Research Article - Der Pharma Chemica ( 2020) Volume 12, Issue 7
Biochemical Study of Hepatoprotective Impact of Aloe Vera Via Overdose of Paracetamol in Albino Rats
Doha Y. Rashed1, Basant M. Morsy1* and MA kandeel22Biochemistry Division, Biochemistry Department, Faculty of veterinary, Beni-Suef University, Egypt
Basant M. Morsy, Biochemistry Division, Chemistry Department, Faculty of Science, Beni-Suef University, Egypt, Email: h_gendy_2010@yahoo.com
Received: 17-Aug-2020 Accepted Date: Nov 22, 2020 ; Published: 28-Nov-2020
Abstract
Aloe Vera is an evergreen perennial plant species of the genus Aloe, it is cultivated in the Arabian Peninsula but germinates wild in equatorial climates around the world and is implanted for agricultural and medicinal uses. This study aimed to demonstrate the hepatoprotective impact of Aloe vera extract against paracetamol [PCT] in albino rats. Hepatotoxicity was induced in rats by gastric intubation of 500 mg/kg b.wt /day for 4 weeks. These rats were orally administered with Aloe vera extract at dosage of 500 mg/kg b.wt /day for 6 weeks. The treatment with Aloe vera remarkably improved the deleterious effect of PCT on oxidative stress and antioxidant defense system. That was reflected by increase of glutathione and deficiency in lipid peroxidation and catalase. The pro-inflammatory cytokine, TNF-α, and tumor marker, AFP, levels exhibited a marked increase in serum of PCT-administered rats comparable to the normal ones. The treatment of PCT-administration rats with Aloe vera caused marked amelioration of these estimated variablesIn conclusion, Aloe vera extracts attenuate hepatotoxicity of Paracetamol by decreasing oxidative stress and reducing liver injury.
Keywords
Acetaminophen, Oxidative stress, Antioxidant, Hepatotoxicity, Hepatoprotective effect, Aloe Vera
Introduction
Liver is a major organ system involved in the digestion of various drugs, xenobiotics & toxins. During the metabolism, many free radicals are produced & may cause liver damage [1]. The liver is responsible for the metabolic homeostasis of the body including biotransformation, detoxification and excretion of many endogenous and exogenous compounds [2]. Also [3] stated that therapeutic dose of acetaminophen or paracetamol [PCT] or N-acetyl-p-aminophenol [APAP] is deemed to be safe but an overdose can cause poisoning and a potentially lethal hepatotoxicity. Normally, 85–90% of APAP is eliminated through conjugation with glucuronide or sulfate in human body; about 2% is directly aberuncated into urine unchanged; and only <10% of APAP is digested by cytochrome P450 into toxic metabolite NAPQI. The toxicity of PCT depends on the metabolic activation via cytochrome P- 450, which lead to production of an electrophilic reactive metabolite, N-acetyl-p-benzoquinone imine [NAPQI] in hepatocytes and sinusoidal endothelial cells. NAPQI is quickly turned by hepatic glutathione into a nontoxic paracetamol-mercaptate compound that is renally excreted. Paracetamol overdose causes glutathione depletion and covalent protein modifications leading to mitochondrial dysfunction with generation of reactive oxygen species [ROS] and peroxynitrite. In addition, oxidative stress, comprised lipid peroxidation can cause irreversible membrane injury and cell death. The oxidant stress ultimately performs to the opening of the mitochondrial membrane permeability transition [MPT] pore and necrotic cell death [4]. Herbal medicines made of plant extracts are being used overmuch to treat a wide assortment of clinical disease. More alertness has been paid to the protective impacts of natural antioxidants versus chemically induced toxicities. Many scientists suggested that plant-based remedies are safer and more effectual in the medication of numerous liver lesions. Aloe vera is considered the most popular plants used in alternative medicine and chemically consists of polysaccharide, anthraquinone and carbohydrate. It is utilized for the treatment of hepatitis, neoplasms, ulcer and wound. The plant has anti- inflammatory properties and antigenotoxic, chemo preventive, antioxidative and antiviral effects. Aloe vera acts as treatment for liver problems and as a hepatic stimulant [5], also it is one of the herbal remedies known for its remedial and therapeutic properties for various diseases. It possesses remarkable protective and therapeutic potential versus diverse cancers. The bioactive components of Aloe vera with chemo preventive potential such as aloin, lectin, aloe-emodin, barbaloin, and aloesin have shown to possess immune- potentiating, anti-mutagenic, anti-proliferative, apoptosis-inducing, antioxidant, and anti- metastatic potential [6].
Materials and Methods
Experimental Animals
Male albino rats weighing between 100-120 g were utilized as experimental animals in the present research. Rats were procured from the Animal House of Abou Rawash, El-Giza, Egypt. They were conserved under remarking for about 15 days before the beginning of the experiment to preclude any intercurrent infection. The collected animals were kept in metal [stainless steel] separate bottom cages at normal atmospheric temperature [25 ± 5°C] also under good ventilation and taken water and standard balanced diet. All the procedures were performed according to the Institutional Animal Ethics Committee in Beni-Suef University recommendations.
Collection and Preparation of Plant Materials
Method of preparation of aqueous extract of aloe vera
Aloe vera leaves were rinsed in ordinary water and the juice was obtained by gently pressing the leaves. The yield was computed based on the weight of the extract compared to the weight of the leaves. The yield was 90 g per 100 g of the leaves. The sample of the extract was standardized by the method of Patil RM [7].
Experimental Design and doses
The number of rats utilized in the present study is 18 rats. They were divided into 3 groups, six for each group, designed as follow:
Group 1: This group was regarded as normal control group and was given the equivalent volume of dist. Water as a single dose daily by gastric intubation [stomach tube].
Group 2: It was given dose of PCT 500 mg/kg b.w. [8] liquefied in distilled water daily by gastric intubation for 4 weeks.
Group 3: The rats of this group were administered dose of PCT [500 mg/kg b.w.] daily for 4 weeks and were also treated with Aloe vera [500 mg /kg b. w. /day] [9], dissolved in dist. Water daily by gastric intubation for 6 weeks.
Sampling and tissue preparation
At the fine of this experiment, six animals of normal, PCT-administered control rats and PCT-administered rats treated with Aloe vera were sacrificed under mild diethyl ether anesthesia and then blood from these rats was collected in a centrifuge tube from jugular vein and kept to clot for 45 minutes at room temperature. Sera were separated in centrifuge at 3000 r.p.m. for 15 minutes at 30ºC and preserved frozen at -30ºC waiting for biochemical analyses. Liver from these animals was quickly eviscerated after dissection.
