Review Article - Der Pharma Chemica ( 2023) Volume 15, Issue 6
Cardiac Glycoside Poisoning: Pharmacological Therapy
Hari Prasad Sonwani*, Pragya Sahu and Rashi BandeyHari Prasad Sonwani, Department of Pharmacology, Apollo College of Pharmacy, Anjora, India, Email: sonwanihari438@gmail.com
Received: 28-Oct-2023, Manuscript No. Dpc-23-120776; Editor assigned: 31-Oct-2023, Pre QC No. Dpc-23-120776 (PQ); Reviewed: 14-Nov-2023, QC No. Dpc-23-120776; Revised: 17-Nov-2023, Manuscript No. Dpc-23-120776 (R); Published: 15-Dec-2023, DOI: 10.4172/0975-413X.15.6.134-141
Abstract
Because cardiac glycosides are found in natural sources and are widely used in therapeutic settings, they are a significant cause of poisoning. Toxicological manifestations of poisoning can range in intensity. Bradycardia, heart bloc and gastrointestinal symptoms are the main clinical characteristics. Ventricular fibrillation or tachycardia causes death. Numerous treatments have been employed; the most popular ones include activated charcoal, atropine, β-adrenoceptor agonists, magnesium, anti-digoxin Fab and temporary pacing. More unusual treatments include fructose-1,6-diphosphate (which is undergoing a clinical trial) and anticalin. Even with therapies that have been around for decades, there is still disagreement over the best way to use them, when to use them and what dosage would produce the best results. This adds to the variation in use that exists worldwide. Access is another aspect that affects utilization. Accessibility obstacles include the need for financial resources (such as anti-digoxin Fab in resource poor nations) or transfer to a specialized center (for example, to obtain temporary pacing). According to recent findings, people with chronic digoxin poisoning may not benefit much from anti-digoxin Fab and the current methods for determining the dose in cases of digoxin toxicity may be oversimplifying the necessary dosage. More affordable and potent medications are needed, especially to treat yellow oleander toxicity, which is a major issue in nations with limited resources.
Keywords
β-adrenoceptor; Anti-digoxin; Bradycardia; Cardiac glycoside
Introduction
Source, prevalence and significance
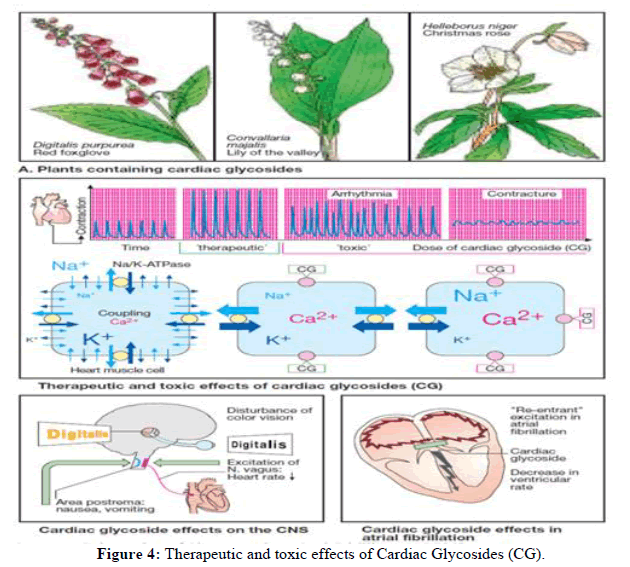
Natural substances known to exist in a variety of plant and animal species are known as cardio active steroids. A large amount of structural variability exists and they are divided into two groups according to the aglycone steroid moiety: Cardenolides, which have a five membered lactone ring and bufanolides, which have a six membered lactone ring [1]. Ouabain and bufanolides share structural similarities with substances that are increased in preeclampsia, hypertension and chronic kidney disease in humans and are likely derived from the adrenal glands [2,3]. The term "cardio active steroids" (also known as "cardio glycosides") will be used throughout this analysis because many of these steroids are coupled to sugars and are the primary class of steroids linked to poisoning. Regretfully, cardiac glycoside poisoning is an international phenomenon. This is a reflection of both the widespread and long standing usage of digitalis glycosides, especially digoxin, for therapeutic purposes, as well as the occasional and widespread poisoning using oleander plants. For instance, hundreds of cases of cardiac glycoside poisoning were reported to US poison control centers in the US alone in 2013; most of these cases were treated in medical facilities [4]. Inter current illnesses, mistakes in prescription or distribution, as well as unintentional or deliberate poisoning, can all result in digoxin poisoning. The tropics and subtropics are home to both common and yellow oleanders [5]. In some parts of India and Sri Lanka, yellow oleander poisoning is a serious public health concern [6,7]. Consumption of yellow oleander seeds or leaves has the potential to cause fatal poisoning. Treatment is challenging because due to changes in toxic threshold, diagnostic test variability, delayed onset of toxicity (especially with yellow oleander), need for hospital transfer and accessibility of cost effective and effective treatments (Table 1).
