Research Article - Der Pharma Chemica ( 2021) Volume 13, Issue 4
Chemical Speciation of Binary Complexes of L-Proline and L-Valine with Essential Metal Ca(Ii), Zn(Ii) and Mn(Ii) Ions in Sodium Lauryl Sulfate -Water Mixtures
Chaluma Sori*, Rajalakshmanan Eswaramoorthy and Hadgu Hailekiros BelayChaluma Sori, Department of Applied Chemistry, Adama Science and Technology University, Adama, Oromia, Ethiopia, Email: chalisori2021@gmail.com
Abstract
Chemical speciation of Ca (II), Zn(II)and Mn(II) complexes of L-Proline and L-Valine in 0.0-2.5 % w/v SLS-water mixtures has been studied pH-metrically at a temperature of 303K and at an ionic strength of 0.16 mol L−1. The selection of best fit chemical models is based on statistical parameters and residual analysis. The predominant species detected were ML+, MLH2+ and ML2H+ for Ca(II), Zn(II) and Mn(II). Models containing different numbers of species were refined by using the computer program MINIQUAD75. The appropriateness of the experimental conditions was verified by introducing errors deliberately. The chemical speciation, metal bioavailability and transportation have been explained based on stability constant and distribution diagrams drawn using HYSS HYPERQUAD.
Keywords
Chemical speciation, HYSS HYPERQUAD, L-Proline, L-Valine and SLS
Introduction
Chemical species is specific form of an element defined as to isotopic composition, electronic or oxidation state, or complex or molecular structure. Speciation analysis is analytical activities of identifying and measuring the quantities of one or more individual chemical species in a sample [1]. The chemical speciation of an element, either essential or toxic is relevant, because the chemical form in which an element enters the body, mostly determines its absorption and transport properties, and hence its biological and physiological activities. Speciation study of essential metal ion complexes is useful to understand the role played by the active site cavities in biological molecules and the bonding behavior of protein residues with the metal ion [2].
Zinc is an essential trace element for both animals and humans. Zinc plays vital roles in biological systems [3]. It can play either a predominantly catalytic role or a solely structural role to maintain the protein configuration. It is a versatile ion, as it can bind with different combinations of ligand types resulting in a broad range of stability, reactivity and functions. It is an essential trace element that functions as a cofactor for certain enzymes involved in metabolism and cell growth, it is found in nearly 300 specific enzymes [4].
Manganese is an essential trace element for human beings. It is essentialfor normal bone structure. The classes of enzymes that have manganese cofactors are very broad and include oxidoreductases, transferases, hydrolases, lyases, isomerases, and ligases. It is a component of several enzymes, for example superoxide dismutase, pyruvate carboxylase and glutamine synthetase [5]. Manganese exhibits a large scale of oxidation states from -III to +VII which makes this element an important cofactor of many enzymes, in particular for the catalysis of electron transfer reactions, e.g. Mn superoxide dismutase [6].
Calcium is the most abundant metallic element in the human body, one of the most abundant in other organisms and the third most abundant element in vegetation. Calcium is a structural component of bones, teeth and soft tissues and activates many metabolicprocessesIt helps in blood clotting, nerve signaling and the release of certain hormones. It plays an important role in the cell membranes and as a structural component and also in muscle contraction. Ca2+ ions are essential components of plant cell walls and cell membranes and are used to balance organic anions in the plant vacuole [7].
L-Proline (P) is a non-essential imino acid containing pyrrole type of nitrogen rather than the amino nitrogen of the amino acids [8]. It acts as a bidentate ligand and actively participates in acido-basic equilibria especially in physiological pH conditions. Proline is one of the main components of the collagen. It promotes the formation of bone, skin and cartilage. It is extremely important for the proper functioning of joints and tendons. It has several significant applications in biological systems [9].
L-Valine (V) is an essential proteinogenic amino acid and not synthesized in mammals. It acts as a bidentate ligand with one carboxylic group and one amino group. It is essentialin humans, meaning the body cannot synthesize it: it must be obtained from the diet. Valine promotes muscle growth and tissue repair. It is a precursor in the penicillin biosynthetic pathway. It also lowers elevated blood sugar levels and increases growth hormone production. It has wide applications in pharmaceutical and food industries [10].