Homogenate preparation
0.5g from liver was homogenized utilizing Teflon homogenizer [Glas-Col, Terre Haute, USA] in 5 ml 0.9% sterilized sodium chloride [NaCl] [10% w/v] for biochemical analyses.
Assessment of Biochemical assays
Liver tissues were rapidly separated after dissection of rats. Part of these tissues was homogenized for determination of Liver lipid peroxidation by measuring thiobarbituric acid reactive substances [TBARS] [10-12]. Catalase activity determined [13] which follows the first-order kinetics as given by the equation: K = log [S0 / S3] X 2.3 / t. Liver glutathione [GSH] was measured according to the procedure of [14]. Sera were quickly isolated for subsequent biochemical analyses; pro-inflammatory cytokine [TNF-α] was determined in accordance with the method [15-17]. And alfa feto protein [AFP] [18-21]. by using specific ELISA kits Quantikine Rat Total Adeponectin Immunoassay [USA], liver functions that include [ALT, AST] were measured in accordance with the procedure of Burtis 2005 [22], ALP was determined according to the procedure [23], GGT was measured in accordance with the process of Szasz [24], [total protein and albumin], [total, direct and indirect] Bilirubin] were determined in accordance with the method of Doumas [25], kidney functions that include [urea was determined by the procedure of Patton and Crouch [26], creatinine was measured by the procedure of Henry [27] and uric acid was determined by the method of Fossati [28], heart function parameters in serum that include LDH was measured by the method of Witt and Trendelenburg [29] and CK-MB was determined according to the process[30].
Statistical analysis
The Statistical Package of the Social Sciences [IBM SPSS for WINDOWS7, version 20; SPSS Inc, Chicago] was used for the statistical analysis of the results. Comparative analysis was conducted by using the general linear models’ procedure [IBM SPSS]. p>0.05 were considered statistically non-significant, while p<0.05 considered statistically significant.
Results
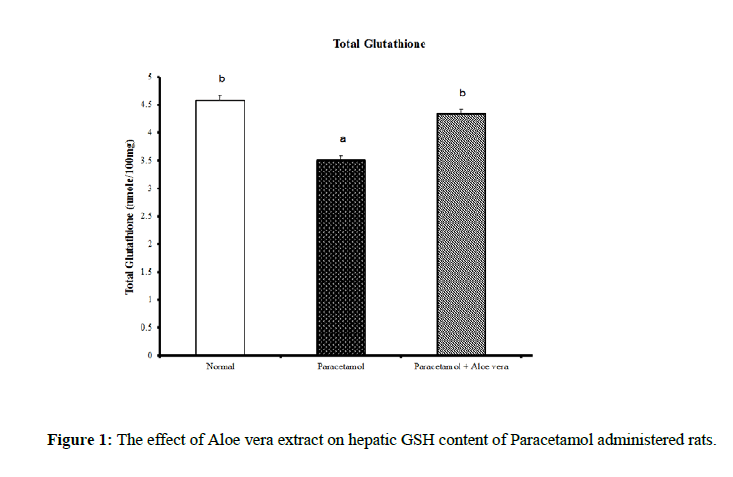
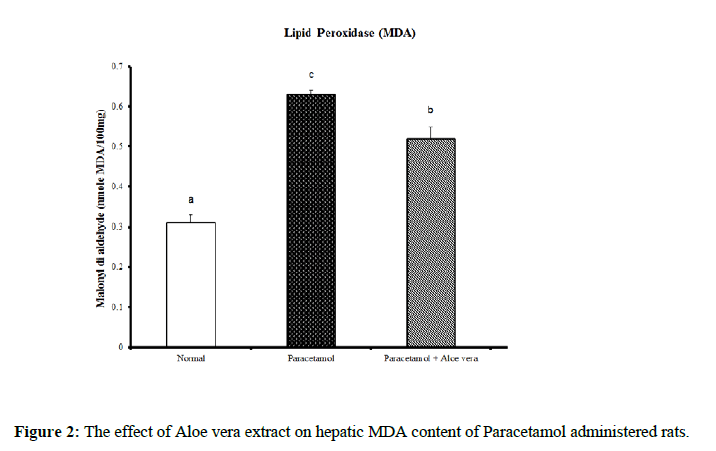
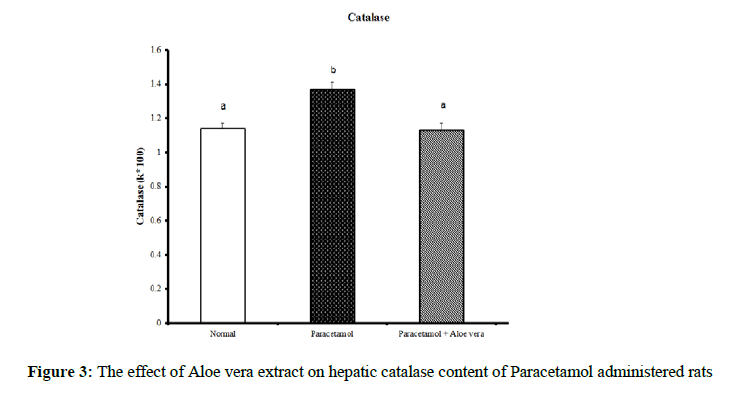
Data summarized in Table 1 and (Figure 1) showed the impact of Aloe vera extract on the liver oxidative stress markers and antioxidant defense system of paracetamol-administered rats. Hepatic glutathione content [GSH] was significantly decreased because of PCT overdosing But after the treatment of PCT-administered rats with Aloe Vera for 6 weeks produced a significant increase of these oxidative stress values comparably to the corresponding PCT administered group pointing to a marked normalization in comparison of normal group. Over, rats mainlined with PCT exhibited a noticeable increase in Hepatic lipid peroxidase [LPO] and catalase [CAT] activity (Figures 2 & 3) were significantly increased because of PCT overdosing however the treatment of PCT-administered rats with Aloe Vera for 6 weeks significantly decreased these raised values and becomes adjacent to the normal values.
| parameter Group |
T.Glutathione [n.mol/100mg] |
MDA [n.mol/100ml] |
CATALASE [k*100] |
|---|---|---|---|
| Normal | 4.58 ± 0.09 b | 0.31 ± 0.02 a | 1.14 ± 0.03 a |
| Paracetamol | 3.51 ± 0.07 a | 0.63 ± 0.01 c | 1.37 ± 0.04 b |
| Paracetamol + Aloe vera |
4.34 ± 0.08 b | 0.52 ± 0.03 b | 1.13 ± 0.04 a |
| LSD | 0.23 | 0.03 | 0.05 |
*Values significantly different to control at [p≤0.05].*Data are written as mean ± SE. *Values that have the same superscript symbol are not significantly different. *F-Probability: P < 0.05
Table 1: The impact of Aloe vera extract on hepatic antioxidants of paracetamol-administered rats.