| Botanic sources of cardiac glycosides | |
|---|---|
| Common name | Botanic name |
| Foxglove | Digitalis purpurea, Digitalis lanata |
| Common oleander | Nerium oleander |
| Yellow oleander | Thevetia peruviana |
| Lily of the valley | Convallaria majalis |
| Red squill | Urginea maritima |
| Ouabain | Strophanthus gratus |
| Dogbane | Apocynum cannabinum |
| Wallflower | Cheiranthus cheiri |
| Milkweed | Asclepias spp |
| Mock azalea | Menziesia ferruginea |
| Pheasant's eye | Adonis spp |
| Star of bethlehem | Ornithogalum umbellatum |
| Wintersweet, bushman's poison | Carissa acokanthera, Cerbera manghas |
| Sea mango frangipani | Plumeria rubra |
| King's crown | Calotropis procera |
| Rubber vine | Cryptostegia grandiflora |
Table 1: Botanic sources of cardiac glycosides.
Literature Review
Drug related kinetics (Pharmacokinetics)
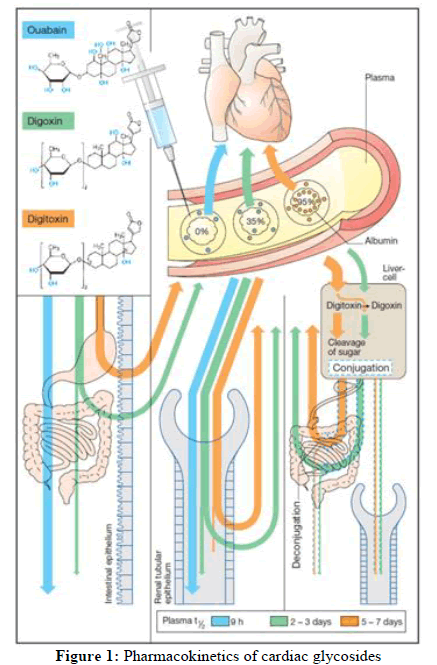
Despite structural similarities, the pharmacokinetic characteristics of individual cardiac glycosides differ greatly [8]. Digoxin's pharmacokinetics might differ, including absorption (may be related to the formulation [9]), half-life of elimination (mean 40 h, range 20 h-50 h) and renal elimination predominate [10]. Digoxin takes around 6 hours to start acting; this is because it takes time for it to be distributed to a peripheral compartment and/or for time dependent binding to the Na+-K+-ATPase [11]. Because full distribution has not taken place, the initial serum digoxin concentration in acute poisoning may be extremely high and will not accurately reflect the entire body burden. Given that Multiple Dosages of Activated Charcoal (MDAC) enhance clearance (explained later); it is assumed that digitalis cardiac glycosides undergo enterohepatic recycling. Digitoxin, for instance, has a lengthy elimination half-life of 7.5 days, which is indicative of significant enterohepatic recirculation [10]. As opposed to consuming in yellow oleander extract, the concentration time profile is dominated by extended absorption (which in some cases extends beyond 50 hours post ingestion) of digoxin cross reacting compounds, which exhibits an irregular pharmacokinetic profile. The apparent terminal half-life, which has a median duration of 42.9 hours, is likewise quite varied. The area under the concentration time curve or the degree of cardiotoxicity has a weak correlation with the quantity of seeds consumed, indicating heterogeneity in bioavailability [8]. Pharmacokinetic information on other cardiac glycosides in humans is scarce (Figure 1).
