Research Article - Der Pharma Chemica ( 2020) Volume 12, Issue 5
Chromium Adsorption Using Modified Locust Bean and Maize Husk
Ugwoke O. Chijioke1, Dauda Benjamin2, Ezugwu A. James3, Abugu H. Onyeka3*, Alum L. Ogechi3, Eze S. Ifeanyi3 and Odewole O. Abiola32Department of Textile Science and Technology, Ahmadu Bello University, Zaria, Nigeria
3Department of Pure and Industrial Chemistry, University of Nigeria, Nsukka, Nigeria
Abugu H. Onyeka, Department of Pure and Industrial Chemistry, University of Nigeria, Nsukka, Nigeria, Email: hillary.abugu@unn.edu.ng
Received: 30-Jun-2020 Accepted Date: Aug 14, 2020 ; Published: 30-Aug-2020
Abstract
Carbon-based adsorbent from the hardwood of locust bean and maize husks were assessed for their potential in reducing Cr6+ ions in contaminated industrial effluent waters. Two samples of activated carbon (AC) were successfully prepared by a simultaneous two step carbonization and activation processes using maize husk (Zea mays) and locust bean (Entada africana). Chemical activation was achieved by first impregnating the prepared raw materials with 60 %(v/v) and 80 %(v/v) H3PO4 acid and thereafter subjected to carbonization at 550 oC and 950 oC for a period of 1½ hr and 4 hr, respectively. The ACs were characterized and isotherm plot was produced for the adsorption of methylene blue (MB) from which the adsorption capacity was calculated. The effect of pH, adsorbent dosage and time were studied. The results showed large surface area (115.69 to 143.74 m2/g), as suggested by the SEM result, which indicates the possibility of high adsorption of Cr6+ at pH 2 – 6, and that was due largely to HCrO4- than by any other Cr6+ species. The pseudo-first order kinetic best described the data obtained and so suggest physisorption mechanism involved in the adsorption of Cr6+ unto LBAC and MHAC. The relatively high percentage ion removal by LBAC and MHAC suggests that they can be used as inexpensive, efficient and environmentally friendly alternatives in the cost-effective removal of Cr6+ from industrial wastewaters when these modification conditions are used.
Keywords
Activated carbon, Adsorption, Chromium, Locust bean, Maize husk
Introduction
Heavy metal pollution is a serious problem today and its removal from the environment is of serious concern due to their bioaccumulation and persistence in the environment [1]. Like organic pollutants, most heavy metals do not undergo biological degradation into harmless end products [2]. Chromium’s applications are in chrome plating, stainless steel and in metal ceramics alloys. Chromium plating was once widely used to give a polished mirror finish steel [3]. In metallurgy, Cr is used to give a shiny finish, corrosion resistance and to harden stainless steel [4]. It is also used as industrial catalyst and pigments in dyeing and tanning of leather. Cr (IV) oxide (CrO2) is used in the production of magnetic tape [5].
People could be exposed to Cr via inhalation, ingestion and through dermal contact with Cr or Cr compounds since Cr is produced in environmental matrices such as air, water and soil [6]. The level of Cr in air and water is generally low. In drinking water, the level of Cr is low as well, but Cr contaminated well water may contain the dangerous Cr (IV) [7]. For most people eating food that contains Cr (III) is the main form of Cr uptake, as chromium (III) occurs naturally in many vegetables, fruits, meats, yeasts and grains [7]. Chromium (III) is an essential nutrient for humans and shortages may cause heart conditions, disruptions of metabolisms and diabetes [8], but the uptake of too much chromium (III) causes skin rashes. Cr (VI) is dangerous to health especially for those that work in chromium-plating and textile industries. Tobacco smokers also have a higher risk of exposure to chromium effects [9]. Cr can cause nose irritations and nose bleeds after breathing in Cr (IV). Other health problems that are associated with chromium (VI) are: stomachs ulcers and upset, respiratory complications, genetic material alteration, lowering of the immune system, liver and kidney problems and even death [10].
The oxidation state of Cr determines the health hazards associated with its exposure. Chromium metal ions cause diseases such as liver damage, nephritis and stomach distresses. Cr. (VI) ions are the major cause of nasal mucous ulcer [11].