Sodium lauryl sulphate (SLS) or sodium dodecyl sulphate is an anionic surfactant used in many cleaning and hygiene products, food, pharmaceuticals, and cosmetics. It is an anionic surface active agent and it is the sodium salt of lauryl sulfate that conforms to the formula: CH3(CH2)10CH2OSO3Na [11]. It is an anionic surfactant and profoundly influences the bulk properties of physiological systems. Sodium lauryl sulfate can solubilise, concentrate and compartmentalize ions and molecules [12]. Sodium lauryl sulfate–water mixtures are chosen in these studies to maintain the dielectric constants of the medium comparable to those of the physiological fluids since the polarity of the active site cavities should generally be applicable, to compare ligand binding to the metal ion in protein. Some studies were performed in aqua-organic mixtures to mimic the low dielectric constant at active site of bio systems but no attempt was made to account for the compartmentalization. Hence, in this study the speciation of the binary complexes of essential metal ions (Ca, Zn and Mn) with L-Proline and L-Valine has been studied in surfactant media where micellisation leads to compartmentalization.
Experimental
Chemicals and Standard Solution
All the chemicals used in this experiment were of analytical reagent grade purity. Triple distilled deionized water was used for the preparation of all the solutions. 0.1 mol dm-3 Aqueous solutions of Ca(II), Zn(II) and Mn(II) chlorides (Merck, India) were prepared maintaining 0.05 mol L-1 hydrochloric acid to suppress the hydrolysis of metal salts. 0.05 mol dm-3 aqueous solutions of L-proline and L-valineAR grade (SRL, India)were prepared by maintaining a 0.05mol L-1 hydrochloric acid concentration to increase its solubility. Sodium lauryl sulphate (SLS, Qualigens, India) was used as received and its purity was checked by determining critical micellar concentration (CMC). Solutions of 0.2 mol L-1 hydrochloric acid (AR grade, Merck, India) and 0.4 mol L-1 sodium hydroxide (E-Merck, India) were prepared. A solution of 2.0 mol L-1 sodium chloride (Merck, India) was prepared to maintain the ionic strength in the titrand. All the solutions were standardized by standard methods. To assess the errors that might have crept into the determination of the concentrations, the data were subjected to analysis of variance of one way classification (ANOVA) using the computer program COST (concentration of solution by titration) [13]. The strength of alkali and miner acid were determined using the Gran plot method [14].
Apparatus
The materials used in this study were: Test tubes, pipette, pH meter, burette, glass electrode, funnel, 250 Erlenmeyer flasks, mass balance, measuring cylinder, dropper, volumetric flask, magnetic stirrer, beaker and water bath.
Alkalimetric Titrations
The pH measurements of metal-ligand binary systems were carried out in media containing varying amounts of sodium lauryl sulfate in the ranges of 0-2.5% (w/v) maintaining an ionic strength of 0.16 mol dm-3 with NaCl at 310 K by using a digital pH meter (ModelAd8000, India) of readability (0-14 pH). The electrode of the cell was calibrated with 0.05 mol L-1 potassium hydrogen phthalate solution in the acidic region and with 0.01 mol L-1 borax solution in the alkaline region. The effect of variation in asymmetry potential, liquid junction potential, activity coefficient, sodium ion error and/or dissolved carbon dioxide on the response of glass electrode is to be considered for accurate determinations. The correction factor proposed by McBryde and others to account for the above parameters was used in the present electrometric titrations [15]. To verify whether the electrode was equilibrated, a strong acid was titrated with an alkali every day until no appreciable differences were observed between the pH values of two titrations at the corresponding volumes of titrant. Free acid titrations were performed before the metal -ligand titrations to calculate the correction factor. In each of the titrations, the titrand consisted of 1 mmol hydrochloric acid in a total volume of 50 cm3. Titrations with different metal-to-ligand ratios (1:2.5, 1:3.75, and 1:5) were carried out with 0.4 mol dm−3 sodium hydroxide.
Modeling Strategy
The binary and ternary stability constants were calculated from pH metric titration data using the computer program MINIQUAD75. The best-fit chemical model for each system was investigated by using non-linear least-squares computer program, MINIQUAD75 [16]. The chemical speciation, metal bioavailability and transportation were explained based on the distribution diagrams drawn using HYSS HYPERQUAD [17].