Action mechanism and toxicity
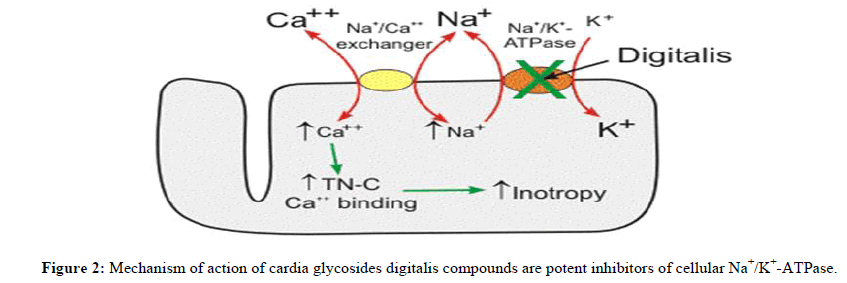
Digoxin and ouabain have been extensively used to describe the widely accepted mechanism of action of cardiac glycosides. Individual cardiac glycosides, however, may have distinct modes of action [2], which could affect toxicity or the way a patient reacts to medication. For instance, insulin seems to counteract the effects of ouabain and digoxin on Na+-K+-ATPase, however this may be because of distinct binding sites [12]. Cardiac glycosides cause intracellular retention of Na+ through inhibition of the Na+-K+-ATPase in cardiac and other tissues. This is followed by a rise in intracellular Ca2+ concentrations via the action of the Na+-Ca2+ exchanger. Increased intracellular Ca2+ concentrations generate bradycardia and inotropy, while intracellular Na+ and Ca2+ buildup results in partial membrane depolarization, which boosts automaticity and epicardial ectopy. Digoxin may also block voltage-gated Na+ channels and raise vagal tone, which can lead to bradycardia and poor atrioventricular node conduction. Additional activities, such as the activation of intracellular signal transduction systems and the endocytosis of Na+-K+-ATPase, have also been observed [3,13] (Figure 2).
This ion transport system moves sodium ions out of the cell and brings potassium ions into the cell. This transport function is necessary for cell survival because sodium diffusion into the cell and potassium diffusion out of the cell down their concentration gradients would reduce their concentration differences (gradients) across the cell membrane over time. Loss of these ion gradients would lead to cellular depolarization and loss of the negative membrane potential that is required for normal cell function. The Na+/K+-ATPase also plays an active role in membrane potential generation. This pump generates electro genic hyperpolarizing currents because it transports 3 sodium ions out of the cell for every two potassium ions that enter the cell. This can add several negative millivolts to the membrane potential depending on the activity of the pump.
The symptoms and signs of poisoning
Acute poisoning is characterized mostly by gastrointestinal symptoms (vomiting, nausea, vomiting, abdominal pain, diarrhea), hyperkalemia, generalized weakness, sleepiness and, most significantly, cardiotoxicity (dysrhythmias, bradycardia and heart block). These could show up a few hours after acute overdose. Rarely, chronic digoxin toxicity has been linked to alterations in vision, including green or yellow discoloration [6,14-18]. However, because of coexisting illnesses and drug interactions, patients with chronic poisoning may exhibit other electrolyte abnormalities [19]. Sinus bradycardia is the most prevalent heart anomaly associated with poisoning. ECG variations in medicinal dosage (or moderate poisoning) include the ST segment being lowered and the T wave flattening or inverting. Prolonged PR interval (first degree heart block) or sinus bradycardia are signs of moderate poisoning. Because of the suppression of the atrioventricular node, severe poisoning presents as a second or third degree heart block. There are also reports of sinus arrest and exit block. Ventricular fibrillation refractory to electrical cardioversion or a systolic arrest are the causes of death [6,14-19]. 56% of 162 patients who purposefully poisoned themselves with yellow oleander underwent treatment requiring dysrhythmias in a series of intentional poisonings [15]. The above-discussed pharmacokinetics was reflected in the varying time course of cardiotoxicity progression and remission. For instance, a patient experienced a 72-hour sinus rhythm before experiencing second degree heart block, whereas at 92 hours, another experienced a severe dysrhythmia [14,15]. Still, most deaths happened in less than a day [20]. Digoxin poisoning, especially chronic cases, has also been linked to atrial fibrillation, ventricular ectopic and tachycardia. These side effects may also be related to pre-existing cardiac conditions, electrolyte imbalances from concurrent diuretic therapy or concurrent medical conditions [16-18].
Results and Discussion
According to earlier reports, the death rate from digitalis compounds might reach 20% and acute toxicity from digoxin overdose could take up to five days or up to 24 hours after admission. Following the introduction of fab antibody fragments, mortality was shown to be lower [18] and it has since decreased even further [4]. When patients arrive to tertiary centers, the case fatality rate for yellow oleander poisoning can reach 10% [21], although it can only reach 3% at secondary care hospitals [15,20]. This variation in the result could be caused by a number of variables, such as the resources, therapies and referral standards.