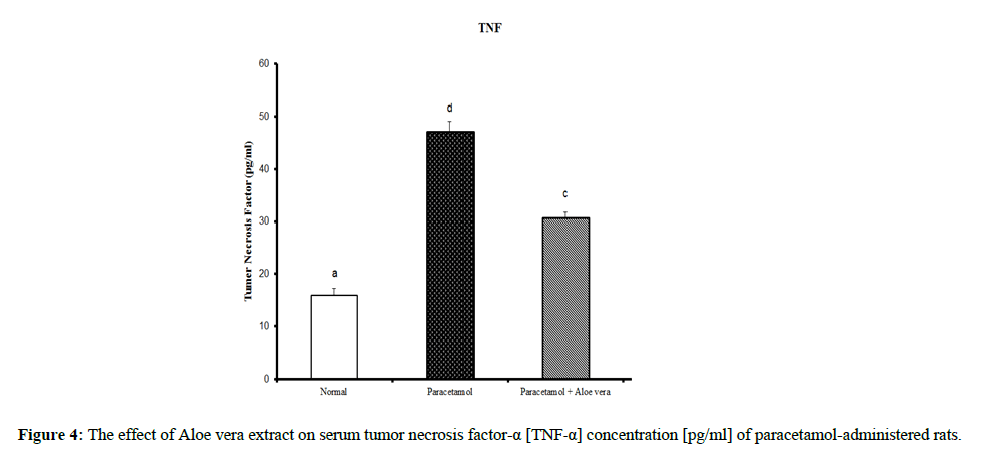
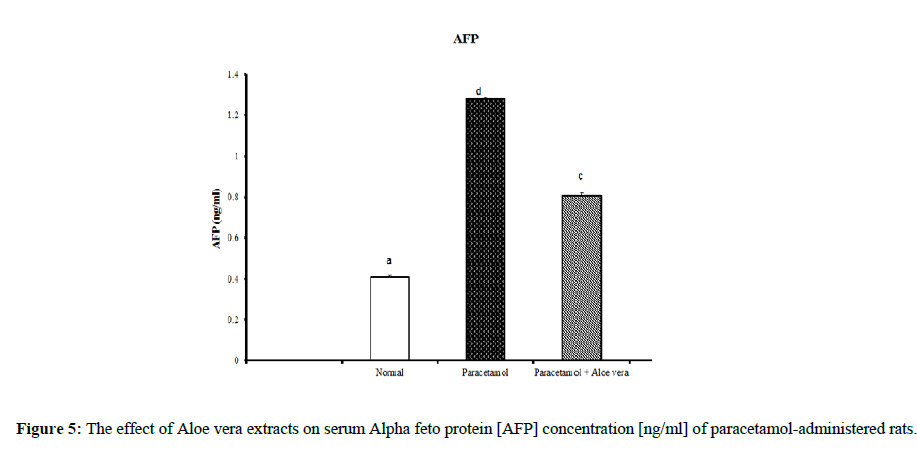
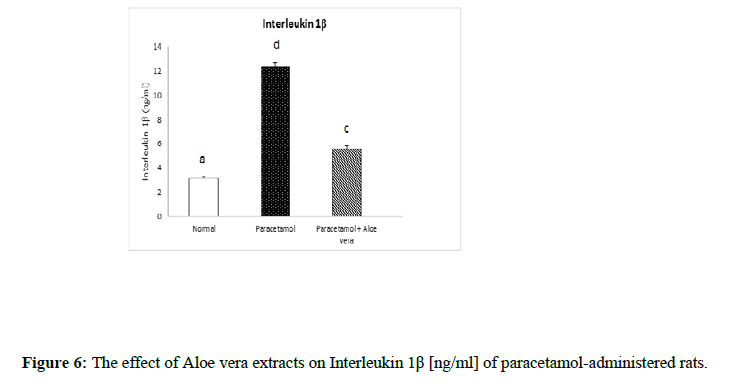
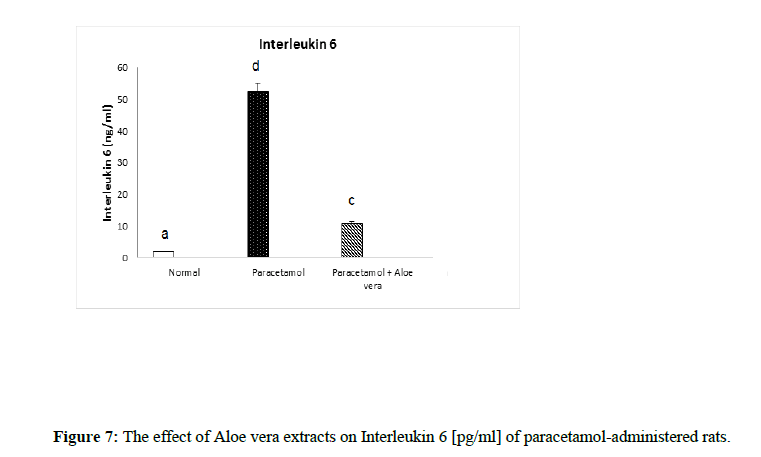
Table 2 and (Figure 4 & 5) showed the impact of Aloe vera extract on serum tumor necrosis factor-α [TNF-α] concentration [pg/ml] and alpha feto protein [AFP] concentration [ng/ml] of paracetamol- administered rats. Table 3 (Figure 6 & 7) showed the impact of Aloe vera extract on Interleukins [IL 1β and IL 6] of paracetamol-administered rats. Our results revealed that the administration of PCT produced marked impairment demonstrated by significant increase in these hepatic inflammatory factors comparable to normal rats, while Oral administration of Aloe Vera significantly minimized these elevated levels of Hepatic TNF-α, AFP, IL-6 and IL-1β when compared with the PCT administered rats recording noticeable amelioration comparably to normal ones.
| Parameter Group | TNF [pg/ml] |
AFP [ng/ml] |
|---|---|---|
| Normal | 15.88 ± 1.38 a | 0.41 ± 0.008 a |
| Paracetamol | 47.13 ± 1.92 d | 1.28 ± 0.009 d |
| Paracetamol + Aloe vera | 30.66 ± 1.14 c | 0.81 ± 0.01 c |
| LSD | 2.02 | 0.02 |
*Values significantly different to control at [p≤0.05]. *Data are written as mean ± SE.