Results and Discussion
The results of the best fit models that contain the stoichiometry of the complex species and their overall formation constants along with some of the important statistical parameters are given in Tables 1 & 2.
Ucorr = U/(NP-m)X108, where m = number of species; NP=Number of experimental points; SD=Standard deviation
Very low-standard deviation in overall stability constants (log β) signifies the precision of these data. Small values of mean, standard deviation, and mean deviation for the systems corroborate that the residuals are around a zero mean with little dispersion.A perusal of Tables 1 & 2 indicates that the χ2 values range between 10.09 – 13.9 for SLS-water mixtures. The values of kurtosis and skewness range from 1.6 – 4.0 and -1.0 – 0.9 for SLS-water mixtures, respectively. Deviation of the values of kurtosis and skewness from three and zero, respectively, show the tendency of these residuals to concentrate more to the left or right of the mean and broadening of the peak. However, the values of Ucorrin all the three mass-balance equations are very low confirming the adequacy of the chemical model to represent the experimental data.
| %w/v | log βmlh(SD) | NP | Ucorr | Skew- | χ2 | Kurto- | R- | pH- | ||
|---|---|---|---|---|---|---|---|---|---|---|
| ML+ | MLH2+ | ML2H+ | 8 *10 |
ness | sis | factor | Range | |||
| Ca(II) | ||||||||||
| 0 | 2.15(2) | 12.71(4) | 15.10(1) | 100 | 0.1 | 0.6 | 12 | 2.5 | 0.005 | 2.6-11.4 |
| 0.5 | 2.30(0) | 12.96(1) | 15.50(2) | 120 | 1.4 | 0.1 | 11.8 | 3 | 0.002 | 2.4-11.0 |
| 1 | 3.55(1) | 12.98(9) | 15.89(0) | 99 | 0.6 | 0.5 | 12.5 | 2.5 | 0.006 | 1.5-11.0 |
| 1.5 | 3.49(6) | 13.12(0) | 16.55(2) | 90 | 0.5 | 0.9 | 11.2 | 1.6 | 0.001 | 1.5-11.0 |
| 2 | 2.31(8) | 13.20(2) | 15.40(1) | 103 | 3.7 | 0.3 | 11 | 3.1 | 0.005 | 1.7-11.0 |
| 2.5 | 3.09(3) | 12.87(3) | 15.75(0) | 107 | 3.1 | 0 | 12.1 | 2.8 | 0.004 | 1.7-11.0 |
| Zn(II) | ||||||||||
| 0 | 6.70(0) | 13.30(3) | 20.35(1) | 99 | 1.9 | 0 | 12.09 | 3.5 | 0.002 | 1.5-8.7 |
| 0.5 | 6.55(9) | 12.56(9) | 19.40(0) | 100 | 2.5 | 0.3 | 12.08 | 3.9 | 0.005 | 1.5-9.2 |
| 1 | 6.61(1) | 13.77(0) | 19.75(9) | 130 | 2 | 0.9 | 11.09 | 3.1 | 0.009 | 1.5-9.2 |
| 1.5 | 6.60(0) | 13.30(3) | 20.29(9) | 88 | 3.8 | 0.4 | 11.08 | 2.8 | 0.005 | 1.5-9.2 |
| 2 | 6.92(1) | 13.99(6) | 20.38(8) | 90 | 3 | 0.1 | 12.01 | 2.5 | 0.01 | 1.5-9.2 |
| 2.5 | 6.45(2) | 14.19(2) | 20.00(1) | 104 | 3 | 0.2 | 10.09 | 2.3 | 0.013 | 1.5-9.2 |
| Mn(II) | ||||||||||
| 0 | 4.00(1) | 12.39(9) | 16.71(0) | 99 | 1.1 | 0.6 | 12.03 | 3 | 0.001 | 1.6-9.7 |
| 0.5 | 4.80(0) | 12.50(0) | 17.67(7) | 100 | 3.6 | 0.2 | 11.09 | 3 | 0.004 | 1.6-9.7 |
| 1 | 5.24(9) | 13.57(9) | 17.90(2) | 103 | 3.3 | 0.1 | 12.06 | 2.9 | 0.005 | 1.6-9.7 |