Controlling poisoning
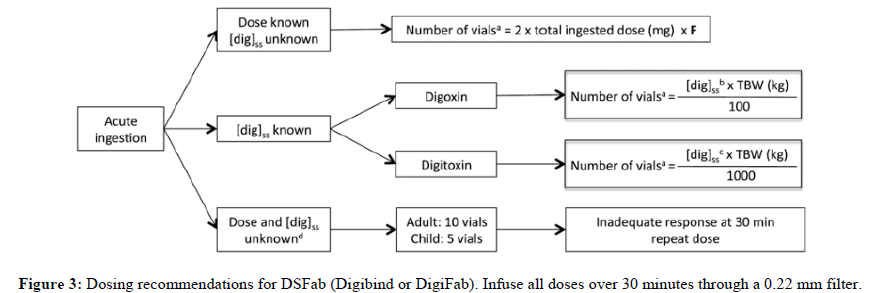
The management of patients with suspected or confirmed cardiac glycoside poisoning is made more difficult by the erratic dose reaction relationship; variable toxicity time course and need for costly or invasive treatments and demand for inter hospital transfers. Since the cardiac glycosides share a similar structure, digitalis poisoning remedies are often applied to it. Nonetheless, a growing number of clinical researches evaluating yellow oleander poisoning have been conducted in recent years. As of right now, only yellow oleander toxicity is the subject of randomized controlled trials, so information supporting suggestions for poisoning with other cardiac glycosides including digoxin is of low quality (Figure 3) [22].
If cardiac arrest is imminent, give via slow intravenous push. [dig]SS, serum digoxin concentration (nanogram per milliliter) at steady state; F, estimated bioavailability (if intravenous digoxin or digitoxin use 1, if digoxin tablets use 0.8); TBW, total body weight:
•Round number of vials upward.
•If measurement in nanomole per liter, multiply by 0.781.
•If measurement in nanomole per liter, multiply by 0.765.
•Ingestions of cardiac glycosides other than digoxin or digitoxin should be treated with empiric dosing recommendations.
Overview and evaluation of risks
For every patient experiencing acute poisoning, a risk assessment concerning the possibility of developing toxicity and treatment planning should be carried out. Due to variations in the lethal dose in the case of cardiac glycoside poisoning and the previously mentioned variable in the beginning of toxicity in the case of yellow oleander, things can get tricky. For instance, consumption of one or two yellow oleander seeds has been known to cause mortality, but ingestion of ten or more seeds has been known to result in survival without the need for pacing or anti-digoxin fab [6,8,14,21]. Digoxin overconsumption of 10 mg is frequently linked to severe toxicity and death in the absence of therapy; however, outcome data supporting this are few and complicated by the medical care obtained. Patients experiencing acute poisoning should, wherever feasible, be admitted to a critical care unit for ongoing cardiac monitoring, diagnostics and treatment planning. Yellow oleander may produce a delayed start of major dysrhythmias, so patients may need to be monitored for up to 72 hours after intake. Whether it is acute or chronic poisoning, the symptoms of digoxin toxicity often appear six hours after the last dosage. The likelihood of poisoning is low and the patient can be medically managed if they are asymptomatic, the ECG shows no brady or tachyarrhythmias, potassium is within the reference range and the digoxin concentration is less than 2.3 ng ml~1 (3 nmol l~1) wiped out. Important interventions to take into account include bradycardia and nausea management, intestinal decontamination and brief cardiac pacing or ant digoxin Fab administration for severe dysrhythmias. Investigations have a major role in guiding risk categorization and treatment decisions.
Chemical biology
A higher potassium content is linked to an increased risk, but the exact relationship between potassium concentration and cardiotoxicity is not well understood. Hyperkaliemia is a sign of cardiac glycoside poisoning. When hyperkaliemia is observed after acute digoxin exposure, it is a sign of poisoning [23]. Numerous factors, including renal failure, concurrent use of angiotensin blocking medications or spironolactone, may exacerbate hyperkaliemia in chronic digoxin poisoning [24]. While hyperkaliemia is linked to toxicity in the yellow oleander case, a mean concentration of 5.4 mmol l^1 was discovered in cases of severe cardiotoxicity and 4.3 mmol l~1 in cases of mild cardiotoxicity. Each group's reliability is diminished by significant variability, including hypokalemia [14]. Dioxin test higher plasma digoxin concentrations are linked to more severe poisoning in digoxin exposures, however there are no set standards for determining if a patient is poisoned. For instance, some individuals with digoxin concentrations less than 2 ng ml^1 (2.6 nmol l~1) but not others with values above 2 ng ml~1 (2.6 nmol l~1) showed signs of toxicity [19]. As was previously mentioned, distribution kinetics may have a role in this weak association. Digoxin assays, which are mostly based on ELISA platforms that exhibit cross-reactivity with comparable structures, can be employed to detect the presence of non-digoxin cardiac glycosides, such as those derived from oleander [1,8]. However, because it represents an unknown quantity, the reported "digoxin concentration" might not be correlated with toxicity. One of a number of cardiac glycosides that vary in cross reactivity and potency. Furthermore, it's possible that more contemporary ELISA platforms are less effective at measuring non-digoxin cardiac glycosides because they cross-react less than older platforms. Insights about the degree of cross reactivity with the assay they employ, such as whether it has been evaluated against positive controls from non-digoxin sources, may be obtained by speaking with the laboratory (Figure 4).
