages of 88.78%, 74.22%, 71.65% and 69.78%, respectively and the total BTEX biodegradation is 81.97%. It has been known that; most filamentous fungi are able to mineralize or even transform monoaromatic compounds into other products of lowered toxicity [34,35].
| Compounds | Residue concentration (ppm) | Degradation percentage (%) | |
|---|---|---|---|
| Control | Sample | ||
| Benzene | 206.62032 | 23.19031 | 88.78 |
| Toluene | 95.69141 | 24.67295 | 74.22 |
| Ethyl-Benzene | 25.38381 | 7.67067 | 69.78 |
| Xylene | 34.37181 | 9.74495 | 71.65 |
| Total | 362.06735 | 65.27888 | 81.97 |
Table 3: Assessment of BTEX biodegradation using Aspergillus flavus
In accordance with our results Prenafeta-Boldu et al. [36] have reported the use of the fungus Cladophialophoria sp in BTEX mineralization and found that; toluene, ethylbenzene and m-xylene were intensely decomposed than benzene. Another example is the common fungus Phanerochaete chrysosporum with its ability of biodegradation of mono aromatic compounds due to the production of extracellular enzymes that participate in their decomposition [35].
Assessment of the degradation efficiency of PAHs mixture
PAHs are widely distributed environmental contaminants that have detrimental biological effects. These groups of compounds are environmentally stable due to their aromatic nature including stabilization by multiple rings [37]. Despite these properties a variety of fungal species have the ability to synthesize unspecific enzymes that can be used in their degradation.
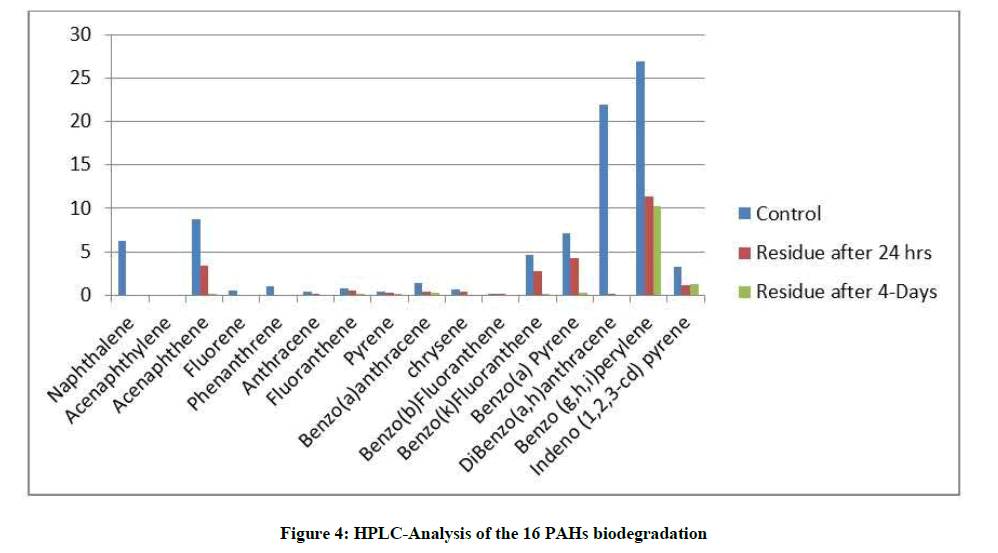
By comparing the HPLC profiles at different time periods Table 4 and Figure 4 it was observed that there was a significant decrease in the peak areas corresponding to each compound reflecting its degradation. After 24 h of incubation 70% of the total poly aromatic mixture was degraded, three compounds (Naphthalene, fluorine and Phenanthrene) were completely degraded as the low molecular weight poly aromatic compounds were easily degraded than the higher molecular weight ones [38]. After 4 days of incubation the degradation percentage was increased to nearly 85.4% with the disappearance of four additional compounds. Fast growth of the isolate A. flavus and its ability to completely mineralize a number of compounds reflects its affiliation to what is called observed adapted communities [39].