Industrial applications of adsorbents became a common practice following the widespread use of charcoal for decolorizing liquids and, in particular, its use in gas mask during the 1914-1918 World War for the protection of military personnel from poisonous gases [12]. Adsorbents for the drying of gases included alumina, bauxite, and silica gel; bone char and other carbons were used for sugar refining and the refining of some oils, fats and waxes. Activated charcoal was employed in the recovery of solvents, elimination of odours and the purification of air and industrial gases. Fuller's earth and magnesia were found to be active in adsorbing contaminants of petroleum fractions and oils, fats and waxes [13]. Base-exchanging silicates were used for water treatment while some chars were capable of recovering precious metals [14]. A major limitation to the use of non-carbon-based sorbents in water purification is that they are expensive. Hence, there is a need for the development of safe and low-cost alternatives to the expensive and commercially available adsorbents. Many researchers have successfully investigated the production of carbon-based adsorbents using agricultural wastes [15] such as sugarcane bagasse [16], African canarium seed [17], Oil bean and snail shell [18], rice husk [19], sawdust, coconut husk [20], oil palm shell [21], for the removal of heavy metals from wastewater. According to Choudhari [15], a low-cost adsorbent requires less processing, abundant in nature and could be waste material and by-products and, higher adsorption capacity may offset the cost of additional processing if required. Ajayi-Banji [22], modified Locust bean to char and used it in heavy metal sequestration but not chromium. Jalil et al, [23], chemically (Ca (OH)2) modified and calcined maize husk and used it to adsorb malachite green, but Indah et al, [24] did not modify it and used it to remove iron from aqueous solution. Therefore, this study seeks to explore Locust bean and Maize husk, as heavy metal (chromium) adsorbents from aqueous solution at these different modification conditions.
Experimental
Standard stock solutions
Standard Stock Solution of K2Cr2O7 (1000 mg/L); Activating Chemical, H3PO4 (Concentrations of 40%, 60% and 80%); 1,5-Diphenylcarbohydrazide solution (DPC) (1%): Methylene Blue stock (1000 mgL-1) Solution Blank solution for control. All reagents were purchased from Sigma-Aldrich Company.
Adsorbent
The activated carbons used in this study were produced from the hard wood of a locust bean and from maize husks. The raw materials were first cut into small sizes before washing thoroughly with water and allowed to dry in an oven at 1050C. After cooling to room temperature, they were soaked in 80% and 60% phosphoric acid (H3PO4), respectively. Each mixture was allowed to impregnate for 48 hours after which the acid was drained off and the base materials dried to constant weight at 1050C. The locust bean and maize husks impregnated materials were simultaneously carbonized and activated in a muffle furnace at the optimized conditions of 9000C for 4 hours and 5500C for 90 minutes, respectively. When the activation time was reached, the furnace was disconnected from the power supply and the carbon allowed to cool in the absence of air. The cooled carbon was pulverized further, filtered with 125 - 45μm sieve and then washed with de-ionized water till a near neutral pH (6.3) was achieved on the carbon surfaces. In the designation of the carbons, LBAC refers to locust bean based activated carbon and MHAC represents activated carbon from maize husks.
Characterization of Activated Carbons (AC)
Ash content determination
The percentage ash content was determined according to the ASTM D2866-94 method. Dry AC sample (1.0 g) was placed in ceramic crucible and moved into a heated muffle furnace at 1000 0C. The furnace was left on for one hour after which the crucible and its content was transferred to desiccator and allowed to cool. The crucible and content were reweighed and the weight lost was recorded as the ash content of the AC sample (W). Thereafter, percentage ash content (dry basis) was calculated using equation 1.
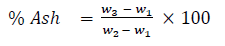 (1)
(1)
where, W1 = Weight of the crucible (g); W2 = Weight of the crucible + Activated carbon sample (g); W3 = Weight of the crucible + ash containing sample (g).
Moisture content determination
Approximately 1.0 g of powdered AC was weighed into a crucible. The crucible was positioned in a hot electric oven maintained at about 110 0C. The sample was constantly removed and reweighed at a 30 min interval until a constant weight (W) was obtained. The crucible and its content was retrieved and cooled in desiccator. The percentage moisture content was calculated using equation 2.
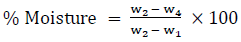 (2)
(2)
where, W1 = Weight of the crucible (g); W2 = Weight of the crucible + Activated carbon sample (g); W4 = Weight of the crucible + ash containing sample (g).