*Values that have the same superscript symbol are not significantly different. *F-Probability: P < 0.05
Table 2: The impact of Aloe vera extract on serum tumor necrosis factor-α [TNF-α] concentration [pg/ml] and alpha feto protein [AFP] concentration [ng/ml] of paracetamol-administered rats
| parameter Group |
IL 1 β [ng/ml]]L |
IL 6 [ng/ml] |
|---|---|---|
| Normal | 3.16 ± 0.10 a | 1.91 ± 0.15 a |
| Paracetamol | 12.36 ± 0.27 d | 52.41 ± 1.08 d |
| Paracetamol + Aloe vera |
5.59 ± 0.23 c | 11 ± 0.79 c |
| LSD | 0.31 | 0.05 |
*Values significantly different to control at [p≤0.05]. *Data are written as mean ± SE.
*Values that have the same superscript symbol are not significantly different. *F-Probability: P < 0.05
Table 3: The impact of Aloe vera extract on Interleukins [IL 1β and IL 6] of paracetamol-administered rats.
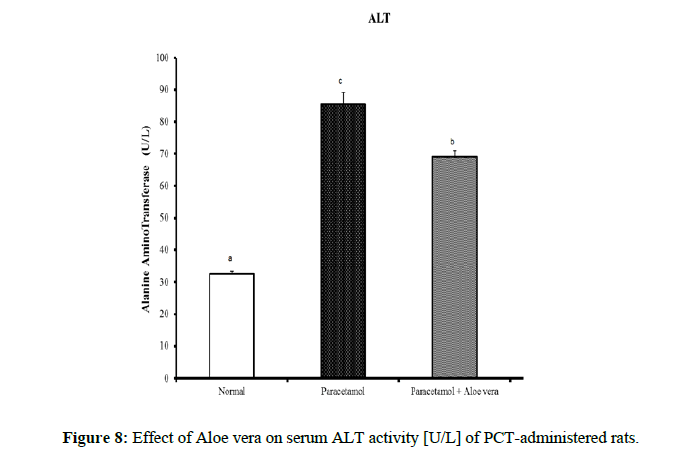
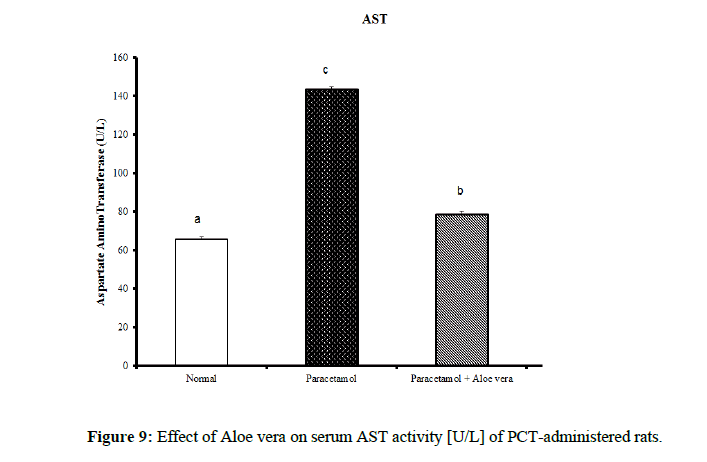
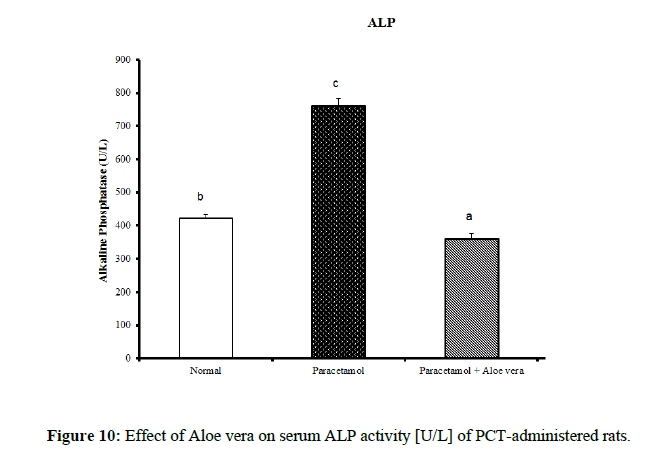
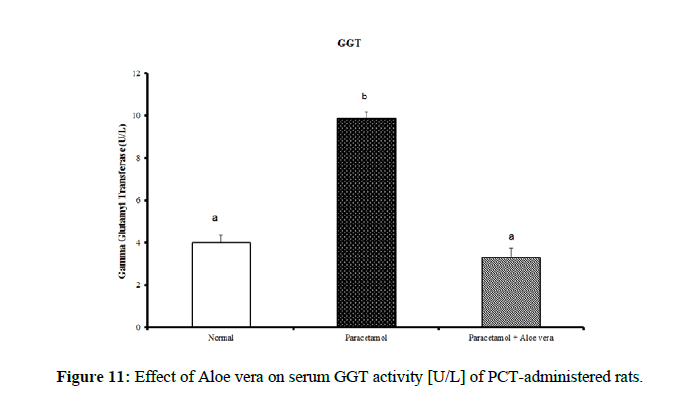
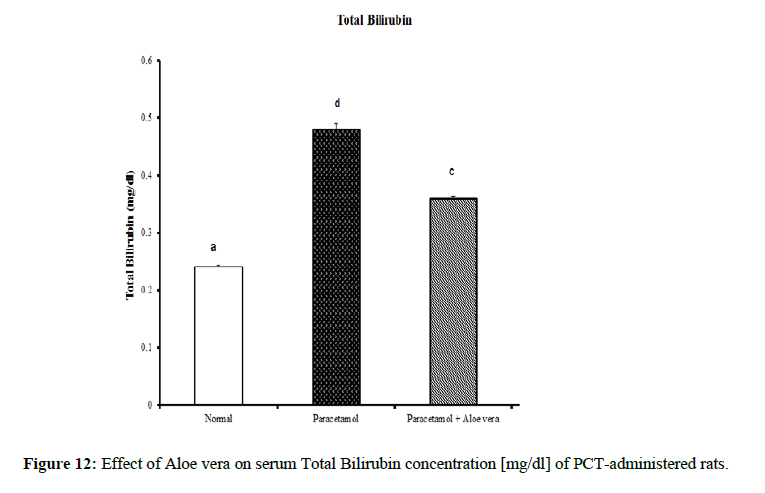
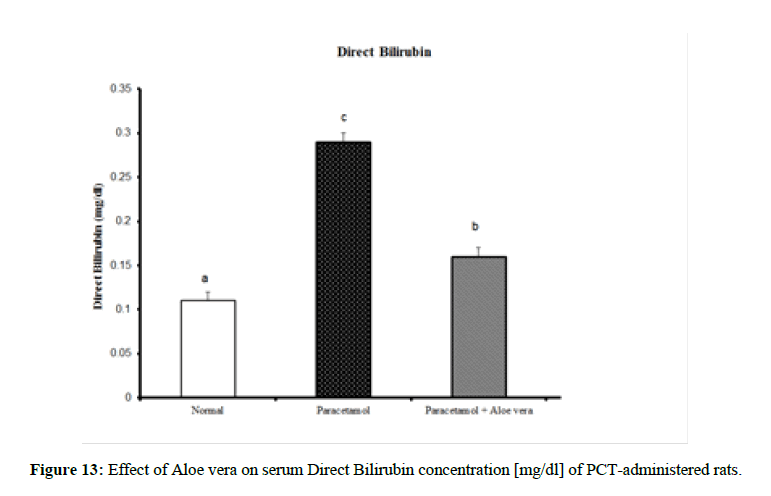
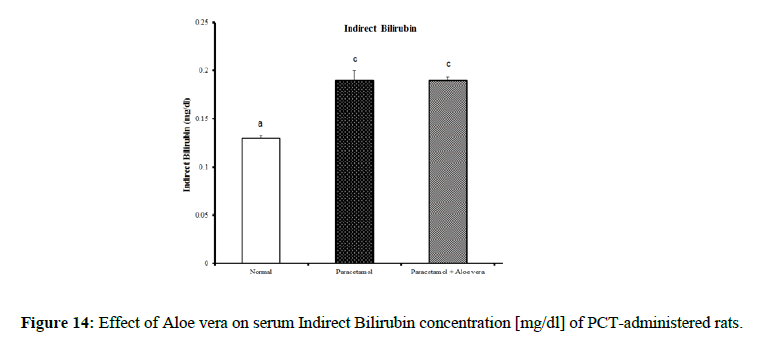
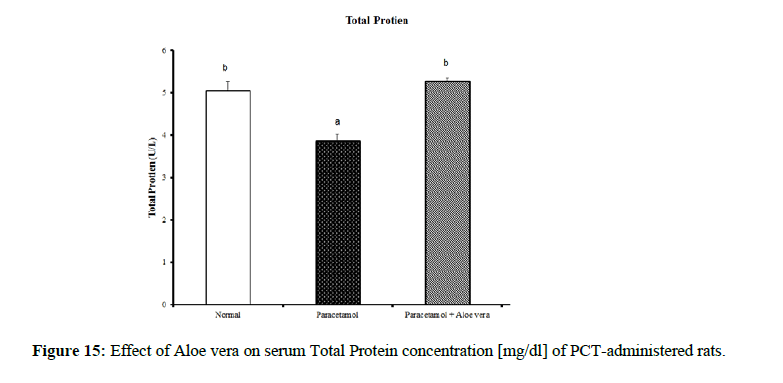
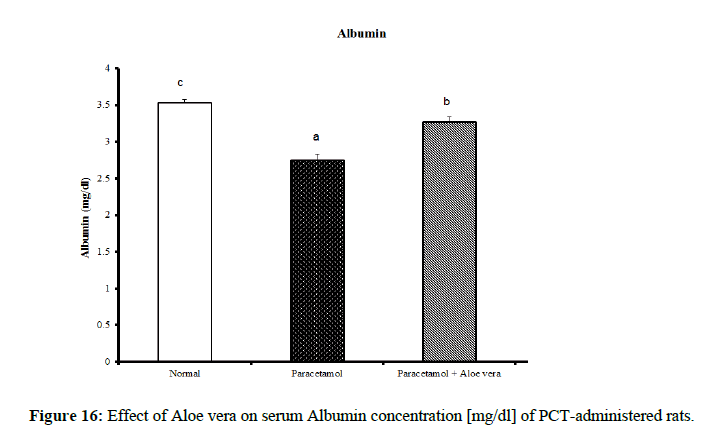
