Mini Review - Der Pharma Chemica ( 2022) Volume 14, Issue 11
Catalytic Applications of Aminophenol Based Ligand Containing Transition Metal Complexes
Ganesh Chandra Paul*Ganesh Chandra Paul, Department of Chemistry, ICFAI University Tripura, Agartala, India-799210, India, Email: gcp.iitgchem@gmail.com
Received: 27-Oct-2022, Manuscript No. dpc-22-78432; Editor assigned: 29-Oct-2022, Pre QC No. dpc-22-78432; Reviewed: 12-Nov-2022, QC No. dpc-22-78432; Revised: 14-Nov-2022, Manuscript No. dpc-22-78432; Published: 21-Nov-2022, DOI: 10.4172/0975-413X.14.11.14-24
Abstract
Aminophenol based ligands have had a tremendous and continually growing impact on catalysis research. Their utility spans over a number of areas including homogeneous catalysis, small molecule activation, carbon dioxide reduction, and hydrogen evolution processes. For the development of a catalyst metal-ligand covalency is necessary via mutual cooperation and strong electronic coupling between a metal center and coordinating ligands. The role of o-aminophenol based ligands in different metabolic/enzymatic reactions in biological systems is now well documented. Their application is now expanding in the field of synthetic chemistry and highlighted recent applications in catalysis research. The prospective role of various o-aminophenol ligand-based redox levels and their influence on metal centers in catalysis has been widely studied. The present short review covers the catalytic applications of transition metal complexes based on sterically hindered functionalized o-aminophenol type ligands.
Keywords
Redox non-innocent ligand; Homogeneous catalysis; Electronic structure; Electron transfer; Reaction mechanism.
INTRODUCTION
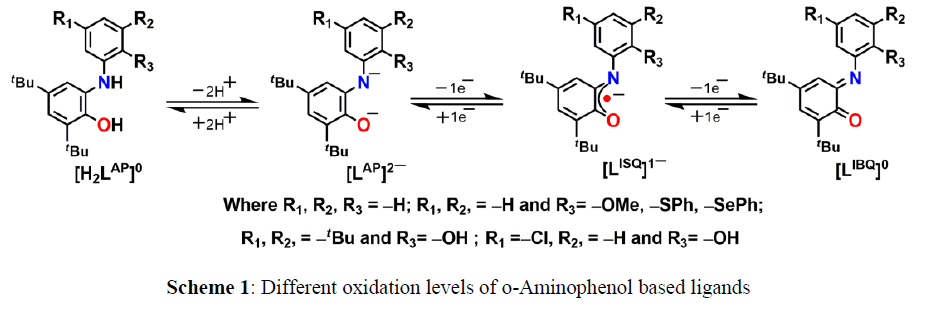
The metal complexes of o–aminophenol based ligands are currently being studied intensively. Studies on the coordination chemistry and structure–bonding correlation of o–aminophenol based redox non–innocent ligands have been a flourishing research field over several years and continue to be extensively explored. It is observed that properties of a metal multi-electron reactivity at a well-defined metal complex and play a pivotal role in catalysis. o–Aminophenol-based ligands span redox–levels from dianionic o–amidophenolate (LAP)2– to o–iminosemiquinonate(1–) monoanion (LISQ)•– π-radical to neutral o–iminoquinone (LIBQ)0 forms. Designing of non-innocent ligands and thereafter synthesis of radical complex depend on the interaction between the metal ion and the coordinating ligand. In order to prepare new coordination complexes having useful application first and foremost thing is to design new multifunctional ligand. The formation of tyrosyl radicals in the mononuclear copper enzyme galactose oxidase is a prototypical example of a class of metalloproteins to use free radicals as cofactors to promote oxidation reactions. Redox–active ligands offer an intriguing way to approach –containing transition metal complexes have biological relevance to interpret the electronic structure of several radical–containing metalloenzyme intermediate. For example, different substituent’s based 2–anilino–4,6–di–tert–butylphenol (H2L) has been found to behave as a non-innocent ligand. It provides the corresponding radical–containing manganese (IV), iron (III), cobalt (III), copper (II), nickel (II), and palladium (II) complexes [1] (Scheme 1).
Ligand H2L and their corresponding metal complexes showed different activity like enzymatic mimicking, small molecule activation, H2 gas generation and C-N bond forming reaction.
Bioinspired Alcohol Oxidation Mediated by o-Aminophenol Based Ligands:
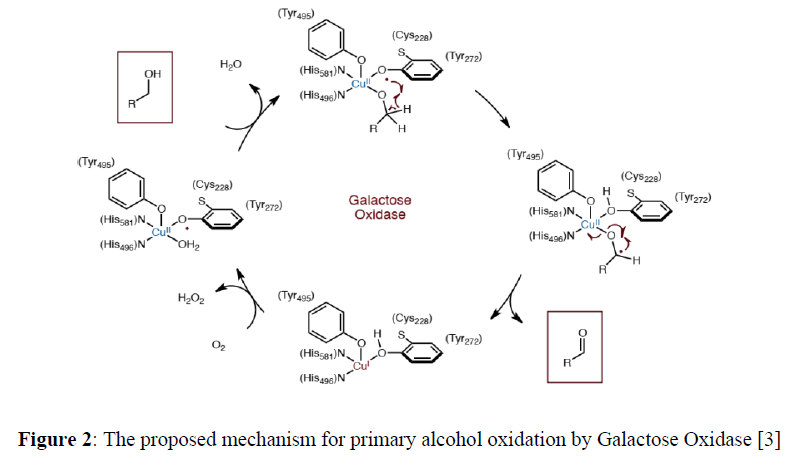
Galactose Oxidase (GO) is a fungal copper containing mononuclear metalloenzyme that catalyzes aerial oxidation of primary alcohols to their corresponding aldehydes with concomitant reduction of molecular oxygen to hydrogen peroxide [2].
RCH2OH + O2 – RCHO + H2O2
The active site of this metalloenzyme contains one copper atom that is surrounded by two histidine imidazole units and two tyrosinate units. The fifth position of the square pyramidal acvtive site is occupied by a solvent molecule (Figure 1). The active form of the enzyme the tyrosine 272 (Tyr272), that is covalently cross-linked to a cysteine residue in a posttranslational oxidative modification step, present in its one electron oxidized form, i.e. tyrosine272 radical. The unpaired in Cu (II) is present at dx2–y2 orbital and should be perpendicular to the radical–containing pZ orbital and hence, a ferromagnetic coupling should result to an EPR active species. In reality, the active form of the GOase is diamagnetic due to antiferromagnetic coupling between the unpaired spins. This is because of the distorted geometry around the Cu (II) center. The copper center is in its +II oxidation state. Both the Tyr272 radical and the Cu (II) centers accept one electron each and facile the two–electron oxidation of primary alcohols to its aldehyde. The mechanistic proposal is shown in Figure 2. GO is probably one of the well-studied enzymes that proceed via radical–type reactions.