| 1.5 | 5.38(5) | 13.79(8) | 17.68(1) | 105 | 2.9 | 0.6 | 12.09 | 3.8 | 0.002 | 1.6-9.7 |
| 2 | 5.90(1) | 13.10(6) | 18.20(0) | 90 | 3 | 0.4 | 10.09 | 3.1 | 0.001 | 1.6-9.7 |
| 2.5 | 6.67(3) | 13.70(6) | 18.99(9) | 80 | 2.6 | 0.8 | 11,99 | 2.9 | 0.003 | 1.5-9.7 |
| %w/v | log βmlh(SD) | NP | Ucorr | Skew- | χ2 | Kurto- | R- | pH- | ||
|---|---|---|---|---|---|---|---|---|---|---|
| ML+ | MLH2+ | ML2H+ | 8 *10 |
ness | sis | factor | Range | |||
| Ca(II) | ||||||||||
| 0 | 2.40(7) | 11.4(0) | 14.00(0) | 88 | 0.7 | -0.6 | 12.9 | 3.1 | 0.007 | 1.7-10.6 |
| 0.5 | 1.80(9) | 11.60(9) | 13.89(1) | 50 | 0.7 | 0.6 | 11.2 | 3.8 | 0.01 | 1.7-10.6 |
| 1 | 2.79(0) | 11.49(8) | 14.40(2) | 56 | 1 | 0.1 | 11 | 2.9 | 0.007 | 1.7-10.6 |
| 1.5 | 2.71(9) | 12.15(6) | 14.37(3) | 49 | 0.6 | -0.2 | 11 | 2 | 0.004 | 1.7-10.6 |
| 2 | 2.89(1) | 12.59(6) | 14.80(1) | 100 | 1.6 | 1 | 12.8 | 3.6 | 0.02 | 1.7-10.6 |
| 2.5 | 3.37(5) | 11.90(3) | 14.63(4) | 76 | 1.4 | -0.3 | 12.8 | 3.3 | 0.001 | 1.7-10.6 |
| Zn(II) | ||||||||||
| 0 | 5.00(5) | 11.61(1) | 16.56(0) | 140 | 0 | 0.2 | 11.7 | 3.5 | 0.005 | 1.5-8.5 |
| 0.5 | 5.60(9) | 11.37(0) | 17.00(0) | 77 | 1.9 | 0.8 | 10.9 | 3.9 | 0.013 | 1.5-8.5 |
| 1 | 5.67(2) | 12.50(7) | 17.49(1) | 110 | 0.6 | 0.4 | 12 | 4 | 0.007 | 1.5-8.5 |
| 1.5 | 6.00(0) | 12.60(8) | 17.51(3) | 40 | 0.9 | 0.7 | 12.7 | 2.9 | 0.009 | 1.5-8.5 |
| 2 | 6.59(3) | 13.19(2) | 18.09(1) | 89 | 1 | 0.9 | 11 | 3.1 | 0.014 | 1.5-8.5 |
| 2.5 | 6.01(1) | 13.09(1) | 18.17(0) | 89 | 1.5 | 0.1 | 12.7 | 2.8 | 0.02 | 1.5-8.5 |
| Mn(II) | ||||||||||
| 0 | 3.0(6) | 11.58(5) | 14.70(0) | 99 | 0.9 | -0.5 | 13.9 | 3.4 | 0.001 | 1.5-9.0 |
| 0.5 | 3.65(1) | 11.80(3) | 15.34(2) | 90 | 0.7 | 0.4 | 13.4 | 3.7 | 0.009 | 1.5-9.0 |
| 1 | 3.60(0) | 12.20(0) | 15.51(2) | 85 | 1.9 | -1 | 11 | 2.1 | 0.003 | 1.5-9.0 |
| 1.5 | 4.79(7) | 12.15(6) | 16.30(9) | 100 | 0.3 | -0.5 | 11.4 | 3.1 | 0.007 | 1.5-9.0 |
| 2 | 4.67(5) | 12.81(2) | 15.80(8) | 106 | 7 | 0.4 | 12.4 | 2.8 | 0.007 | 1.5-9.0 |
| 2.5 | 5.67(1) | 13.14(0) | 17.00(3) | 80 | 9 | 0.1 | 13.9 | 2.7 | 0.003 | 1.5-9.0 |
Effect of systematic errors on best fit model
In order to rely upon the best-fit chemical model for critical evaluation and application under varied experimental conditions with different accuracies of data acquisition, an investigation was undertaken by introducing pessimistic errors in the influential parameters like concentrations of alkali, mineral acid, ligand, metal andtotal volume [18]. The data given in Table 3 show that the order of the ingredients that influence the magnitudes of stability constants due to incorporation of errors is alkali > acid > ligand > metal. The increased standard deviation in stability constants and even rejection of some species on the introduction of errors confirms the correctness of the proposed models Table 3.