Table 4 showed the impact of Aloe vera extract on serum liver function parameters of paracetamol-administered rats, indicated marked elevation in the serum enzymes' activities of liver function [AST, ALT, ALP and GGT] (Figure 8 & 9) together with an allowance (Figure 10 & 11) in total protein and albumin concentration. (Figure 12-16) Also, Table 5 showed the impact of Aloe vera extract on serum kidney function parameters of paracetamol administered rats.
| Parameter | ALT | AST | Alp | GGT | Total Bilirubin | Direct Bilirubin | Indirect Bilirubin | Total Protein | Albumin |
|---|---|---|---|---|---|---|---|---|---|
| Group | [U/L] | [U/L] | [U/L] | [U/L] | [mg/dl] | [mg/dl] | [mg/dl] | [mg/dl] | [mg/dl] |
| Normal | 32.5 ± 1.09 a | 65.67 ± 1.28 a | 422.67 ± 10.52 b | 4.00 ± 0.37 a | 0.24 ± 0.004 a | 0.11 ± 0.01 a | 0.13 ± 0.003 a | 5.05 ± 0.23 b | 3.53 ± 0.05 c |
| Paracetamol | 85.5 ± 3.69 c | 143.33 ± 1.54 c | 762.50 ± 19.12 c | 9.88 ± 0.32 b | 0.48 ± 0.01 d | 0.29 ± 0.01 c | 0.19 ± 0.01 c | 3.87 ± 0.15 a | 2.75 ± 0.08 a |
| Paracetamol + Aloe vera | 69 ± 1.95 b | 78.50 ± 1.65 b | 361.50 ± 15.20 a | 3.33 ± 0.42 a | 0.36 ± 0.004 c | 016 ± 0.01 b | 0.19 ± 0.003 c | 5.28 ± 0.07 b | 3.27 ± 0.07 b |
| LSD | 3.12 | 2.07 | 20.11 | 0.52 | 0.01 | 0.01 | 0.01 | 0.27 | 0.11 |
*Values significantly different to control at [p≤0.05]. *Data are written as mean ± SE.
*Values that have the same superscript symbol are not significantly different. *F-Probability: P < 0.05
Table 4: The impact of Aloe vera extract on serum liver function parameters of paracetamol-administered rats.