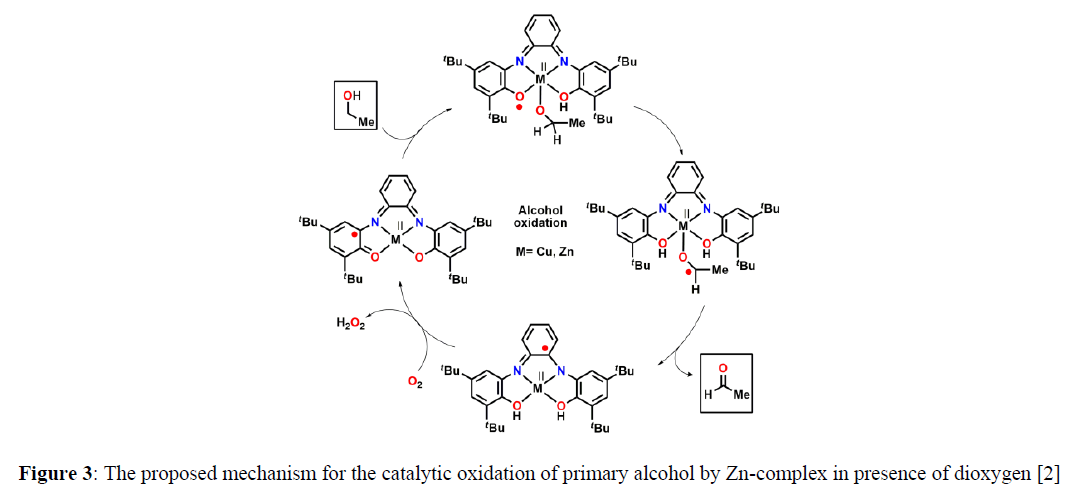
As a biomimic, Chaudhuri and coworkers reported a tetradentate ligand N,N’-bis(3,5-di-tert-butyl-2-hydroxyphenyl)-1,2-phenylenediamine (H4L) and their corresponding square planar Cu(II) and Zn(II) complexes [2]. Both the complexes showed enzymatic mimicking activity as Galactose Oxidase (Figure 2,3).
Ligand-Assisted Activation of Small Molecules
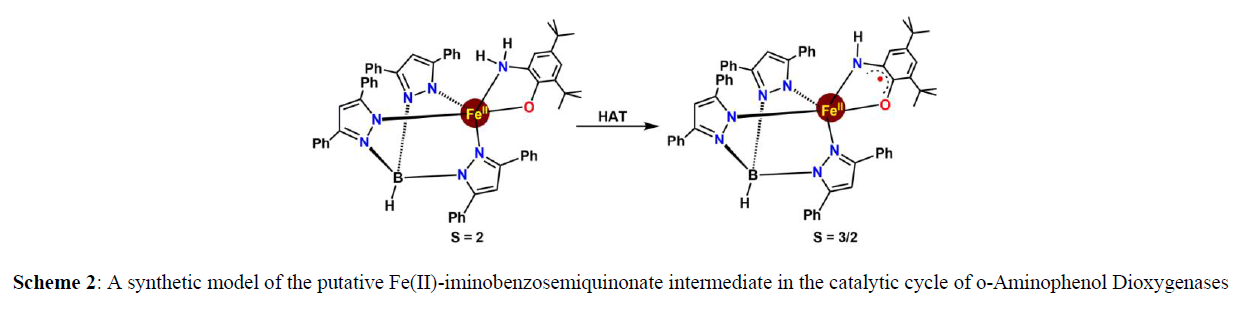
Oxygen Activation Reactions: In the early phase of redox non–innocence applied to small molecule activation, Adam T. Fiedler and coworkers reported a [Fe(Ph2Tp)(ISQtBu)] complex, consisting of a o-aminophenol ligand as electron reservoir, that facilitated oxygen activation. This example demonstrated the viability of Fe2+-ISQ intermediates in the catalytic cycles of o-aminophenol dioxygenases for better understanding of reaction mechanism for the for the aromatic C-C bond cleavage processes by 2-aminophenol dioxygenage by non-heme Fe-complex [3,4] (scheme 2).
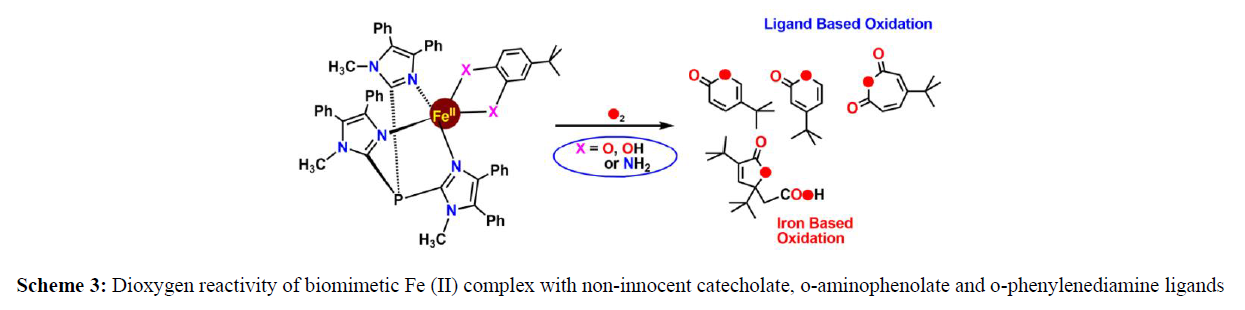
They describe the synthesis of the trigonal–bipyramdial complex, the first synthetic example of an iron(II) center bound to an iminobenzosemiquinonate (ISQ) radical (Scheme 2). The unique electronic structure of the complex and its corresponding oneelectron oxidized derivative with an S = 3/2 have been established on the basis of crystallographic, spectroscopic, and computational analysis. The groups of Adam T. Fiedler and Michael M. Bittner showed spectroscopic, computational, O2 reactivity, and reaction kinetics of three mononuclear Fe(II) complex (Scheme 3) containing “noninnocent” catecholate, o–aminophenolate, and o–phenylenediamine ligands. Depending on the nature of the non-innocent bidentate ligands, the O2 reaction triggers either iron–based oxidation or ligand–based oxidation, or a combination of both processes (Scheme 3) basically ligands are capable of donating protons as well as electrons, and the interplay between these two factors influences rates of reaction with O2 (scheme 3,4).