| log βmlh(SD) | ||||
|---|---|---|---|---|
| Ingredient | % Error | ML+ | MLH2+ | ML2H+ |
| 0 | 3.49(6) | 13.12(0) | 16.55(2) | |
| Alkali | -5 | Rejected | 16.34(55) | Rejected |
| -2 | Rejected | 16.09(34) | Rejected | |
| 2 | Rejected | 16.99(22) | Rejected | |
| 5 | 6.99(34) | 16.10(56) | Rejected | |
| Acid | -5 | 6.90(**) | 15.45(**) | Rejected |
| -2 | 6.45(60) | 15.34(45) | 20.09(30) | |
| 2 | Rejected | 15.90(33) | Rejected | |
| 5 | Rejected | Rejected | Rejected | |
| Ligand | -5 | 4.65(50) | Rejected | 17.45(23) |
| -2 | 4.45(30) | 14.43(40) | 17.45(29) | |
| 2 | 4.08(30) | 14.26(45) | 17.34(20) | |
| 5 | 4.34(66) | 14.62(34) | 17.00(40) | |
| Metal | -5 | 3.40(12) | 13.14(14) | 16.50(12) |
| -2 | 3.41(19) | 13.11(14) | 16.46(19) | |
| 2 | 3.55(18) | 12.13(14) | 15.49(11) | |
| 5 | 3.46(20) | 13.15(14) | 16.40(10) | |
Effect of Surfactant on Metal-Ligand Equilibria
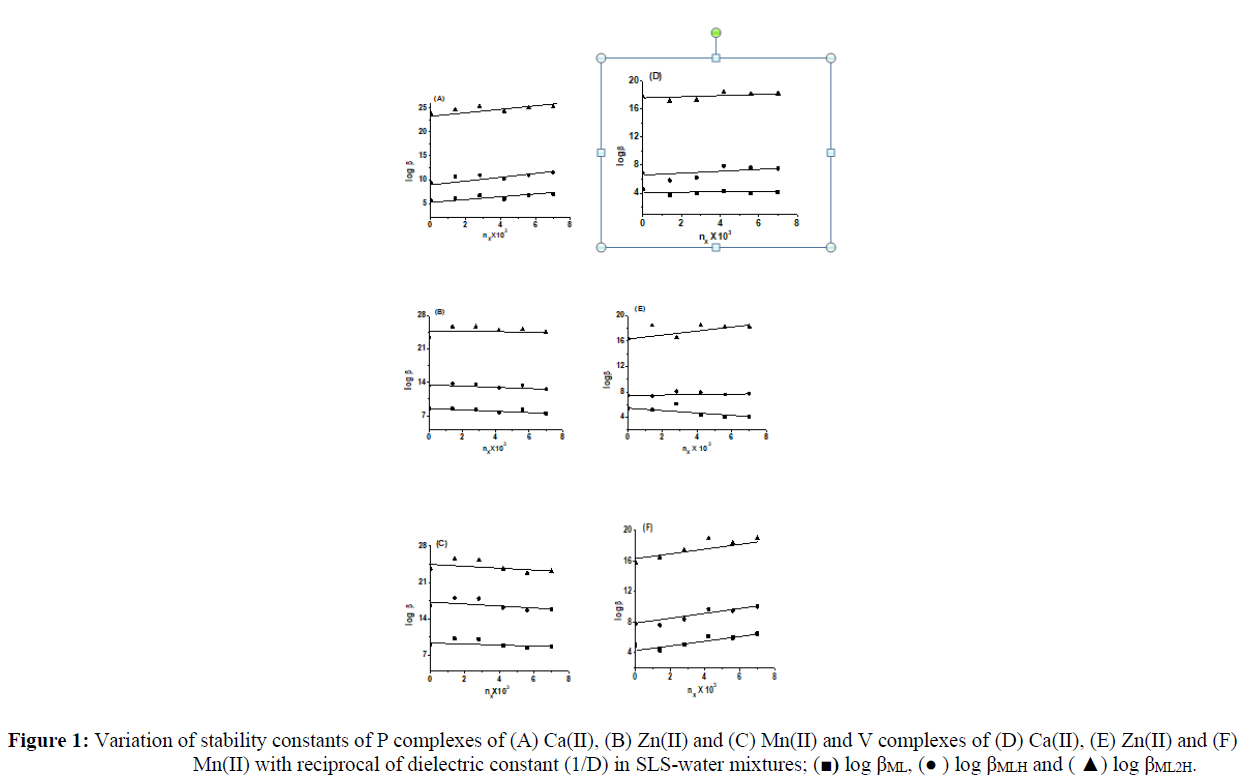
Co-solvent influences the equilibria in solution due to change in the dielectric constant (D) of the medium that varies the relative contribution of electrostatic and non-electrostatic interactions, which in turn vary the magnitude of stability constants. The surfactant in aqueous solution considerably decreases the dielectric constant and these solutions are expected to mimic the physiological conditions. The formation of micelles creates low dielectric environment at the core of the micelles and the reactions taking place at the core [19]. The equilibria in solution is influenced by the surfactants due to change in the dielectric constant (D) of the