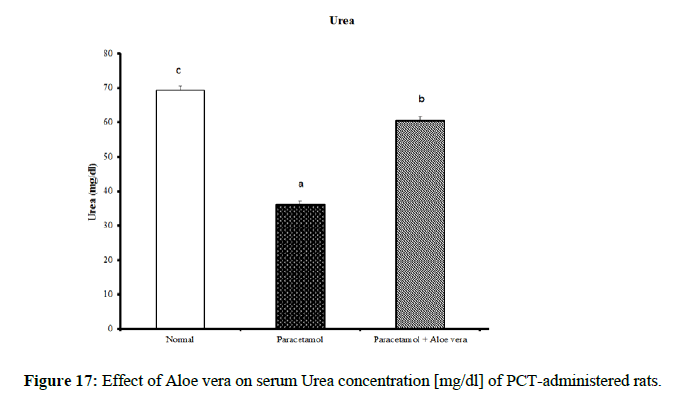
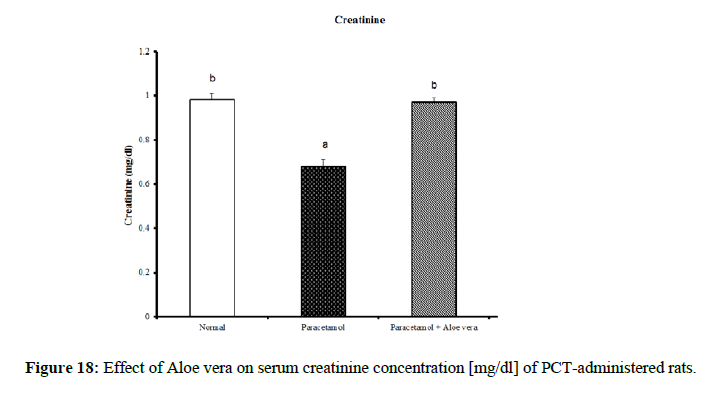
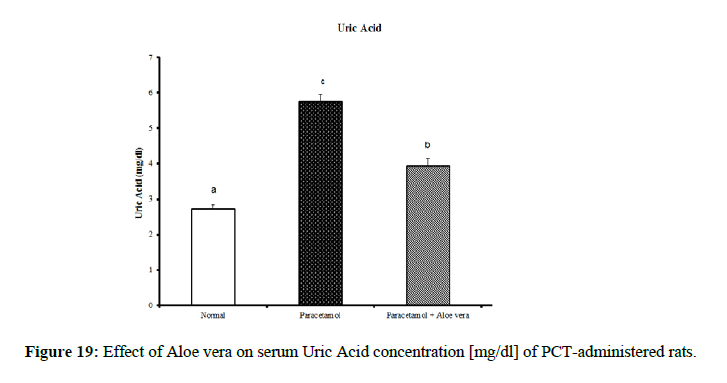
| Parameter Group | Urea [mg/dl] | Creatinine [mg/dl] | Uric acid [mg/dl] |
|---|---|---|---|
| Normal | 69.33 ± 1.2 c | 0.98 ± 0.03 b | 2.73 ± 0.11 a |
| Paracetamol | 36.00 ± 1.15 a | 0.68 ± 0.03 a | 5.75 ± 0.20 c |
| Paracetamol + Aloe vera | 60.50 ± 1.17 b | 0.97 ± 0.02 b | 3.95 ± 0.21 b |
| LSD | 1.85 | 0.046 | 0.22 |
*Values significantly different to control at [p ≤ 0.05]. *Data are written as mean ± SE.
*Values that have the same superscript symbol are not significantly different. *F-Probability: P<0.05
Table 5: The impact of Aloe vera extracts on serum kidney function parameters of paracetamol-administered rats.
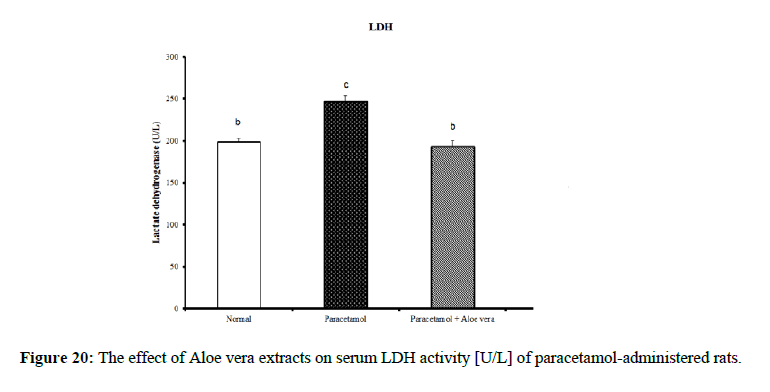
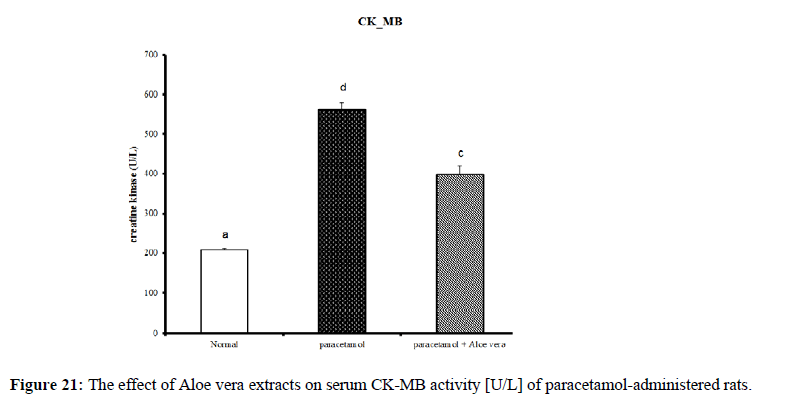
PCT caused a marked decrease in urea and creatinine and increase of uric acid for kidney function parameters (Figure 17-19) Table 6 showed the impact of Aloe vera extract on heart function parameters in serum of paracetamol-administered rats. PCT caused elevation of heart function parameters [LDH and CK_MB]. (Figure 20 & 21).
| Parameter Group | LDH [U/L] |
CK_MB [U/L] |
|---|---|---|
| Normal | 198.46 ± 4.88 b | 208.49 ± 3.99 a |
| Paracetamol | 247.21 ± 7.26 c | 562.15 ± 16.31 d |
| Paracetamol + Aloe vera | 193.72 ± 6.43 b | 399.51 ± 20.12 c |
| LSD | 8.93 | 18.93 |
*Values significantly different to control at [p ≤ 0.05]. *Data are written as mean ± SE.
*Values that have the same superscript symbol are not significantly different. *F-Probability: P < 0.05
Table 6: The impact of Aloe vera extract on heart function parameters in serum of paracetamol-administered rats.