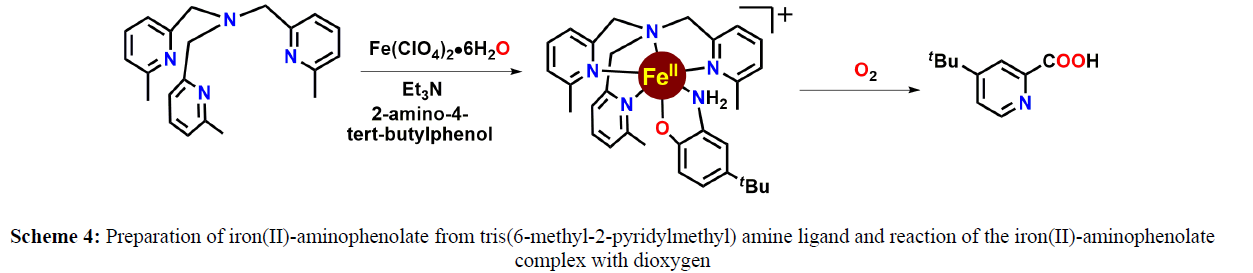
In 2013, T. K. Paine and coworkers have reported the dioxygen reactivity of six-coordinate iron (II)-2-aminophenolate complexes of an N4 ligand using substituted 2-aminophenols. The complexes have been shown to react with dioxygen under ambient conditions to afford substituted 2-picolinic acids mimicking the function of 2-aminophenol dioxygenases (Scheme 5).
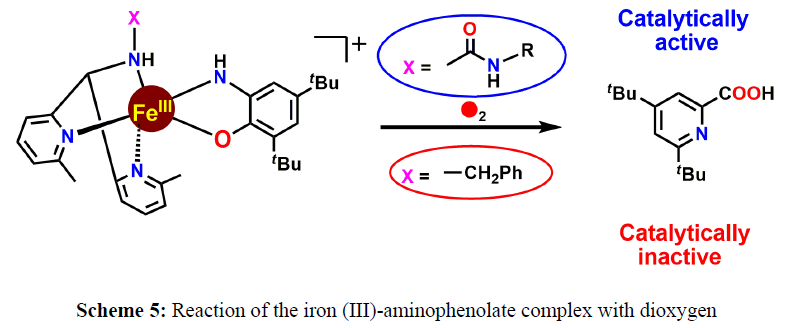
Furthermore, in 2015 the same group has reported two Fe (III)-2-amidophenolate complexes of two different facial tridentate ligands; one ligand contains pendant urea moiety and the other with tethered benzyl moiety (Scheme 5). Both to cleave the ligands react with dioxygen aromatic ring of 2-amino-4,6-di-tert-butylphenol regiospecifically, resulting in the formation of 4,6-di-tert-butyl-2-picolinic acid. The complex supported by urea-bearing ligand shows catalytic C−C bond cleavage with multiple turnovers, whereas the complex without urea group shows no catalysis. The role of the urea group has been implicated to affect the catalytic reactivity (Scheme 6).
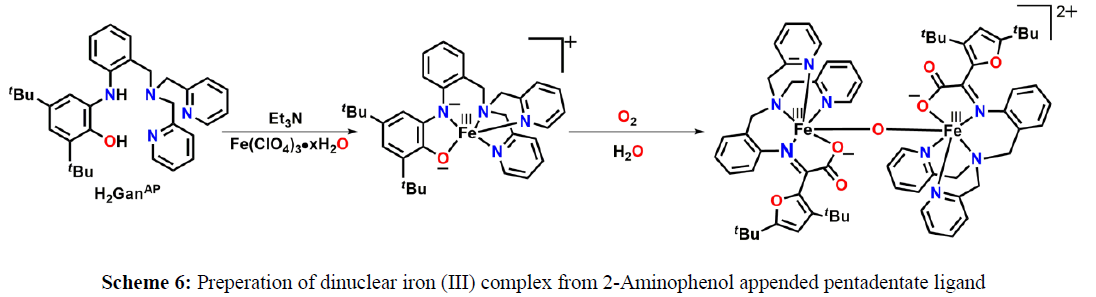
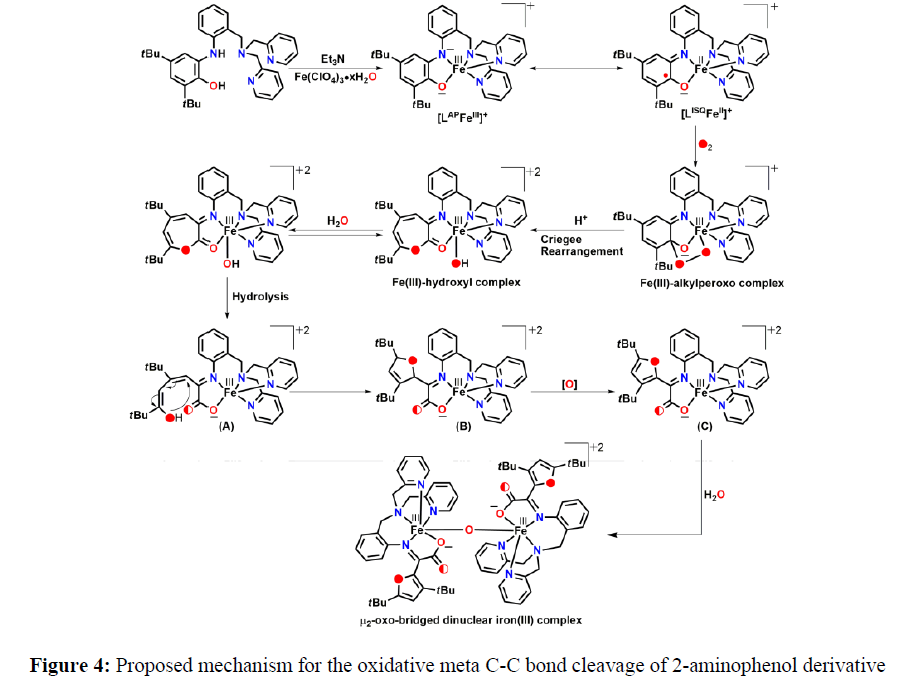
In 2017, Paul and coworkers reported an o–Aminophenol appended pentadentate ligand [H2GanAP]. When 2-Aminophenol appended pentadentate ligand [H2GanAP] reacted with Fe(ClO4)2·6H2O under air it provided a μ2-oxo-bridged dinuclear iron(III) complex [5]. Mechanistic study showed that, initially an iron(III)–amidophenolate complex was formed, further it reacted with molecular oxygen and caused oxidative aromatic C–C bond cleavage take places via a putative alkylperoxo species. Ligand H2LAP upon reacting with iron(III) perchlorate hydrate in the presence of Et3N and tetrabutylammonium perchlorate provided of a five–coordinate [LAPFeIII]+ species. The species, upon exposure to molecular oxygen, initially generated five˗coordinate iminobenzosemiquinone moiety˗containing low˗spin Fe (II) species ([LISQFeII]+). The [LISQFeII]+ species then reacted with molecular oxygen to generate an Fe(III)˗alkylperoxo complex (Figure 4). The Fe (III)˗alkylperoxo complex underwent Criegee rearrangement and species Fe(III)–hydroxyl complex was formed. Cyclization of the product from hydrolysis (A) resulted in 2,5˗dihydrofuran derivative (B). Species E then underwent oxidation to C. Herein, the driving force could be the aromatization via radical pathway and the process might be facilitated by the presence of Fe–salt and in situ generated superoxide or peroxide species. Finally, two units of E combined with a water molecule, as evidences by H2O experiment, and μ2-oxo-bridged dinuclear iron (III) complex was formed (Figure 4).