Cleaning up
Regardless of when the exposure occurred, all patients with acute ingestion of a potentially hazardous substance should get a single dosage of 50 g-100 g of activated charcoal. This proposal is based on pharmacokinetic facts (see above and improved elimination below), as well as the safety of activated charcoal, even though clinical trials has not verified the effectiveness of this technique. Because of the lengthy absorption phase of yellow oleander, some people recommend using gastric lavage [15,21]. However, there is little evidence to support this approach and it may cause problems if activated charcoal is administered first, as it is likely to be more successful in preventing prolonged absorption.
Abnormal electrolyte levels
Because there is little information available, treating hyperkaliemia is debatable. Although there are certain exceptions, as was previously stated, the degree of elevation is generally thought to indicate the severity of poisoning given the mechanism of action of cardiac glycosides. Insulin may have a direct influence on Na+-K+-ATPase, changing the way digoxin works and bringing potassium into cells to treat hyperkalemia. Rats given insulin-dextrose showed a significant increase in survival with reduced cardiotoxicity when compared to the control group and there was a difference in potassium levels (about 7.0 mmol l^1 vs. 4.5 mmol l~1, depending on the model) [25]. Furthermore, the kind of insulin may have a different apparent protective effect on Na+-K+-ATPase activity of cardiac glycoside as a result of variations in the Na+-K+-ATPase subunit that the cardiac glycoside binds to [12]. Patients with cardiac glycoside poisoning may experience hypokalemia due to treatments like diuretics or severe vomiting or diarrhea. Since therapeutic dosage of digitalis enhances cardiotoxicity, hypokalemia should be addressed [16]. Patients with yellow oleander poisoning who were hypokalemic have been known to pass away. In other situations, it is frequently advised to use exogenous calcium to "stabilize" the myocardium in cases of hyperkaliemia. The administration of calcium may theoretically increase toxicity given that the intracellular concentration of calcium is elevated in cardiac glycoside poisoning. Animal data has reported increased toxicity, including death, which may be related to sustain cardiac contraction, also known as "stone heart." Case reports, however, have not a study in pigs with digoxin intoxication found that intravenous calcium chloride 10 mg kg^1 did not alter mortality [27]. The study also indicated problems from intravenous calcium [26,27]. According to some publications, calcium may be hazardous [30], should not be administered [28] or neither [29,30]. We do not currently use exogenous calcium to treat hyperkaliemia in cases of cardiac glycoside poisoning because of its unclear benefits and potential toxicity. This is especially true given the availability of treatments that lower potassium levels, such as insulin-dextrose and anti-digoxin Fab.
Remedies
Pharmacological antagonists of bradycardia, reversal of Na+-K+-ATPase inhibition or increased cardiac glycoside clearance are among the therapeutic approaches for treating cardiac glycoside toxicity. Without particular therapy, up to 40% of patients with severe yellow oleander cardiotoxicity may return to sinus rhythm after a few hours; however, it is impossible to predict which patients would experience this [14,15]. Although there are fewer reports of spontaneous remission from acute digoxin poisoning, the majority that are in the literature are treated, therefore the amount of data is small. It is less evident how antidotes fit into the scheme of treating chronic digoxin overdose. Important counteragents are outlined. Antelope Atropine increases heart rate by opposing cardiac glycoside vagal activation and observational evidence points to a possible advantage. [31,15]. First line doses range from 0.6 to 1 mg, but for chronic bradycardia that is, less than 40 beats per minute accompanied by hypotension doses as high as 2-3 mg have been utilized. Larger doses have been administered, although they may cause an anticholinergic delirium that calls for sedatives and attentive nursing supervision [15,21]. In warm, non-air conditioned wards, hyperthermia can be dangerous [14,15,21]. It serves as a bridge treatment in Sri Lanka before temporary pacing. Dioxin blocking fab digoxin can be bound with high affinity by anti-digoxin Fab, which then extracts it from Na+-K+-ATPase to lessen toxicity. Anti-digoxin fab has been used to treat toxicity from non-digoxin cardiac glycosides, particularly yellow oleander, because it may also bind to other cardiac glycosides, following the same principles as the digoxin immunoassay. The effectiveness and indications for anti-digoxin Fab are unknown because the data on this treatment for digitalis poisoning are restricted to observational data [22]. Benefits from anti-digoxin Fab have been documented in case series; however, there is inconsistent data about the response in cases of acute or chronic poisoning [32]. A clinically significant effect in chronic poisoning has been questioned, although recent observational data show an effect in acute poisoning [24]. Although anti-digoxin Fab was discovered here it seems to be only marginally helpful in reducing cardiac toxicities in cases of chronic digoxin poisoning, but it was successful in binding the free digoxin in the central circulation. Individuals who are diagnosed with chronic "digoxin poisoning" typically have serious co-morbid conditions including cardiac and/or renal failureand they are treated with medications like calcium antagonists and