| After 4 days | After 24 h | Control (mg/l) | Compounds | ||
|---|---|---|---|---|---|
| Degradation | Residue | Degradation | Residue | ||
| Percentage (%) | (mg/ml) | Percentage (%) | (mg/ml) | ||
| 100 | 0 | 100 | 0 | 6.21595 | Naphthalene |
| 0 | 0 | 0 | 0 | 0 | Acenaphthylene |
| 99.75 | 0.0223 | 62.13 | 3.334 | 8.80328 | Acenaphthene |
| 100 | 0 | 100 | 0 | 0.54502 | Fluorine |
| 100 | 0 | 100 | 0 | 1.0018 | Phenanthrene |
| 100 | 0 | 92.44 | 0.0297 | 0.39251 | Anthracene |
| 93.81 | 0.0521 | 39.63 | 0.5081 | 0.84162 | Fluoranthene |
| 99.04 | 0.0045 | 48.36 | 0.2408 | 0.46629 | Pyrene |
| 75.51 | 0.337 | 66.98 | 0.4544 | 1.37601 | Benzo (a)anthracene |
| 100 | 0 | 39.63 | 0.3951 | 0.6544 | Chrysene |
| 100 | 0 | 81.17 | 0.004 | 0.02104 | Benzo (b)Fluoranthene |
| 99.97 | 0.0012 | 39.63 | 2.8055 | 4.64686 | Benzo (k)Fluoranthene |
| 95.81 | 0.2988 | 39.63 | 4.3067 | 7.13338 | Benzo (a)Pyrene |
| 100 | 0 | 99.43 | 0.1255 | 21.89143 | DiBenzo (a,h)anthracene |
| 61.97 | 10.2581 | 57.74 | 11.399 | 26.97177 | Benzo (g,h,i)perylene |
| 61.89 | 1.255 | 63.25 | 1.2104 | 3.29342 | Indeno (1,2,3-cd)pyrene |
| 85.485 | 12.229 | 70.549 | 24.813 | 84.25484 | Total |
Table 4: HPLC analysis of the biodegradation of the most hazardous PAHs using Aspergillus flavus
Similar findings were observed by Bhattacharya et al. [40], Vidali [41] and Olukunle et al. [42] which made the application of A. flavus to bioremediation efforts is suitable, also Damisa et al. [26] reported that A. flavus can degrade a wide range of poly aromatic compounds and revealed this to the higher production of extracellular enzymes and organic acids that enable them to utilize these compounds fastly.
Conclusion
Bioremediation is the tool to transform the compounds to less hazardous/non-hazardous forms with less input of chemicals, energy, and time. It is an approach to remove pollutants in an eco-friendly manner. From the previous study it could be concluded that the isolated fungus A. flavus exhibited significant degradation of a mixture of PAHs (85.4%) after only 4 days of incubation with the complete disappearance of eight hazardous compounds, so it is highly recommended to be used in the treatment of water contaminated by PAHs.
References
- A. Pawar, S. Ugale, M. More, N. Kokani, S. Khandelwal, J. Bioremed. Biodeg., 2013, 4(7), 1-5.
- S. Bisht, P. Pandey, B. Bhargava, S. Sharma, V. Kumar, K.D Sharma, Braz. J. Microbiol., 2015, 46(1), 7-21.
- R.K. Jain, M. Kapur, S. Labana, B. Lal, P.M. Sarma, D. Bhattacharya, I.S. Thakur, Curr. Sci., 2005, 89, 101-112.
- B. Thapa, K.C. Ajay Kumar, A. Ghimire, KUSET., 2012, 8(1), 164-170.
- J. Prathiba, K. Keerthi, A. Deshpande, S. Bhattacharya, I. Priyadarsini, J. Biochem. Tech., 2012, 5(3), 727-730.
- G. Kumar, R. Singla, R. Kumar, Nature and Science., 2010, 8, 89-94.
- P. Trindade, L. Sobral, A. Rizzo, A.S. Leite, A. Soriano, Chemosphere., 2005, 58, 545-522.
- H. Zhao, L. Wang, I. Ren, Z. Li, M. Li, H. Gao, J. Hazard. Mater., 2008, 152, 1293-1300.
- A. Banerjee, A. Roy, S. Dutta, S. Mondal, IJAR., 2016, 4(6), 1303-1313.
- T. April, J. Foght, R. Currah, Canadian J. Microbiol., 2000, 46(1), 38-49.
- S. Le Borgne, D. Paniagua R. Vazquez-Duhalt, J. Mol. Microbiol. Biotechnol., 2008, 15, 74-92.
- K. Bishnoi, R. Kumar, N. Bishnoi, J. Sci. Indust. Res., 2008, 67, 538-542.