Ligand-Assisted ‘C-C’ Coupling Reactions:
Redox non-innocent o-aminophenol ligand backbones can behave as redox reservoirs. Easy oxidation as well as reduction of the radical unit has been successfully utilized in developing oxidation-reduction catalysts (scheme 7).
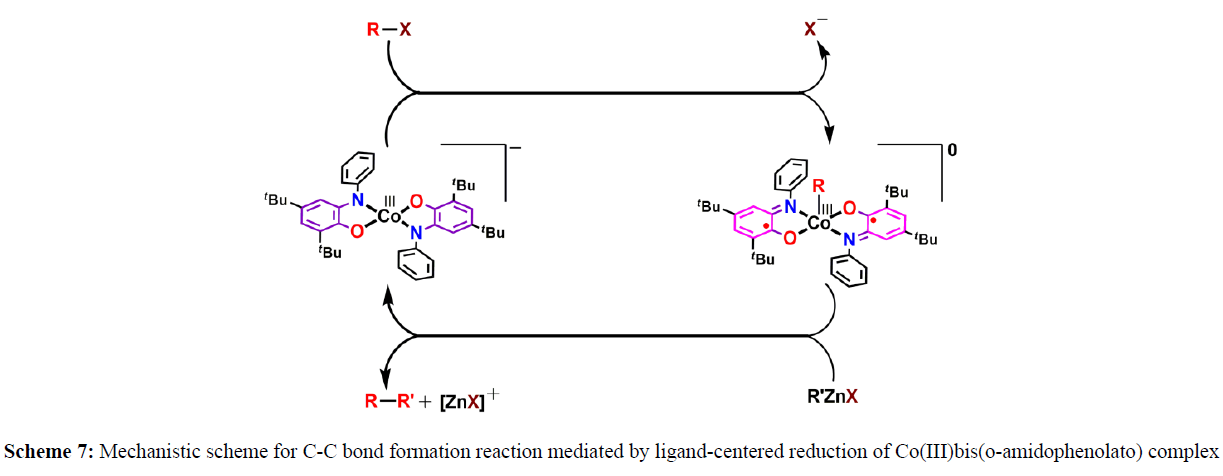
In an elegant example, Soper and coworkers demonstrated that redox-active aminophenol-derived ligands can be used to bring about palladium-like organometallic oxidative addition and reductive elimination reactions at square planar later first row metal centers. In one application of this strategy, they showed that four coordinate cobalt (III) complexes with redox-active amidophenolate ligands are strong nucleophiles that react with alkyl halides, including primary alkyl chlorides, under gentle conditions to generate stable alkylcobalt(III) complexes [6]. The electrophilic addition reactions are unusual because they formally oxidize the cobalt (III) fragment by two electrons, but there are no changes in cobalt oxidation state because the reactions proceed with single electron oxidation of each amidophenolate ligand. Subsequent treatment of the alkylcobalt(III) complexes with organozinc halides results in reductive coupling to form new C–C bonds, regenerating the four-coordinate cobalt(III) starting materials (Scheme 7).
Collectively, these reactions form an unusually well-defined cycle for cobalt Negishi-like coupling of alkyl halides with organozinc halides. Importantly, this reaction scheme can be used for assembly of aliphatic alkyl-alkyl linkages without forming radical intermediates because, despite the thermodynamic bias for sequential one-electron transfer, the kinetically preferred reactions are concerted two-electron processes. Current efforts are optimizing the C–C reductive elimination reaction for development of highly active redox-active ligand complexes for cross coupling. Soper and co-workers established a cobalt-catalyzed methodology for the Negishi cross-coupling reaction, where a crucial oxidative addition at the metal center was governed by the changes in the redox state of the amidophenolate backbone (scheme 8).
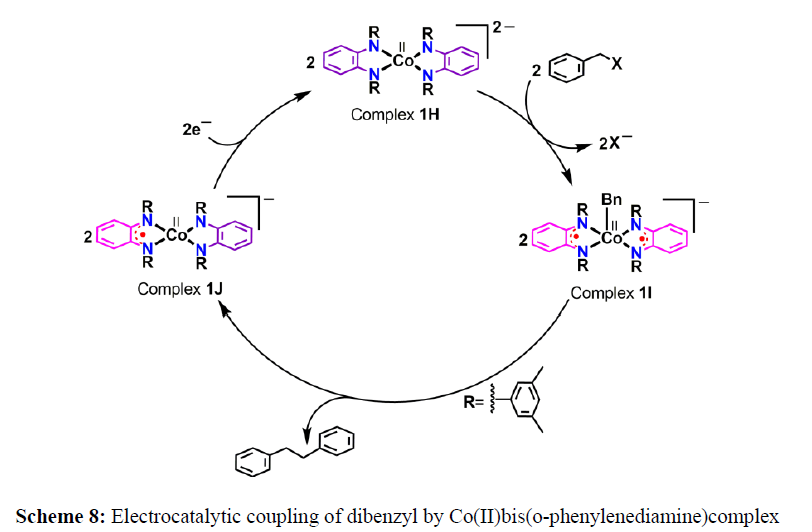
Recently, Sarkar and coworkars reported a four-coordinate cobalt complex 1H capable of electrocatalytic C–C bond formation (Scheme 8). The ligand assisted oxidative addition of benzyl bromide to the metal ion provide a diradical-containing square pyramidal complex 1I, where −CH2Ph (Bn) is axially coordinated to the metal ion. The formation of C−C coupled dibenzyl product results the complex 1J, which upon one-electron reduction regenerates the active catalyst 1H.