β-adrenoceptor blockers. The fact that Fab is ineffective in these situations implies that these additional variables may be mostly responsible for the heart symptoms and mortality risk. In individuals with chronic "digoxin poisoning" who are not treated with Fab, outcomes can be favorable; additional case controlled studies are required to corroborate these findings. Results of acute digoxin poisoning without the use of anti-digoxin fab are also supported by data. A 147 patient case series with a mean 10.1 mg, median 7.5 mg, range 1.25-37.5 mg) in a facility lacking anti-digoxin fab access, with a low mortality rate of 1.4% and symptoms including nausea/vomiting in 70% of cases, ECG abnormalities in 52% and anti-digoxin Fab indications in 43% of cases [33]. The average level of digoxin in 73 patients was 4.3 μg l~1 (5.5 nmol l~1), which was significantly less than what was predicted based on the amount that was given. This implies that anti-digoxin fab may not be necessary in all cases of acute digoxin overdose and that the dosage of anti-digoxin fab should not be determined by the amount taken. On the other hand, even if fab was employed as the first line of treatment, a case series of acute and chronic digoxin and digitoxin toxicity showed a greater mortality rate (7.6%). Additionally, a case control retrospective analysis of chronic digoxin toxicity failed to find any anti digoxin fab's positive impact on mortality. Furthermore, it is unknown what the ideal dosage of anti-digoxin fab is for treating digoxin intoxication. Different strategies are used for dosage regimens; many take into account the kind of poisoning (acute or chronic), the amount consumed and/or the goal of half to complete neutralization depending on serum digoxin concentration [10]. Dosing schedules based on significantly lower starting doses 40 mg in one vial for chronic poisoning and 80 mg in two vials for acute poisoning have recently been proposed. If there is a clinical decline, they can be repeated sooner rather than later or after 60 minutes if there is an insufficient response or recurrence [32]. If the patient is in the peri-arrest state, higher dosages, up to and including the amount that will produce complete neutralization, can be applied. In yellow is an RCT (n=66). The anti-digoxin Fab treatment for oleander toxicity caused an early improvement in cardiac rhythm and hyperkaliemia, which led to the trial's early conclusion. There were no recorded deaths and it lacked the power to identify a shift in mortality. In contrast to digoxin, yellow oleander poisoning typically requires higher dosage due to perhaps decreased cross reactivity and an inability to accurately measure the body load based on blood tests. A dose response research linked to the aforementioned RCT suggested a dosage of 1200 mg, while later findings indicate that 800 mg intravenously may also be useful.
Intermittent heart pacing
Uncontrolled studies indicate that transient cardiac pacing does not restore hyperkaliemia and is linked to greater problems and mortality than anti-digoxin Fab. For instance, a retrospective series found that in 23% of cases, pacing failed to prevent a life-threatening dysrhythmia, whereas in 8% of cases, Fab. It's unclear how much these findings apply to modern treatments, though, given that they are decades old. Ventricular fibrillation may also be brought on by the pacing wire's insertion. Additional potential drawbacks of temporary cardiac pacing include logistical issues, such as the need for procedural knowledge and resources that are frequently absent from rural and developing nations, necessitating the patient's transfer to another hospital, which could result in a delay in treatment that could be fatal [15, 21].
Cardioversion with electricity
When a patient has yellow oleander poisoning-related malignant ventricular dysrhythmias, electrical cardioversion is usually not helpful. Similar recommendations have been made about digitalis poisonings, stating that electrical cardioversion should only be used in situations involving ventricular dysrhythmias that are unresponsive to alternative therapies utilizing low energy levels (e.g., 20-100 J) [16].
Better quality eradication
MDAC are advised in cases of toxic digoxin exposure due to pharmacokinetic information. For instance, MDAC lowered the apparent elimination half-life in patients with chronic digoxin poisoning by almost 50%, but in another study, this was only significant if there was impaired kidney function. MDAC also increased the clearance of intravenous digoxin in volunteers by 47% in one study. In volunteers, MDAC increased the amount of intravenous digitoxin cleared. When compared to single dose activated charcoal (SDAC), MDAC decreased mortality in yellow oleander poisoning, according to an RCT (n=401) [21]. A bigger RCT that followed, nevertheless, that included yellow oleander toxicity (n=1647), found a nonsignificant tendency in better results when using MDAC [20]. There were variations in the MDAC regimen between these investigations, although it was thought that the outcome was not significantly affected by this. Notably, in comparison to no activated charcoal, a pharmacokinetic sub-study of the second RCT revealed a comparable increase in apparent clearance of cardiac glycosides from SDAC or MDAC [8]. When considered collectively, the administration of MDAC seems fair; yet, it should not be chosen over other treatments. While activated charcoal is safe, it shouldn't be given to individuals who have an ileus or an unprotected airway, such as those receiving atropine treatments. Extracorporeal therapies like dialysis are not associated with cardiac glycoside toxicity, according to the data.