Specific surface area by methylene blue (MB) equilibrium adsorption
The experimental standard MB solution was prepared by diluting the stock solution with de-ionized water to obtain concentrations of 10, 20, 30, 40 and 50 mg/L. The initial absorbance of MB was determined at 630 nm by the UV – visible spectrophotometer [25]. The Methylene blue number was determined according to the Method of Joshi[26]. In this test, 0.02 g of each prepared activated carbon was mixed with 25 cm3 of methylene blue (MB) solution in several conical flasks separately at the different concentrations (10 - 50 mg/L). After shaking for 4 hr, the mixture was allowed to stand for additional 20 hr. The suspensions were filtered and the amount of MB remaining in the solution was determined through absorbance measurement.
C1 and C2 represent concentrations of MB in solution before and after its adsorbance by individual AC and A1 and A2 represent their spectrometric absorbance respectively. Then, At equilibrium, A2/A1 = C2/C1 giving C2 = (A2/A1) *C1. The surface area was calculated from equation 3 [25].
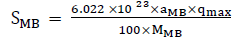 (3)
(3)
where SMB is the specific surface area, qmax is the amount of methylene blue adsorbed at the monolayer of adsorbent, αMB is the occupied surface area of one molecule of methylene blue (197.2 Å2)
Surface morphology and functional group
SEM was used to study the surface morphology of the adsorbents produced. The micrograph was taken at the Department of Chemical Engineering, Ahmadu Bello University, Zaria. Also, FTIR (JASCO FTIR-3500) was used to identify the functional groups present on the surface of the modified LBAC and MHAC.
Equilibrium adsorption
Eight (8) portions of 50 cm3 Cr6+ stock solutions with an initial concentration of 50 mg/L were treated with 0.5 g of the activated carbon. Prior to the addition of the adsorbent, the initial pH of the solutions was adjusted by drop-wise addition of 0.1 M HCl and/or 0.1M NaOH solution. The batch test was carried out for a pH range of 2.0 – 8.0. The study was conducted by introducing 0.5 g of the adsorbent into 50 cm3 of the metal ion solutions contained in beakers. The beakers were placed on an orbital stirrer and the speed set to 125 rpm. Components of the mixtures were allowed to contact and equilibrate for 2hr after which each mixture was filtered. 5 drops of DPC solution were added to every 30 cm3 portion of the filtrates containing Cr6+ ions after acidification with 1.0 cm3 of H3PO4 solution. The solutions were kept for few seconds (during which a red-purple coloration developed) and then analyzed for the equilibrium concentration of Cr6+ in the solution, using UV- Spectrophotometer. Due to the doubtful high values of % adsorption of Cr6+ at very low pH, all the other batch experiments were conducted at pH = 4 for Cr6+.
The effect of adsorbent dosage
Weighed quantities, in the range of 0.1 – 1.1 g, of each carbon were brought into contact with 50 cm3 portions of several 50 mg/L solutions of Cr6+ for a space of 2hr. The adsorption study was performed at a fixed pH = 4.
The study the effect of contact time on the adsorption of Cr6+ 0.5 g of activated carbon was placed in 6 separate conical flasks containing 50 cm3 of 50 mg/L of Cr6+ ions. The mixtures were mixed very well in a shaker and kept to equilibrate under room temperature condition. Each of the flasks was removed and content analyzed at intervals of 20, 40, 60, 80, 100, and 120 min. Filtrates were treated as usual to determine the amount of Cr6+ species left after adsorption.
The pattern of adsorption of these ions on the adsorbents was modeled by adding 50 cm3 of 20, 40, 60, 80, and 100 mg/L solutions of each ion into glass beakers. A fixed amount of 0.5 g of the carbon was introduced. After treatment as in earlier experiments, the mixtures were then separated by filtration.
The final concentrations of ions at equilibrium were measured using the absorption instruments. The amounts of ion adsorbed at equilibrium, qe (mg/g) were calculated from (1).
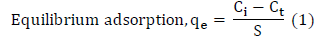
Where, qe is the quantity of ion adsorbed at time, t (mg/g). Ci and Ct (mg/L) is the liquid phase concentration at initial and any time t, respectively, s is the dosage. The adsorption kinetics were investigated using data obtained from “Batch studies on effect of contact time, at 50 mg/L. A number of adsorption kinetics models have been established to understand the adsorption kinetics and rate limiting step. The pseudo-first order model [27] was used for the adsorption of liquid adsorbate on solid adsorbent on the basis of adsorption capacity at different time intervals.