medium that varies the relative contribution of electrostatic and non-electrostatic interactions which in turn vary the magnitude of stability constants. The variations of stability constants (log β) with mole fraction of different SLS-water media are shown in Figure 1. The linear variation of the stability constants of the L-proline and L-valine complexes of Ca(II), Zn(II) and Mn(II) in SLS–water mixtures with mole fraction indicates that electrostatic forces dominate the equilibrium process under the employed experimental conditions. The stability of a complex depends on the polarity of the medium and the electrostatic attraction or repulsive forces
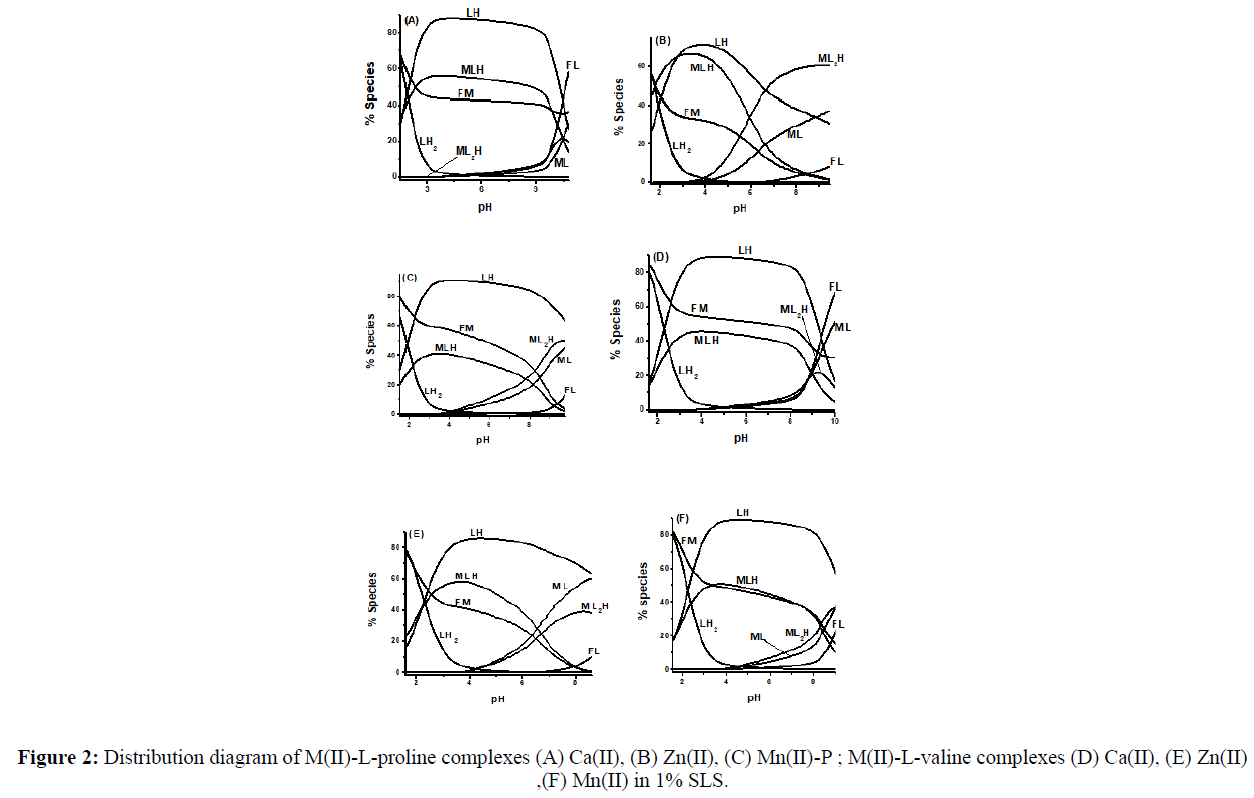
Distribution Diagrams
The binary species that are refined in present study are ML+, MLH2+ and ML2H+ for Ca(II), Zn(II) and Mn(II) with L-proline and L-valine in SLSwater mixtures. The formation of various binary complex species is represented as Equilibria 1-5 for both the ligands.
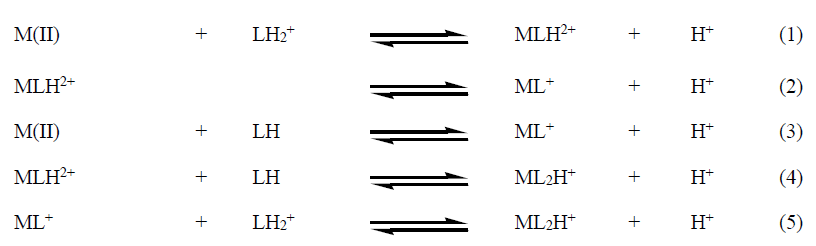
For all metal-ligand systems, MLH2+ species is formed by the interaction of metal ion with LH2 + (Equilibrium 1), where the percentages of FM and LH2 + are decreasing with increasing percentage of MLH2+. The ML+ species is formed by deprotonation of MLH2+ (Equilibrium 2) or by the interaction of FM with LH species (Equilibrium 3). But Equilibrium 2 is more predominant than Equilibrium 3, because the concentration of FM decreases while concentration of LH increases with pH. ML2H+ species is formed through Equilibria 4 and 5. But Equilibrium 4 is predominant than