Alternative therapies
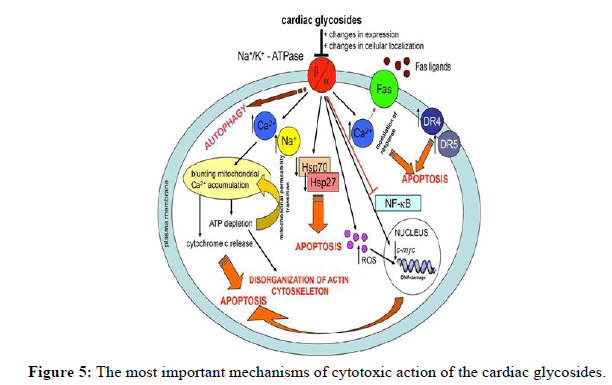
Numerous additional therapies have been tested; however there is little evidence of their effectiveness and their usage looks unusual in standard clinical practice or the information is restricted to animal research. These include magnesium, for which there is insufficient data, fructose-1, 6-diphosphate (FDP; CAS 488-69-7), anticalin and β-adrenoceptor agonists (isoprenaline or salbutamol). Digoxin toxicity and its free plasma levels in rats were both decreased by an anticalin that had a high binding affinity for the drug. As a non-biological substitute for anti-digoxin Fab, anticalins are currently untested in humans. FDP is a reasonably priced medication that enhances Na+-K+-ATPase activity and boosts ATP generation. It is now being evaluated for the treatment of yellow oleander toxicity. There aren't many reasons or supporting data for the other two medications that have been tested, phenytoin and lignocaine (Figure 5).
The first mechanism is based on changes in the homeostasis of Ca2+ and Na+. This leads to mitochondrial dysfunction based on blunting the accumulation of mitochondrial Ca2+. Depletion of ATP causes a mitochondrial permeability transition. These processes are completed by the release of cytochrome c and apoptosis. Disorganization of the actin cytoskeleton is induced by the depletion of ATP. Changes in the homeostasis of Ca2+ modulate the response of the fas receptor to fas ligands. CGs also up regulate two cell death receptors DR4 and DR5. CGs down regulate heat shock proteins that are important in the survival of cells under stress conditions. An increased level of reactive oxygen species (ROS) damages biomolecules, including DNA. The down regulation of the expression of c-myc is among the next mechanisms of CG action. Both the formation of ROS and the down regulation of c-myc lead to apoptosis. The down regulation of NF-B and autophagy are the next processes that lead to cell death.
Conclusion
Heart glycoside poisoning can be treated using a variety of methods, however the effectiveness of these treatments is not well established, which seems to have an impact on how they are used in actual practice. Further information is needed to determine the best course of action for treating cardiac glycoside toxicity, including an assessment of less expensive medications that can be utilized in nations with limited resources. Increasing our knowledge of the dose response of less expensive therapies like insulin-dextrose in people is one of the top research priorities. Additionally, information about more unusual and non-biological countermeasures, such FDP and anticalin, in patients suffering from cardiac glycoside toxicity is noteworthy.
References
- Lalonde RL, Deshpande R, Hamilton PP, et al. Clin Pharmacol Ther. 1985; 37(4): p. 367-371.
[Crossref] [Google Scholar] [PubMed]
- Ibanez C, Carcas AJ, Frias J, et al. Int J Cardiol. 1995; 48(1): p. 27-30.
[Crossref] [Google Scholar] [PubMed]
- Park GD, Goldberg MJ, Spector R, et al. Drug Intell Clin Pharm. 1985; 19(12): p. 937-941.
[Crossref] [Google Scholar] [PubMed]
- Mowry JB, Burdmann EA, Anseeuw K, et al. Clin Toxicol. 2016; 54(2): p. 103-114.
[Crossref] [Google Scholar] [PubMed]
- Eyer F, Steimer W, Nitzsche T, et al. Toxicol Appl Pharmacol. 2012; 263(3): p. 352-359.
[Crossref] [Google Scholar] [PubMed]
- Gawarammana I, Mohamed F, Bowe SJ, et al. BMC Emerg Med. 2010; 10(1): p. 1-6.