Discussion
The treatment with Aloe vera remarkably improved the deleterious effect of PCT on oxidative stress and antioxidant defense system which was reflected by increase of glutathione and deficiency in lipid peroxidase and catalase. Assessment of liver injury was clarified by serum concentrations of ALT and AST; which is the most commonly indicated markers for liver toxicity. While the plasma membrane of liver cell is damaged, many enzymes normally found in the cytosol are released into the bloodstream. Their amount in the serum is a good quantitative evidence of the extent and type of hepatocellular damage. Increased activities of serum enzymes were observed after APAP intoxication comparable to normal rats indicating considerable hepatocellular damage. Serum alpha-fetoprotein [AFP] has been utilized originally as a marker of hepatocellular carcinoma, but it is now known that elevation of AFP evenly found in a range of nonneoplastic liver diseases. AFP increased in chronic inflammation of the liver reason for chemical and dietary hepatotoxins, explicating that it can act as chronic phase reactant [31]. Its decrease in PCT-administered rats treated with Aloe vera may provide evidence for decreased probability for hepatocellular carcinoma as compared with PCT-administered control rats which showed profound elevation of serum AFP. The present study agrees with other studies showing significant elevation of ALT and AST values after overdosing of some drugs [hepatotoxicants] such as APAP [32,33]. The normalization of the above enzymes activities seen in rats took the Aloe vera extract indicates inhibition of liver cell injury and reduction of leakage of these enzymes into the blood. These results proposed that Aloe vera extract reduced the toxicity due to elimination of the toxic products of APAP in rats. This toxicity is evidenced by an elevation in the serum marker enzymes: Alanine aminotransferase [ALT], Aspartate aminotransferase [AST], Alkaline phosphatase [ALP]. In the assessment of liver damage by PCT the determination of these enzyme levels is mostly used. This is because, at any disruption of membranes or necrosis, there is a leakage of these enzymes into circulation and hence can be determined in the serum. Total bilirubin level also increases while there is a decrease in protein level. The presence of flavonoid is considered the major reason for The Hepatoprotective action of most plant materials [34]. Antioxidant characteristics of Aloe extracts have been ascribed to polysaccharides [35,36] as well as aloesin derivatives [37] or anthraquinones. In other studies, modulation of antioxidant enzymes has been associated with antitumor properties of A. vera leaf extract [38] Studies have also proposed that glycoproteins found in Aloe vera proved anti-tumor efficacies [39]. The aqueous extract from A. vera leaves prepared in our laboratory, was shown to contain naturally occurring antioxidant components, including total phenols, flavonoids, ascorbic acid, β-carotene and α-tocopherol. A. vera leaves possess a various array of 75 compounds, comprising anthrones, anthraquinones [e.g. aloe-emodin] and their glycosides [Aloin A and B], proteins, glycoproteins, chromones, carbohydrates, organic acids, lipids, amino acids, saponins and minerals [40–45]. In our opinion, the improvements in heart function and structural integrity in PCT-administered rats treated with Aloe vera may be attributed to the suppression of oxidative stress and improvement of the antioxidant defense system in the heart. This suggestion is supported by the present results which revealed that Aloe vera owns detectable improvement effect on liver LPO, glutathione content and catalase activity. Oxidative stress produces from an imbalance between the cellular generations of reactive oxygen species [ROS] and the antioxidant mechanisms that remove them. Catalase converts harmful hydrogen peroxide into oxygen and water and protects the tissues from highly reactive hydroxyl radicals. The deficiency in the activity of this enzyme may results in many deleterious effects due to accumulation of highly toxic metabolites and hydrogen peroxide on paracetamol administration, which can induce oxidative stress in the cells. In our study, the pro-inflammatory cytokine, TNF-α, and tumor marker, AFP, levels exhibited a marked increase in serum of PCT-administered rats comparable to the normal ones. The treatment of PCT-administration rats with Aloe vera caused marked amelioration of these estimated variables. These results agree well with [46]. Many studies have indicated that inflammatory mediators including TNF-α and IL-6 are decisively involved in the development of acute liver injury by APAP. In addition to hepatocellular necrosis by oxidative stress, APAP hepatotoxicity can also activate Kupffer cells, the phagocytic macrophages of the liver and cause liver inflammation. Cytokines such as IL-6, IL-1β, and IL-10 significantly are elevated in APAP-induced hepatotoxic mice via the production of ROS. Urea is the major excretory consequent of protein metabolism. Urea is transferred by plasma to the kidney, in which it is filtered by the glomerulus from the plasma. Creatinine is a nitrogenous product which is produced from the metabolism of creatinine in the skeleton muscles. Creatinine concentration not only evaluates impairment of kidney function but also acts as a clinical chemistry endpoint to indicate treatment related toxic effects of extracts/compounds on the kidney in empirical models. Although hepatotoxicity is a most common than nephrotoxicity in APAP overdose, acute kidney failure and kidney tubular damage can occur in the obscurity of liver injury [47]. Antioxidant defense mechanisms of liver can neutralize free radicals, Antioxidant defense mechanisms of liver classified into enzymatic antioxidants [SOD, GR, GPx, GST and CAT] and non-enzymatic antioxidants [GSH] [48]. The non-enzymatic antioxidant; GSH is considered the most abounding tripeptide, non-enzymatic biological antioxidants found in the hepatocytes, which is a key constituent of the mostly antioxidant defense system that forfends the membrane protein thiols of hepatocytes from deleterious actions of reactive oxygen metabolites like hydrogen peroxide and superoxide radicals. The drop of GSH level in the PCT treated group might be because of its usage by the extremely generated amount of free radicals in the hepatocytes conducing to hepatic injury [49]. LPO, which is a degenerative process that effectuates cell membranes and other lipid-containing structures under conditions of oxidative stress, increased significantly in hepatic tissue of PCT control rats in the sitting study as compared with the normal group. The major reason of hepatocellular injury and death by over dosage with paracetamol accrues to its transformation to the toxic NAPQI metabolite. This toxic metabolite accumulates because of saturation of the sulfate and glucuronide conjugation pathways. In case of paracetamol overdose, hepatocellular levels of GSH decreased. The cell macromolecules bind covalently to highly reactive NAPQI metabolite, performing to dysfunction of structural and metabolic disarray and enzymatic systems. Over, deficiency of intracellular GSH makes the hepatocytes highly sensitive to apoptosis and oxidative stress [50]. TNF-α is a major endogenous inflammatory mediator of hepatotoxicity in several experimental liver injuries through its direct cytotoxicity, nitric oxide production and the triggering of an inflammatory cascade. The production of TNF-α is known to be one of the earliest events in the hepatic inflammatory response, which stimulates hepatocyte apoptosis, cytotoxicity and necrosis; therefore, TNF-α is considered to be an important target in research to discover hepatoprotective agents. In accordance with this report, our results demonstrate that paracetamol increases serum TNF-α, indicating the role of this cytokine in paracetamol-induced hepatotoxicity. However, our findings reveal that Aloe vera treatment alleviates the progression of the inflammatory mediator TNF-α in paracetamol-exposed mice [51]. Thus, this study assesses the preventive activity of Aloe vera against PCT-induced hepatotoxicity. It extends to evaluate the possible role of oxidative stress and antioxidant defense system in deteriorating the liver, kidney and heart functions by PCT and in prophylactic effects of Aloe vera.