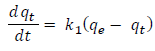 (2)
(2)
Where qe and qt are the adsorption capacity at equilibrium and at time t, respectively. k1 is the pseudo-first order rate constant of adsorption (min-1). After integration and applying boundary conditions t= 0 to t= t and qt= 0 to qt= qt to (2), we get a linear form as:
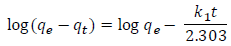 (3)
(3)
log(qe-qt) was plotted against t, and the values of the constants qe & k1 were calculated. The pseudo-second order model is based on certain assumption that the adsorption of adsorbate onto adsorbent supports second order chemisorptions.
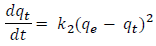 (4)
(4)
where k2 is the pseudo-second order rate constant of adsorption (g/mg/min).
For the boundary conditions t=0 to t= t and qt= 0 to qt= qt, the integrated form of (4) becomes:
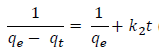 (5)
(5)
(5) is the integrated rate law for pseudo-second order chemisorption reaction, and can be rearranged to obtain
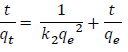 (6)
(6)
By plotting a curve of t/qt versus t, values of qe and the pseudo-second order rate constant of adsorption, k2 (g/mg/min) were evaluated.
The nature of adsorption (i.e monolayer or multilayer adsorption) of these ions on the adsorbents was investigated by adding 50 cm3 of 20, 40, 60, 80, and 100 mg/L solutions of each ion into glass beakers. A fixed amount of 0.5 g of the carbon was introduced. After treatment as in earlier experiments, the mixtures were then separated by filtration. The final concentrations of ions at equilibrium were measured using the absorption instruments. The amounts of ion adsorbed at equilibrium, qe(mg/g) were calculated from equation 1.
Conclusion
Characterization of the ACs showed LBAC had the best surface area, 143.74 m2/g and with the best-defined pores than MHAC. The presence of the oxygen-containing functional groups enhanced the adsorption process on the surface of the LBAC and MHAC. The adsorption isotherms investigation showed that the Langmuir adsorption isotherm is superior to Freundlich adsorption isotherm with higher coefficients of determination (R2) values, although, both isotherm models had comparably high fit for adsorption suggesting a possibility of the adsorption process obeying both models. The magnitude of the Langmuir constant, qe indicates that the amount of Cr6+ per unit weight of LBAC (8.7719 mg/g) required to form a complete monolayer on the surface is significantly higher than its value of 5.9067 mg/g on MHAC. Langmuir constant b, was relatively low overall, confirming high affinity between the adsorbents and adsorbates. Within the pH of our study (2 – 8), the Cr6+ was adsorbed on the carbon as either the HCrO4- or CrO42- species. Although CrO42- ions are very much available and should have contributed more to improve % adsorption at pH > 6, there was rather but a decrease, cuased by the dual competition of both CrO42- and OH- ions to be adsorbed on the active sites of the adsorbent of which OH- was dominant. The pseudo-first order kinetic best fit the data and so suggest a physiosorption mechanism in the adsorption of Cr6+ unto LBAC and MHAC. The relatively high percentage ion removal by LBAC and MHAC suggests that they can be used as inexpensive, efficient and environmentally friendly alternatives in the cost effective removal of Cr6+ from aqueous solution, though LBAC is a better adsorbent.
References
[1] L. Aljerf and M. Aljurf. Nutrition & Dietetics. 2020.
[2] U. Isah and M. Lawal, Advances In Applied Science Research. 2012. 3(6): p. 4033-4035.
[3] T. E. Graedel. J. Ind. Ecol., 2009. 13(1): p. 154-155.
[4] A. Fahim, A.E. Dean, M.D.A. Thomas et al., Materials and Corrosion. 2019. 70(2): p. 328-344.
[5] J.D. Lee, Concise inorganic chemistry. UK: Blackwell Science Ltd; 2006.
[6] Agency for Toxic Substances and Disease Registry (ATSDR), Public Health Service, Toxicological profile for chromium. 2012.
[7] B,V. Lenntech, Environmental Effects of Chromium. 2014.
[8] C.T. William and B.H. Frank. Role of Chromium in Human Health and in Diabetes. 2014.
[9] R.S. Pappas. Metallomics. 2011. 3(11): p. 1181.
[10] W.B. Sharon, K.L Samuel and H.D. Paul. International Programme On Chemical Safety. 1994.
[11] Moreno-castilla, Cadmium ion adsorption on different carbon adsorbents from aqueous solutions. 2004. 20(19): p. 8142-8148.
[12] C.C. Calgon. History of Activated Carbon. 2014.