[Crossref] [Google Scholar] [PubMed]
- Hoffman RS. J Toxicol Clin Toxicol. 2002; 40(3): 285-286.
- Dvela M, Rosen H, Feldmann T, et al. Pathophysiology. 2007; 14(3-4): p. 159-166.
[Crossref] [Google Scholar] [PubMed]
- Lingrel JB. Annu Rev Physiol. 2010; 72: p. 395-412.
[Crossref] [Google Scholar] [PubMed]
- Mowry JB, Spyker DA, Cantilena Jr LR, et al. Clin Toxicol. 2014; 52(10): p. 1032-1283.
[Crossref] [Google Scholar] [PubMed]
- Bandara V, Weinstein SA, White J, et al. Toxicon. 2010; 56(3): p. 273-281.
[Crossref] [Google Scholar] [PubMed]
- Eddleston M, Ariaratnam CA, Meyer WP, et al. Trop Med Int Health. 1999; 4(4): p. 266-273.
[Crossref] [Google Scholar] [PubMed]
- Bose TK, Basu RK, Biswas B, et al. J. Indian Medical Assoc. 1999; 97(10): p. 407-410.
[Google Scholar] [PubMed]
- Roberts DM, Southcott E, Potter JM, et al. Ther Drug Monit. 2006; 28(6): p. 784.
[Crossref] [Google Scholar] [PubMed]
- Jounela AJ, Pentikainen PJ, Sothmann A. Eur J Clin Pharmacol. 1975; 8: P. 365-370.
[Crossref] [Google Scholar] [PubMed]
- Bateman DN. Toxicol Rev. 2004; 23: p. 135-143.
[Crossref] [Google Scholar] [PubMed]
- Roberts DM, Buckley NA. Clin Pharmacokinet. 2007; 46: p. 897-939.
[Crossref] [Google Scholar] [PubMed]
- Oubaassine R, Weckering M, Kessler L, et al. Toxicology. 2012; 299(1): p. 1-9.
[Crossref] [Google Scholar] [PubMed]
- Nesher M, Shpolansky U, Rosen H, et al. Life Sci. 2007; 80(23): p. 2093-2107.
[Crossref] [Google Scholar] [PubMed]
- Eddleston M, Ariaratnam CA, Sjostrom L, et al. Heart. 2000; 83(3): p. 301-306.
[Crossref] [Google Scholar] [PubMed]
- Fonseka MM, Seneviratne SL, De Silva CE, et al. Hum Exp Toxicol. 2002; 21(6): p. 293-295.
[Crossref] [Google Scholar] [PubMed]
- Kelly R, Smith T. Am J Cardiol. 1992; 69(18): p. 108-119.
[Crossref] [Google Scholar] [PubMed]
- Smith TW, Antman EM, Friedman PL, et al. Prog Cardiovasc Dis. 1984; 26(5): p. 413-458.
[Crossref] [Google Scholar] [PubMed]
- Taboulet P, Baud FJ, Bismuth C. Clin Toxicol. 1993; 31(2): p. 247-260.
[Crossref] [Google Scholar] [PubMed]
- Abad-Santos F, Carcas AJ, Ibanez C, et al. Ther Drug Monit. 2000; 22(2): p. 163-168.
[Crossref] [Google Scholar] [PubMed]
- Eddleston M, Juszczak E, Buckley NA, et al. Lancet. 2008; 371(9612): p. 579-587.
[Crossref] [Google Scholar] [PubMed]
- De Silva HA, Fonseka MM, Pathmeswaran A, et al. Lancet. 2003; 361(9373): p. 1935-1938.
[Crossref] [Google Scholar] [PubMed]
- Roberts DM, Buckley N. Cochrane Database Syst Rev. 2006; 12(4): p. 44-49.
[Crossref] [Google Scholar] [PubMed]
- Pap C, Zacher G, Kárteszi M. Orvosi hetilap. 2005; 146(11): p. 507-513.
[Google Scholar] [PubMed]
- Oubaassine R, Bilbault P, Roegel JC, et al. Toxicology. 2006; 224(3): p. 238-243.
[Crossref] [Google Scholar] [PubMed]
- Levine M, Nikkanen H, Pallin DJ. J Emerg Med. 2011; 40(1): p. 41-46.
[Crossref] [Google Scholar] [PubMed]
- Hack JB, Woody JH, Lewis DE, et al. Clin Toxicol. 2004; 42(4): p. 337-342.
[Crossref] [Google Scholar] [PubMed]
- Hoffman RS, Howland MA, Lewin NA, et al. Goldfrank's Toxicol Emerg. 2015; 23(7): 57-66.