Conclusion
Aloe Vera administration exhibited a beneficial therapeutic effect on rats with hepatotoxicity due to the existence of anthraquinones, anthrones and their glycosides, proteins, chromones, glycoproteins, carbohydrates, organic acids, lipids, amino acids, saponins and minerals. That showed hepatoprotective anti-inflammatory, immunomodulatory, anticancer and gastro protective effects.
Compliance with Ethical Standards
Conflict of interests. The authors declare that they have no conflict of interest. Statement on the welfare of animals. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
References
[1] D Arshed Iqbal, S Ramesh Chandra and B Suresh Kumar. Am J Plant Sci. 2012.
[2] WA Saka, RE Akhigbe, OS Ishola et al., Pakistan J Biol Sci. 2011, 14[14]: p. 742.
[3] SB Subramanya, B Venkataraman, MFN Meeran et al., Int J Mol Sci. 2018, 19[12]: p. 3776.
[4] CD Williams, A Farhood, H Jaeschke. Toxicol Appl Pharmacol. 2010, 247[3]: p. 169–78.
[5] AM Garba, B Mohammed, SH Garba. IOSR J Pharm Biol Sci. 2012, 3[2]: p. 6–10.
[6] N Suhail, N Bilal, S Hasan et al., Nutr Cancer. 2019, 71[2]: p. 272–84.
[7] NV Madhav and K Bairy. Carbon N Y. 2011, 2[3].
[8] L Wang, X Li and C Chen. Int Immunopharmacol. 2018, 61: p. 20–8.
[9] B Sharma, S Siddiqui, G Ram et al., J Diabetes Metab. 2013, 4[9]: p.1–6.
[10] H Aebi. Elsevier. 1974, p. 673–84.
[11] SM HARVEY, JR GREENWOOD. Int J Syst Evol Microbiol. 1983, 33[2]: p. 275–84.
[12] JW Hartz, S Funakoshi and HF Deutsch. Clin Chim Acta. 1973, 46[2]: p. 125–32.
[13] A Griesmacher, M Kindhauser, SE Andert et al., Am J Med. 1995, 98[5]: p. 469–75.
[14] E Beutler, MKY Yeh. Blood. 1963, 21[5]: p. 573–85.
[15] BK Pedersen, K Ostrowski, T Rohde. Et al., Can J Physiol Pharmacol. 1998, 76[5]: p. 505–11.
[16] JG Conway, JA Wakefield, R Brown et al., J Exp Med. 1995, 182[2]: p. 449–57.
[17] M Lantz, S Malik, ML Slevin et al., Cytokine. 1990, 2[6]: p. 402–6.
[18] A Adinolfi and M Adinolfi. J Med Genet. 1975, 12[2]: p. 138–51.
[19] JG Cannon, RG Tompkins, JA Gelfand et al., J Infect Dis. 1990, 161[1]: p. 79–84.
[20] Y Fong, KJ Tracey, LL Moldawer et al., J Exp Med. 1989, 170[5]: p.1627–33.
[21] DJ Herzyk, AE Berger, JN Allen et al., J Immunol Methods. 1992, 148[1–2]: p. 243–54.
[22] MT Burtis, JL Ulmer, GA Miller et al., Am J Neuroradiol. 2005, 26[1]: p. 34–8.
[23] GP Young, S Friedman, ST Yedlin et al., Am J Physiol Liver Physiol. 1981, 241[6]: p. 461–8.
[24] G Szasz. Clin Chem. 1969, 15[2]: p. 124–36.
[25] BT Doumas, PP Kwok-Cheung, BW Perry et al., Clin Chem. 1985, 31[11]: p. 1779–89.
[26] CJ Patton and SR Crouch. Anal Chem. 1977, 49[3]: p. 464–9.
[27] RJ Henry. Clin Chem Princ Tech 2nd ed Harper Row Publ New York. 1974.
[28] P Fossati, L Prencipe and G Berti. Clin Chem. 1980, 26[2]: p. 227–31.
[29] I Witt and C Trendelenburg. J Clin Chem Clin Biochem. 1982, 20[4]: p. 235–42.
[30] JF Tucker, RA Collins and AJ Anderson. Acad Emerg Med. 1997, 4[1]: p. 13–21.
[31] HA El-Bakry, G El-Sherif and RM Rostom. Biomed Pharmacother. 2017, 96: p. 798–811.
[32] PS Lee, R Yoshida, DC Ekiert et al., Proc Natl Acad Sci. 2012, 109[42]: p. 17040–5.
[33] F Anwar, S Latif, M Ashraf. Int J Devoted to Pharmacol Toxicol Eval Nat Prod Deriv. 2007, 21[1]: p. 17–25.
[34] VU Ezeonwu. J Med Appl Biosci. 2012, 4: p. 26–31.
[35] JH Wu, C Xu, CY Shan. Life Sci. 2006, 78[6]: p. 622–30.
[36] L Chun-hui, W Chang-hai, X Zhi-liang et al., Process Biochem. 2007, 42[6]: p. 961–70.
[37] A Yagi, A Kabash, N Okamura et al., Planta Med. 2002, 68[11]: p. 957–60.
[38] HA El-Shemy, MAM Aboul-Soud, AA Nassr-Allah et al., Curr Med Chem. 2010, 17[2]:129–38.
[39] Y Gao, KI Kuok, Y Jin et al., Crit Rev Food Sci Nutr. 2019, 59: p. 244–56.
[40] BK Vogler and E Ernst. Br J Gen Pr. 1999, 49[447]: p. 823–8.
[41] S Choi and MH Chung. Elsevier; 2003, p. 53–62.
[42] U Nandal and RL Bhardwaj. Int J Pharm Sci Rev Res. 2012, 13[1]: p. 59–67.
[43] B Raksha, S Pooja and S Babu. J Plant Sci. 2014, 2[3]: p.102–7.
[44] S Shrestha, K Yang, C Guy et al., Nat Immunol. 2015, 16[2]: p. 178–87.
[45] N Akev, A Can, N Sütlüpınar et al., İstanbul Üniversitesi Eczac Fakültesi Derg. 2015, 45[2]: p. 191–215.
[46] BO Cho, HH Yin, CZ Fang et al., Food Sci Biotechnol. 2015, 24[6]: p. 2205–12.
[47] AJ Sit and JW McLaren. Google Patents. 2009.
[48] SA El-Newary, RF Ismail, NM Shaffie et al., Int J PharmTech Res. 2016, 9: p. 327–41.
[49] D Singh, PV Arya, VP Aggarwal et al., Antioxidants. 2014, 3[3]: p. 569–91.
[50] N Mohammed, AH Yaro, AB Nazifi. Trop J Nat Prod Res. 2018, 2[5]: p. 220–6.
[51] MK Rasool, EP Sabina, SR Ramya et al., J Pharm Pharmacol. 2010, 62[5]: p. 638–43.