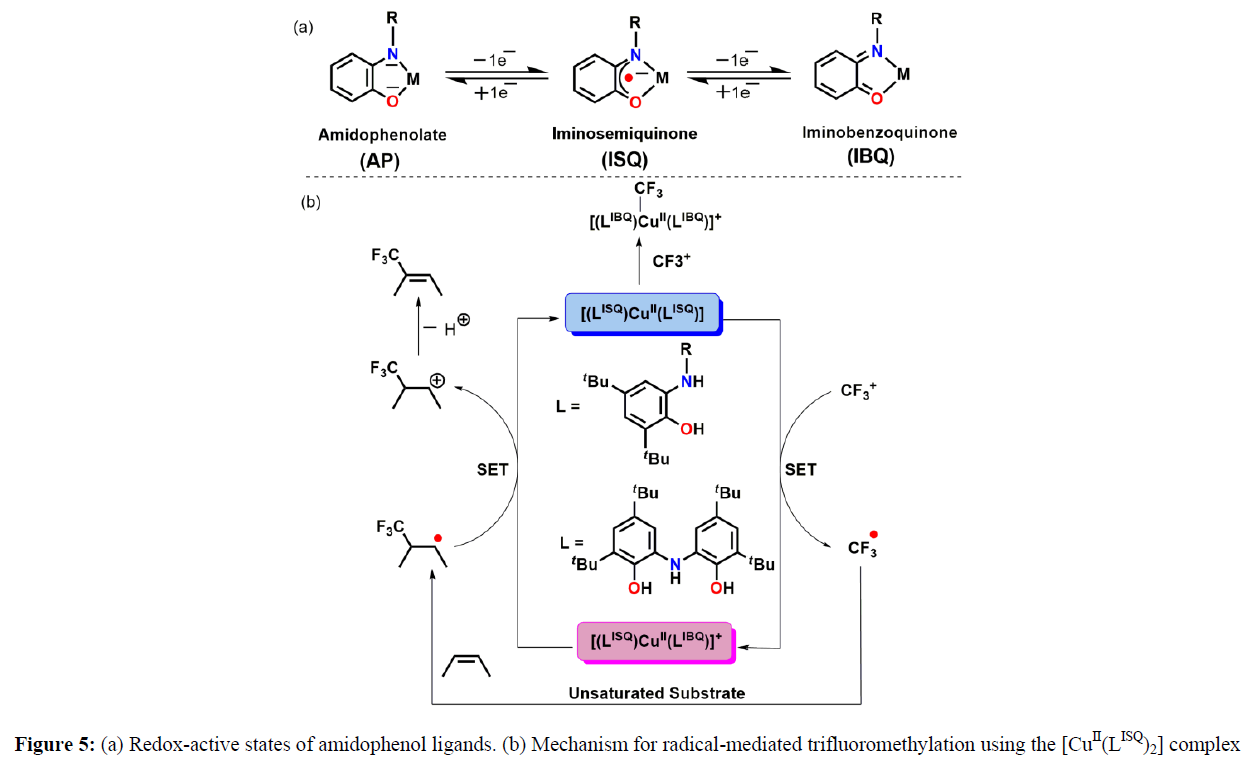
Another iconic class of ligand as a redox reservoir that remains at the central part of ligand-mediated redox processes is the group of amidophenolates. Since 2000, bulky amidophenolate ligands (AP2–;) have been significantly popularized by Wieghardt later Fensterbank and his group due to the easy access of two sequential one-electron redox processes affordable on the ligand backbone (Figure 5) [7].
Homolytic Bond Activation
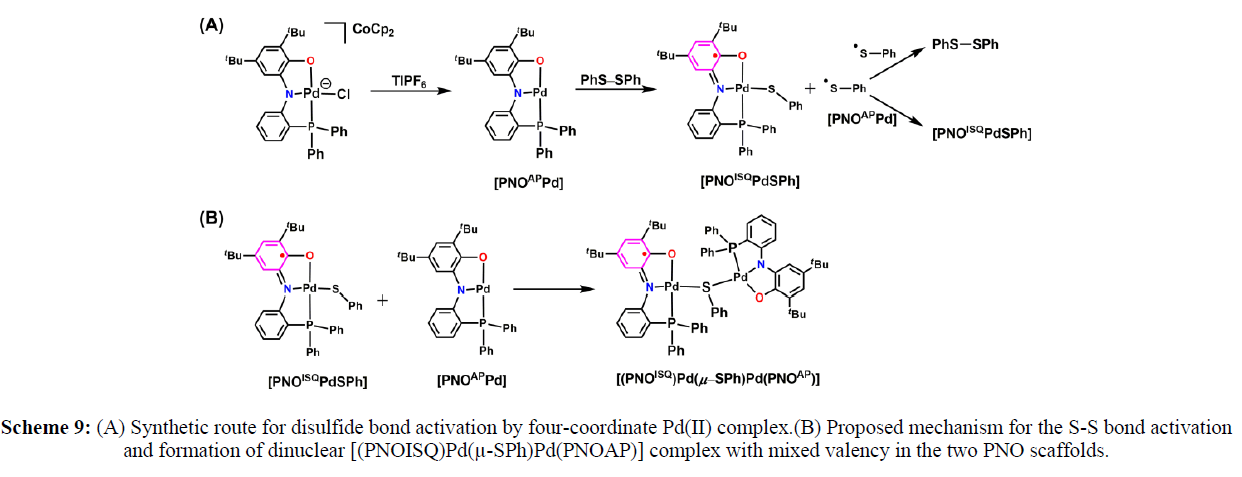
A new strategy for hemolytic bond activation is done by an o–aminophenol appended Pd(II) complex. Coordination of the novel redox–active phosphine–appended aminophenol pincer ligand (PNOH2) to PdII generates a paramagnetic complex with a persistent ligand-centered radical. The complex undergoes fully reversible single–electron oxidation and reduction. Homolytic bond activation of diphenyldisulfide by the single-electron reduced species leads to a ligand–based mixed–valent dinuclear palladium complex with a single bridging thiolate ligand. Mechanistic investigations support an unprecedented intramolecular ligand-to-disulfide single-electron transfer process to induce homolytic S–S cleavage, thereby releasing a thiyl (sulfanyl) radical (scheme 9).