[13] N. Le-Minh, E. C. Sivret, A. Shammay et al., Critical Reviews in Environmental Science and Technology. 2018. 48(4): p. 341-375.
[14] W. J. Thomas and B. Crittenden. Adsorption Technology & Design, 1998. p. 1-7.
[15] D. Choudhari, D. Sharma and A. Phadnis. Eur. Chem. Bull. 2013. 2(11): p. 880-886.
[16] W. Chandra, S. Chris and H. Hirofumi. Int. J. Eng. Sci., 2012. 3(1): p. 35-40.
[17] H. O. Abugu, P.A.C. Okoye, V.I.E. Ajiwe et al., Int. J. Innov. Res. Dev., 2014. 3(13): p. 418-446
[18] H. O. Abugu, P.A.C. Okoye, V.I.E. Ajiwe et al., J. Environ. Anal. Chem. 2015. 2(6): p. 1-17.
[19] R. Okpuwhara. M.Sc. Thesis. Nigeria: Ahmadu Bello University, Zaria. 2013.
[20] Z. Hu and M. Srinivasan. Micropor. Mesopor. Mater. 1999. 27: p. 11-18.
[21] E. Abechi, C. Gimba, A. Uzairu et al., Arch. Appl. Sci., 2011. 3(1): p. 154-164.
[22] A. A. Ajayi-Banji, A. Temitayo, A.T., Ewemoje et al., Environmental Research, Engineering and Management. 2015. 71(4): p. 5-10
[23] A. A. Jalil, S. Triwahyono, M. R. Yaakob et al., Bioresource Technology. 2012. 120: p. 218-224
[24] S. Indah, D. Helard and A. Sasmita. Water Science & Technology. 2016. 73(12): p. 2929-2935
[25] A. Itodo, F. Abdulrahman, L.G. Hassan et al., Newyork Science Journal. 2010. 3(5): p. 25-33.
[26] S. Joshi, M. Adhikari, B.P. Pokharel et al., J. Che Sci, 2013. 3(5): p. 19 - 24.
[27] S. Lagergren. Ksver Veterskapsakad Handligar. 1898. 24(4): p. 1-39.
[28] O. A. Ekpete, A. C. Marcus and V. Osi. J. Che., 2017. 6 pages.
[29] S. De Gisi, G. Lofrano, M, Grassi & M. Notarnicola. Sustainable Materials and Technologies. 2016. 9: p. 10-40.
[30] A. H. Abdullah, A. Kassim, T. Zainal et al., Malaysian J. Anal. Sci. 2000. 7 (1): p. 65-68.
[31] H.A, Aziza, M.N. Adlan and K.S. Ariffin. Bioresources Technology. 2008. 99: p. 1578-1583.
[32] A. H. Karoyo, L. Dehabadi and L. D. Wilson. ACS Sustainable Chemistry & Engineering, 2018. 6(4): p. 4603-4613.
[33] O. A. Ekpete and M. Horsfall Jr., Res J Chem Sci, 2011. 1(3): p. 10-17
[34] R.M. Rao, R.R. Bansode, J.N. Losso et al., Bioreources Technology. 2003. 90: p. 2629-2635.
[35] L. Aljerf. J Environ Manage. 2018. 225: p. 120-132.
[36] J. Rivera-Utrilla, I. Bautista-Toledo, M. Ferro-Gracia et al., Carbon. 2003. 41: p. 323-330.
[37] K. Mohanty, M. Jha, B. C. Meikap et al., Chem. Eng. J. 2006. 117: p. 71.
[38] T. Nassima and A. Moussa. Water Practice and Technology. 2009. 4(2): p.1-13.
[39] T. Karthiheyan, S. Rajgopal and L. R. Miranola. J. Hazard. Mater. 2005. 124: p. 192.
[40] Y.B. Zhan, A. Shukla, S. Shukla et al., J. Hazard. Mater. 2000. 80: p. 33-42.
[41] A.R. Mohammad, O.F. Mohammad and A.A. Shafiqul. Dhaka Univ. J. Sci. 2012. 60(1): p. 15-20.
[42] D.W. O’Connell, C. Birkinshaw and T.F. O’Dwyer, Bioresour. Technol. 2008. 99: p. 6709-6724.
[43] P. Magne and P. L. Walker Jr. Phenol adsorption on activated carbons. 1986. 24 (2): p. 101.
[44] J. Coates, In Encyclopedia of Analytical Chemistry. 2006. p. 10815-10837.