Equilibrium 5, because the concentration of MLH2+ and LH are decreasing with increasing concentration of ML2H+. An observation made from the distribution diagrams (Figure 2)is that the free metal ion concentration is more in case of L-valine than L-prolinefor Ca(II), Zn(II) and Mn(II) in SLS-water mixtures. This infers the stronger complexing ability of L-proline than L-valine.
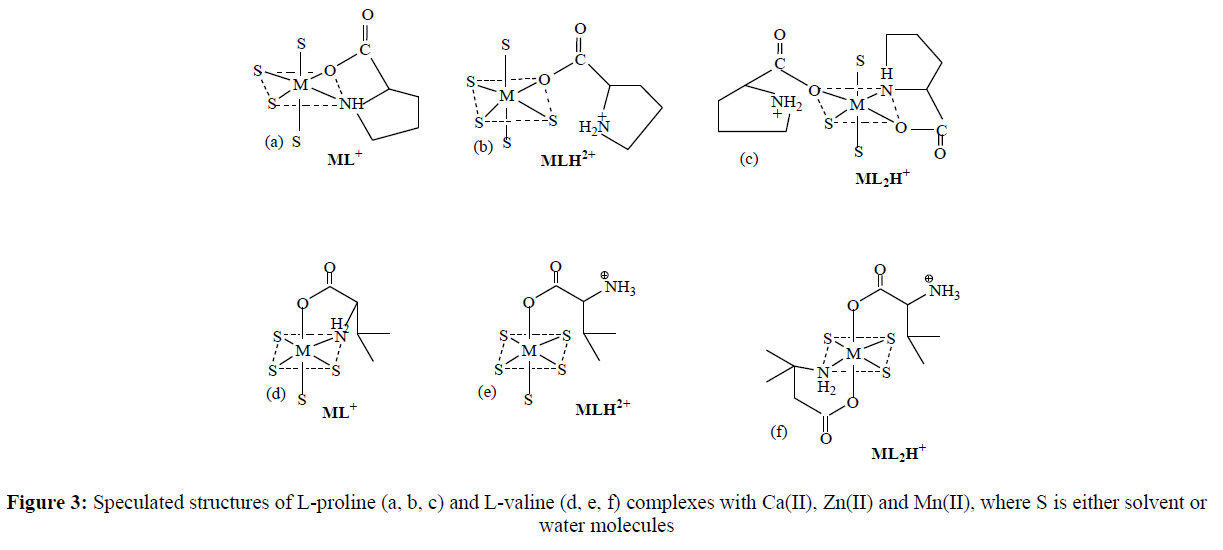
Structures of Binary Complexes
Although it is not possible to elucidate or confirm the structures of the complex’s pH metrically, they can be proposed based on literature reports and chemical knowledge. Amino nitrogen and carboxyl oxygen of P and V participate in bonding with metal ions. . Literature shows that, in aqueous solution, Ca(II), Zn(II) and Mn(II) ions typically form octahedral complexes. Thus, octahedral structures have been proposed as given in Figure 3.
Conclusion
The following conclusions have been drawn from the modeling studies of the speciation of binary complexes of Ca(II), Zn(II) and Mn(II) with Lproline and L-valine in SLS-water mixture. The binary species refined for both ligands are ML+, MLH2+ and ML2H+. The stability constants of these species are found to follow the trend Ca(II)<Mn(II) < Zn(II). The linear variation of the stability constants with mole fraction of SLS indicates the dominance of electrostatic forces. Errors introduced in the concentrations of ingredients affected the magnitudes of stability constants of metal complexes which were in the order: alkali > acid > ligand > metal.
Acknowledgment