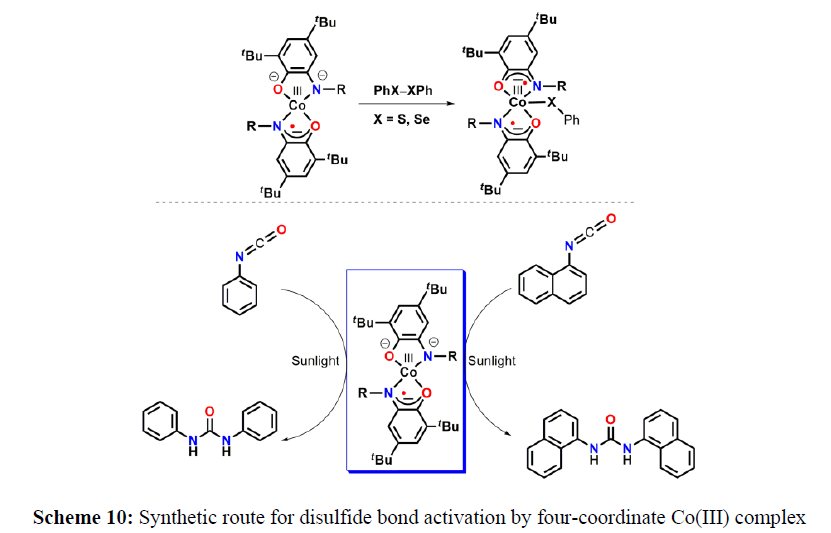
In 2015, van der Vlugt, de Bruin, and coworkers have utilized the redox active nature of a coordinated-2-amidophenoate derivative in a four-coordinate Pd(II) complex for the one-electron homolytic S–S bond cleavage of diphenyl disulfide. A similar strategy of using amidophenolate backbones as a redox–responsive ligand has been leveraged by Paul and coworkars for a series of homolytic S–S bond cleavage of diphenyl disulfide and homolytic Se–Se bond cleavage of diphenyl diselenide compounds (scheme 10).
Further the four-coordinate, monoradical-containing Co(III) complexes participated in the non-innocent ligand driven homolytic cleavage of S–S and Se–Se bonds and catalyzed the conversion of RNCO (R = phenyl and naphthyl) to the corresponding urea derivatives (TON 480) in dry CH2Cl2 under sunlight stimulus (Figure 6).
Further in 2016, Mondal and coworkers proposed and synthesize a Cu (II)-bis(iminosemiquinone) complex. When the Cu(II)-bis(iminoquinone) complex was subjected to react with NaBH4 in dry CH3CN, H2 gas was formed along with the generation of the corresponding Cu(II)-bis(iminosemiquinone) complex. Identification of H2 gas was confirmed by GC analysis [8].
Catalytic C-H amination of organic azides
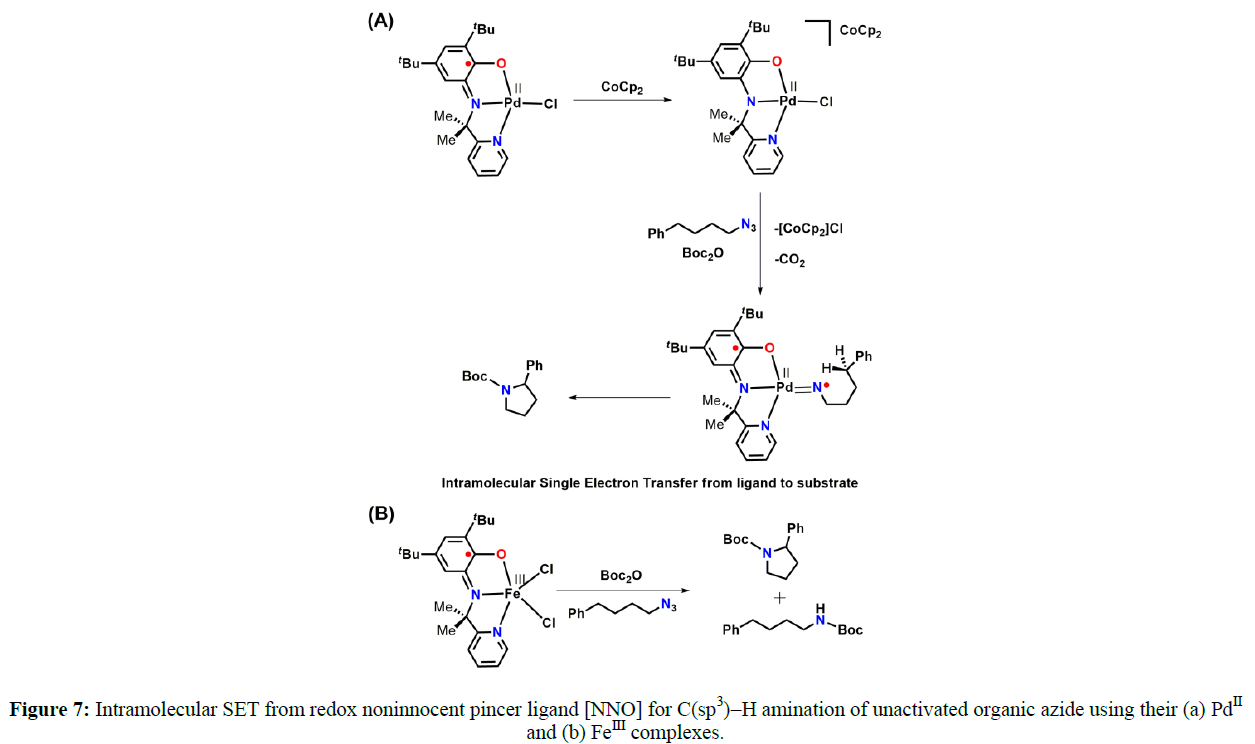
The amidophenolate-containing pincer backbone has been utilized profusely where three of its redox states, namely the quinone, semiquinone, and catecholato forms, have been exploited. Vlugt introduced a pincer where the electronic conjugation between two aryl moieties has been disconnected by the placement of a tertiary carbon with a dimethyl substituent (Figure 7A). The catalyst employs Pd(NNO)AP complex with an aminophenol-based redox-active pyridine-aminophenolate NNO ligand. The reaction proceeds via formation of an open-shell Pd-nitrene adduct via SET from ligand to the substrate. This was possible because the ligand can be conveniently oxidized to its iminosemiquinonate form. This intermediate step is followed by an intramolecular H atom abstraction and radical rebound to release the N-heterocycle pyrrolidine. This observation is very interesting, as SET takes place from the ligand, even in the presence of palladium, which itself performs efficient two electron chemistry. As a subsequent effort, the palladium was replaced with the base metal iron, where pyrrolidine formation was observed via C(sp3)-H amination that was achieved with a TON of 620. The same aminophenolate NNO ligand was reacted with FeCl3 to form air-stable (NNO)ISQFeCl2 (74) (Figure 7B) [9]. The magnetic moment measurement by the Evans method revealed a value of 4.86 μB, which was indicative of a S = 2 state. This was completely consistent with an electronic structure where a high spin Fe (III) was strongly anti-ferromagnetic coupled to a ligand-centered NNOISQ radical. Furthermore, a temperaturedependent solid-state magnetic moment by SQUID and zero-field Mössbauer measurement further substantiated the proposed electronic structure of the molecule (Figure 7).