- H. Li, Y.H. Liu, N. Luo, X.Y. Zhang, T.G. Luan, J.M. Hu, Res. Microbiol., 2006, 157, 629-636.
- F. Ameen, M. Moslem, S. Hadi, A. Al-Sabri, Plastics & Recycling Technology., 2015, 31(2), 125-144.
- M. Wu, M. Nie, X. Wang, J. Su, W. Cao, Spectrochimica Acta., 2010, 75, 1047-1050.
- L. Ajello, L.K. Georg, W. Kaplan, Laboratory manual for medical mycology, PH SPNO. 994. US. Government printing office, Washington, Dc., 1962.
- D.E. Riddle, Mycologia., 1950, 42, 265.
- L. John, Pitt J.I.A Laboratory Guide to Common Penicillium Species, 2nd Ed., Commonwealth Scientific and Industrial Research Organization, 1994.
- G.S. De Hoog, J. Guarro, Atlas of Clinical Fungi, 1st Edi., CBS, Baarn, Netherland, 1995.
- B. Kendrick, The Fifth Kingdom, 5th Edi., Focus publishing, Newbury port MA 01950USA, 2000.
- A. Nicholson, B.Z. Fathepure, Appl. Environ. Microbiol., 2004, 70(2), 1222-1225.
- B. Lal, S. Khanna, J. Appl. Bacteriol., 1996, 81, 355-362.
- B. Shaw, Oil and Gas J., 1995, 4, 85-88.
- R. Keeler, Reseach and Development Magazine., 1995, 2, 139-140.
- M.I. Ali, N.M. Khalil, M.N. Abd El-Ghany, Afric. J. Microbiol. Res., 2012, 6(16), 3783-3790.
- D. Damisa, T.S. Oyegoke, U.J.J. Ijah, N.U. Adabara, J.D. Bala, R. Abdulsalam, IJABPT., 2013, 4(2), 136-140.
- B. Meera, P. Madhanraj, A. Panneerselvam, C. Chandran, Int. J. Res. Sci. Res., 2016, 7(12), 14828-14830.
- K. Saranya, T. Palanisami, M. Mallavarapu, N. Ravi, Int. Biodeterior. Biodegrad., 2016, 108, 149-157.
- A. Sharma, S.B. Singh, R. Sharma, P. Chaudhary, A.K. Pandey, R. Ansari, V. Vasudevan, A. Arora, S. Singh, S. Saha, J. Environ. Manag., 2016, 181, 728-736.
- N. Othman, N.H. Hussain, A.T. Abd Karim, S. Abdul-Talib, International conference on sustainable infrastructure and built environment in developing countries, Bandung, West Java, Indonesia, 2009, 101-105.
- C.A. Baraniecki, J. Aislabie, J.M. Foght, Microb Ecol., 2002, 43, 44-54.
- J.D. Moody, J.P. Freeman, P.P. Fu, C.E. Cerniglia, Appl. Environ. Microbiol., 2004, 70(1), 340-345.
- R. Gopinathan, M. Prakash, R. Bharathirajan, Int. J. Curr. Microbiol. Appl. Sci., 2012, 1, 12-16.
- R. Singh, S.M. Celin, J. Ecobiotech., 2010, 2(4), 27-32.
- A.A. Olajire, J.P. Essien, J. Pet. Environ. Biotechnol., 2014, 5(5), 1-22.
- F.X. Prenafeta-Boldú, J. Vervoort, J.T.C. Grotenhuis, J.W. Van Groenestijn, Appl. Environ. Microbiol., 2002, 68, 2660-2665.
- Y.I. Tatyana, I.S. Olesya, O.N. Maxim, L.S. Sergei, A.K. Irina, Res. Microbiol., 2015, 164, 244-253.
- P. Oleszczuk, S. Baran, Pol. J. Environ. Stud., 2003, 12(4), 431-437.
- B. Gerdes, R. Brinkmeyer, G. Dieckmann, E. Helmke, FEMS Microbiol. Ecol., 2005, 53, 129-139.
- D. Bhattacharya, P.M. Sarma, S. Krishnan, S. Mishra, B. Lal, Appl. Environ. Microbiol., 2003, 69(3), 1435-1441.
- M. Vidali, J. Appl. Chem., 2005, 73(7), 1163-1172.
- O.F. Olukunle, T.S. Oyegoke, Nig. J. Biotech., 2016, 31, 46-58.