Thanks to Adama Science and Technology University for funding this research project under the grant number ASTU/SM-R/007/19, Adama, Ethiopia
References
- Jiwan Singh and Ajay S Kalamdhad, Int. J Env Eng Re, 2013. 2: p. 27-37.
- Belay HH, Sailaja BBV and Rao GN. Der Pharma Chemica, 2015. 7(12), pp. 232-240.
- Dudev T and Lim C. J Chin Chem Soc, 2003. 50: pp. 1093-1102.
- Osredkar N. Sustar, J Clinical Toxicology, 2011. 53: pp. 1-18.
- Szpetnar M, Luchowska-Kocot D, Boguszewska-Czubara et al., Neurochemical Research, 2016. 41: pp. 2129-2139.
- Michalski B. Biomed Appl J Chromatogr, 1996. 684: pp. 59-75.
- White PJ and Martin RB. Ann Bot, 2003. 92: pp. 487-511.
- Bhushanavathi P, Veeraswamy B, Rao GN et al., J Chem, 2012. 9(2): pp. 517-524.
- Iwamura H, Mathew SP and Blackmond DG. J American Chem Soc, 2004. 126(38): p. 11770-11771.
- Bartek T, Blombach B, Zönnchen E et al., Biotechnol.Prog.2010. 26(361).
- Nikitakis JM, McEwen GN, Wenninger JA. The Cosmetic, Toiletry, and Fragrance Association Inc., 1991.
- Ramanaiah M, Rao CN and Sailaja BB. Physical Sciences, 2014.84(4): p. 485-494.
- Rao RS and Rao GN. Himalaya Publishing House.,2005.
- Gans P and O Sullivan B. Talanta, 2000. 51(1): p. 33-37.
- Havel J and Meloun M., Talanta, 2007. 32(3): p. 171.
- Gans P, Sabatini A and Vacca A. Inorganica Chimica Acta, 1976. 18: p. 273.
- Alderighi LP, Gans A, Ienco D et al., Coord Chem Rev, 1999. 184: p. 311.
- Surya SunithaCh and Nageswara Rao, Int. J. Adv. Res., 2017.5(11): p. 449-456.
- Gandham Hima Bindu and Gollapalli Nageswara Rao. Chemical Speciation & Bioavailability, 2011. 23(2): p. 88-95.