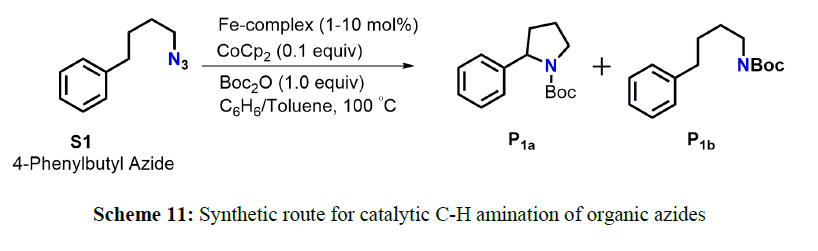
The iron complex was used as the catalyst for sp3 C-H amination using 1-azido-4-phenylbutane as the substrate and Boc2O as the in situ protecting group for the product pyrrolidine. Upon 10 mol % loading of the catalyst, at 100ºC, Boc-protected pyrrolidine was obtained in 70% yield alongside the straight-chain side-product formed in 30% yield. In the tested reaction, the active catalyst reacted with azide to form an open-shell nitrene radical that spearheaded a HAT step to yield N-heterocycles.9b In 2014, using the Pd-complex as a catalyst, Broere and coworkers investigate C(sp3)-H amination of different organic azides via a radical-type mechanism involving C-H abstraction followed by a radical rebound step, or alternatively via direct insertion of the radical nitrene fragment in the C-H bond and they established the concept of ligand-based single-electron transfer to generate a substrate radical [9]. But the main problem of this reaction is poor yield (scheme 11).
Thereafter in 2017, Bagh and coworkers set out to investigate the activity of well-defined air-stable Fe-complex for catalytic C(sp3)-H amination, using 1-azido-4-phenylbutane as standard substrate and ditert-butyl dicarbonate (Boc2O) as in situ protecting group to avoid catalyst deactivation by pyrrolidine coordination. Heating an equimolar mixture of both reagents (100 µmol) at 100ºC in benzene for 24 h in the presence of 10 mol% 1 as catalyst in a pressure tube resulted in complete conversion of 1-azido-4-phenylbutane to the desired Boc-protected pyrrolidine P1a (70 %) and Boc-protected amine P1b(30%) as side-product (entry 1). Lowering the catalyst loading to 5 mol% led to a slightly different product ratio of 63:37 for P1a: P1b(entry 2) (scheme 12).
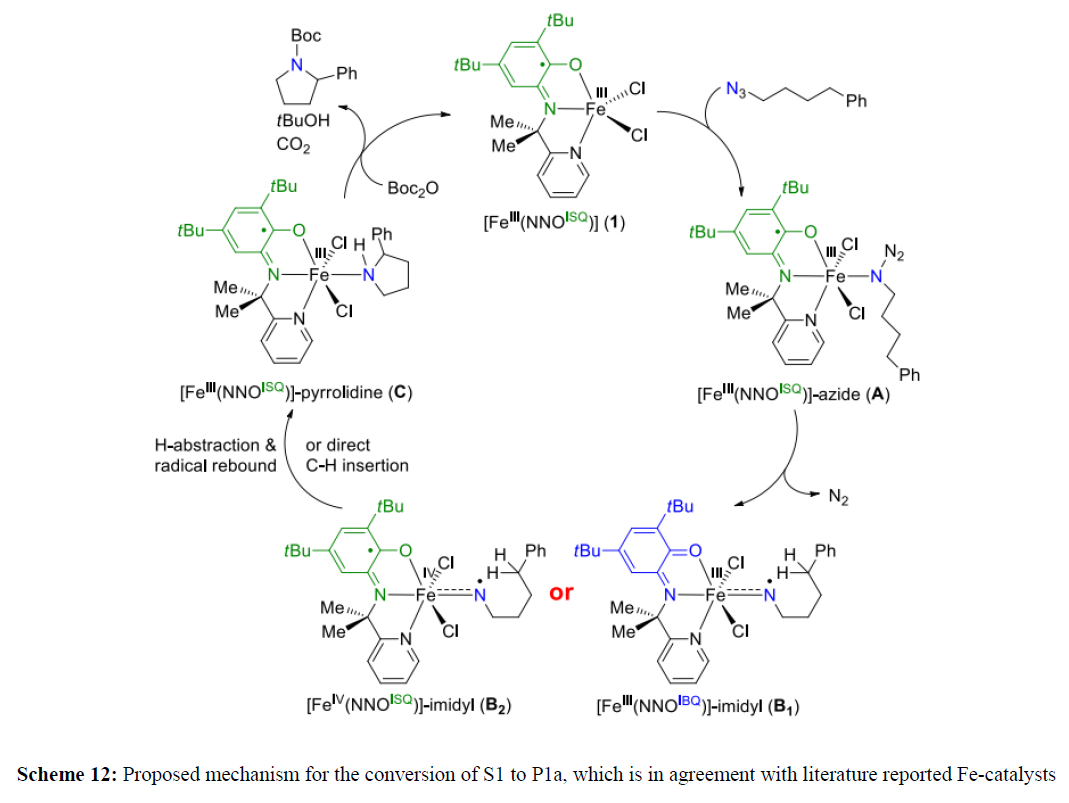
Mechanistic investigation suggested that initial activation of Fe complex by Boc2O (present in slight excess relative to substrate) at elevated temperature (rate determining step) leads to the activated FeIII catalyst with a higher affinity for the substrate. Two potential ways for Boc2O to interact with 1 are depicted in Scheme 9: either chloride abstraction or reaction with the phenolate fragment of the redox-active H2LNNO ligand can (pseudo)reversibly generate a cationic FeIII species 1. Thereafter, facile coordination of the azide-substrate to the metal center gives adduct A and subsequent N2 elimination generates iron(III)-nitrenoid species B1. Either direct nitrene insertion (preferred for (homo)allylic substrates) B2 or H-atom abstraction and radical rebound forms the FeIII(pyrrolidine) adduct C. Finally, reaction with a Boc-containing species (denoted “Boc” in Scheme 9) -either the in situ generated tert-butoxycarbonyl chloride or the carbonate derivative of the NNO ligand; see Scheme 9 - releases Boc-protected N-heterocycle (P1a) and tert-butanol with regeneration of complex 1.
Multi-component Coupling Reaction
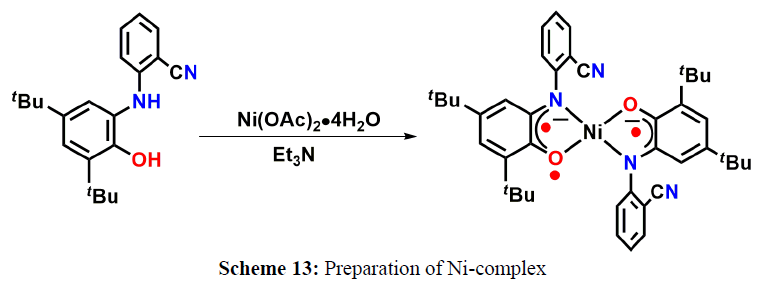
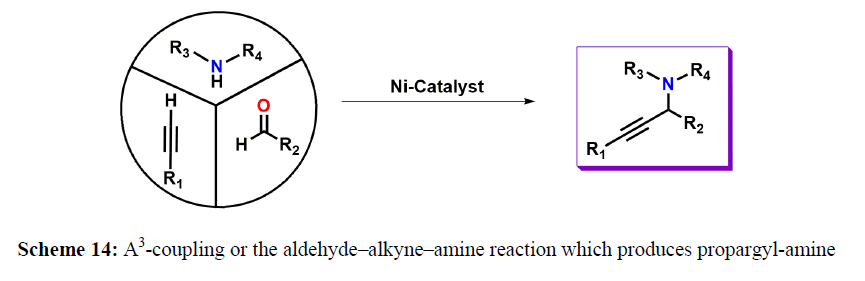
M. Nasibipour and coworkers demonstrated Ni-catalyzed coupling of aldehyde, alkyne, and amine that is usually referred as A3-coupling, has received greater attention due to its atom economy, step efficiency, and high chemical selectivity. This reaction was recommended to be carried out via the addition of in situ generated metal-alkynylide to iminium ion, that is formed in situ, via a reaction between amine and aldehyde and water molecule is the only side product (scheme 13-15).
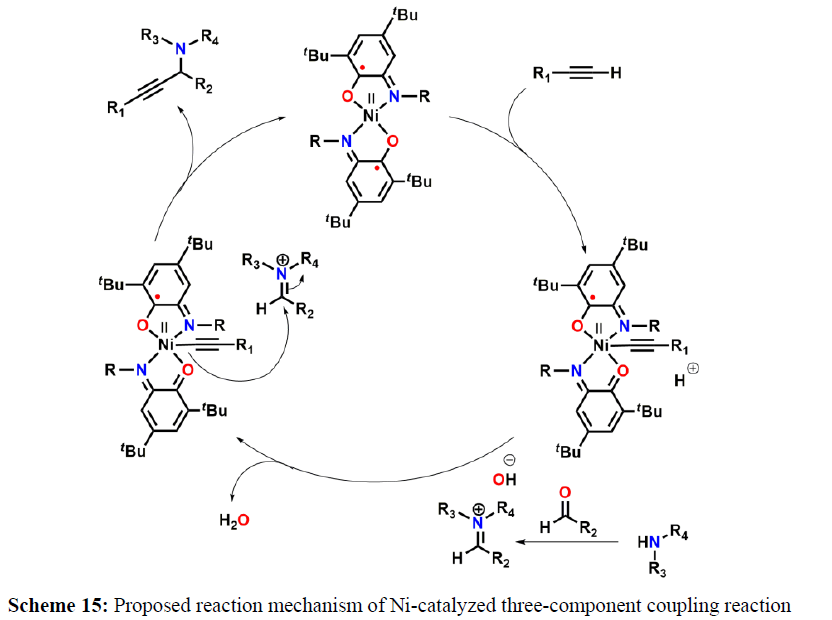
A plausible reaction pathway is proposed as shown in Scheme 15. In the present work NiL2ISQ itself acts as the active catalyst generating an initial nickel(II) acetylide intermediate by the reaction between a Ni(II) center in the NiL2ISQ complex with a terminal alkyne. One of the non-innocent iminosemiquinone LISQ ligands undergoes changes in its oxidation state and iminosemiquinonate/iminoquinonate form of the NiII complex, [NiIILISQLIBQ]+, is achieved and keeps the total oxidation state unchanged. Moreover, complexation of this nickel acetylide intermediate with iminium ion, which is generated in situ from the aldehyde and amine. Finally, the addition of acetylide to the iminium ion within the coordination sphere of nickel(II) gives propargylamine product and regenerates the nickel catalyst for the subsequent reactions [10].
CONCLUSION
All chemicals were employed are commercial products (Aldrich Chemical Co.) and were used without purification. All yields refer to isolated products after purification. 1H (300 MHz) NMR and 13C (75 MHz) NMR spectra were recorded on Varian mercury XL-300 and Bruker spectrometer instruments using TMS as internal standard. The solvent used for NMR spectra was CDCl3 and DMSO-d6. Infra-red spectra were taken on Shimadzu FTIR–408 on KBr pellet. The mass spectra were recorded on Shimadzu GC-MS QP trap 2010A mass spectrometer with an ionization potential of 70 eV. Column chromatography was performed on silica gel (230–400 mesh) supplied by Acme Chemical Co.
Notes
The authors declare no competing financial interest.
Acknowledgements
GCP thank ICFAI University Tripura for the laboratory facility. The author also thanks Prof. (Dr.) Chandan Mukherjee for some initial help in writing this review.
References
- Kaim W. Inorg Chem. 2011, 50(20): p. 9752;
- Wang Y, DuBois JL, Hedman B, et al., Science. 1998, 279: 537.
- Wachter RM, Montague-Smith MP, Branchaud BP. J Am Chem Soc. 1997, 119 (33): p. 7743.
- Bittner MM, Lindeman SV, Fiedler AT. J Am Chem Soc. 2012, 134: p. 5460.
- Chkraborty B, Paine TK. Angew Chem Int Ed. 2013, 52: p. 920.
- Singh K, Kundu A, Adhikari D. ACS Catal. 2022, 12: p. 13075.
- Jacquet J, Salanouve E, Orio M, et al., Chem Commun. 2014, 50: p. 10394.
- Broere DLJ, Metz LL, de Bruin B, et al., I Angew Chem Int Ed. 2015, 54: p. 1516.
- Broere DLJ, van Leest NP, de Bruin B, et al., I Inorg Chem. 2016, 55: p. 8603;
- Nasibipour M, Safaei E, Moaddeli A, et al., RSC Adv. 2021, 11: p. 12845.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref