Research - Der Pharma Chemica ( 2020) Volume 12, Issue 4
Comparative Characterisation of Ground and Surface Water Contamination by Anthropogenic Activities
Krishnaveni J1*, Kamalakannan N2, Ramapandi K3, Vanavan M3 and Prakash Kumar R32Faculty of Civil Engieering, Suguna College of Engineering, Coimbatore, Tamilnadu, India
3Department of Civil Engieering, Suguna College of Engineering, Coimbatore, Tamilnadu, India
Krishnaveni J, Faculty of Chemistry, Suguna College of Engineering, Coimbatore, Tamilnadu, India, Email: venijosh73@gmail.com
Abstract
Ground and surface water samples have been collected from the local area tanks. Totally 16 samples were collected and the chemical characteristics of the sample has been studied namely pH, Electric conductivity, Total Dissolved Solids, Total Hardness, Chloride, Alkalinity and Fluoride. These tested parameters are compared with BIS standard parameters, so that the level of anthropogenic agents was identified. Semi structured interview was conducted and the impact of the pollution on the human health has been assessed.
Keywords
Electric conductivity, Titration method, Anthropogenic activities
Introduction
Waste disposal has always been an important issue for human societies. Solid wastes are disposed on or below the land surface resulting in potential sources of ground water contamination. Coimbatore city is one of the rapidly developing cities in Tamil nadu which needs some definite waste disposal management techniques in order to maintain the ecosystem.
The survey works done by the investigators pertaining to the topic, their findings and recommendations are reviewed. Barde, et al. (2014) examined that waste disposal facilities are mainly responsible for the gradual quality degradation of subsurface fresh water reservoirs [1]. Balachandar, et al (2010) examined that the problem of water quality has become more important than quantity, as the environmental problems are becoming more serious in different parts of the world due to the intervention of human activities [2]. Jin-zhu, et al. (2002) addressed the effect of the population growth, development of industrial and agricultural production and the petroleum exploitation, brought about the unceasing expansion of artificial oasis and abrupt increase of water demand [3]. Ei-Hames et al (2011) examined one of the critical problems that hinder the sustainable development in developing countries located in arid zones [4]. The suitability of ground water for domestic use was determined on the basis of pH, TH, and TDS and by comparing them with the Bureau of Indian Standards (BIS) requirements [5]. Patel and Chaudhari (2014) examined that ground water is an important source of water supply for municipalities, agriculture, and industry [6]. Coimbatore district in western part of Tamilnadu, which lies between 10013’N and 11023’ N latitudes 76039’E and 77030E longitudes. It covers a total geographical area of 7,649 km2.
Methodology
The methodology comprises of data collection, and assessment of sociological consequences
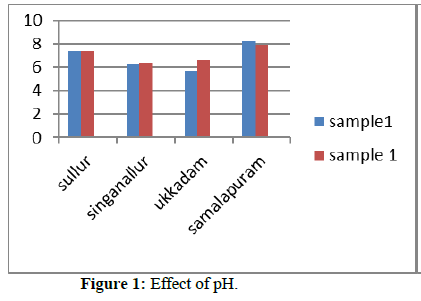
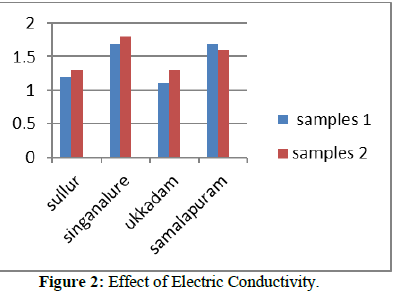
pH metry and Electric conductivity
pH and Electric conductivity for all the collected samples were measured using pH meter and conductivity meter. pH value of all the collected samples are well within the permissible limit. Effect of high and low concentration of pH and the treatment required to decrease the pH is Tables 1 & 2. Electrical conductivity for all the collected samples was measured using conductivity meter. Electrical conductivity values for most of the sample are above the permissible limits.
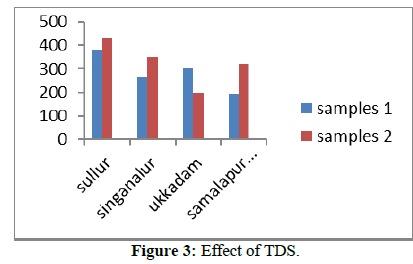
Total Dissolved Solids
TDS value for some of the samples is exceeding the permissible limits. Tables 1&2 shows the possible sources for high TDS, effects of high TDS and the treatment required for reducing TDS.
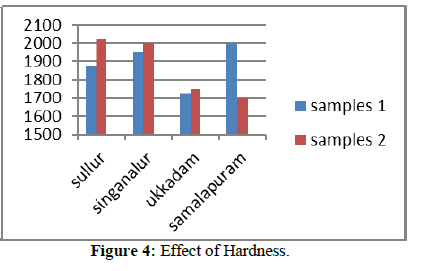
Total hardness
The total hardness is measured by titration method. EDTA is taken as burette solution, 20 ml of collected sample is taken in a conical flask and two drops of EBT is added as indicator, color change from red to blue. The burette reading is noted down to precede the calculation of total hardness. Sources, effects and treatment methods for reducing hardness are shown in Table.
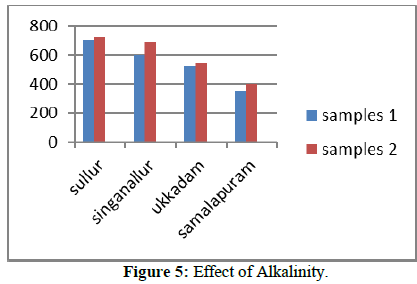
Alkalinity
The presence of carbonates, bicarbonates, and hydroxides are the main cause of alkalinity in nature water. Titration method is used to measure alkalinity. The calculated alkalinity values of both surface and groundwater samples of all the four tanks are compared to BIS values of Tables 1 & 2.
Table 1: Effect of Chemical Characteristics of Ground Water Samples
| SAMPLE | pH | EC | HARDNESS | TDS | CHLORIDE | ALKALINITY | FLOURIDE |
|---|---|---|---|---|---|---|---|
| C.B.S.1 | 7.69 | 2.0 | 1650 | 400 | 538.0 | 850 | 0.51 |
| C.B.S.2 | 7.54 | 2.2 | 1700 | 250 | 538.8 | 800 | 0.23 |
| UK.S.1 | 6.50 | 2.1 | 2200 | 210 | 452.7 | 250 | 0.43 |
| UK.S.2 | 6.51 | 2.0 | 1950 | 280 | 457.2 | 260 | 0.20 |
| S.P.S.1 | 7.05 | 1.9 | 1900 | 180 | 396.1 | 400 | 1.83 |
| S.P.S.2 | 7.01 | 2.0 | 1850 | 240 | 395.2 | 490 | 1.49 |
| S.N.S.1 | 7.07 | 2.0 | 1900 | 110 | 376.5 | 1900 | 0.58 |
| S.N.S.2 | 7.05 | 2.2 | 1990 | 155 | 375.7 | 1850 | 0.91 |
C.B.S = COIMBATORE BIG TANK(SULUR)
UK. S = UKKADAM
S.P.S = SAMALAPURAM
S.N.S = SINGANALLUR
Table 2: Effect of Chemical Characteristics of Surface Water Sample
| Sample | pH | EC | HARDNESS | TDS | CHLORIDE | ALKALINITY | FLOURIDE |
|---|---|---|---|---|---|---|---|
| C.B.S.1 | 7.35 | 1.2 | 1875 | 380 | 585.5 | 700 | 0.44 |
| C.B.S.2 | 7.36 | 1.3 | 2025 | 430 | 586.6 | 725 | 0.32 |
| UK.S.1 | 5.70 | 1.1 | 1925 | 300 | 426.1 | 525 | 0.24 |
| UK.S.2 | 5.80 | 1.3 | 1750 | 290 | 428.9 | 550 | 0.23 |
| S.P.S.1 | 8.44 | 1.7 | 2000 | 190 | 420.0 | 350 | 0.46 |
| S.P.S.2 | 8.10 | 1.6 | 1700 | 320 | 416.5 | 400 | 1.18 |
| S.N.S.1 | 6.32 | 1.7 | 1950 | 265 | 105.0 | 600 | 0.67 |
| S.N.S.2 | 6.35 | 1.8 | 2000 | 350 | 100.5 | 690 | 0.87 |
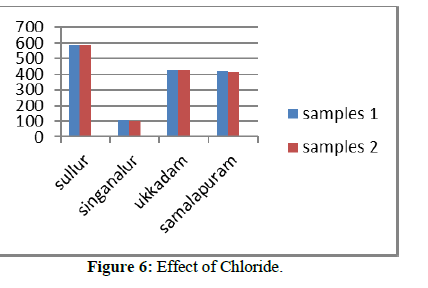
Chloride
The permissible limit for chloride is 250 mg/l to 1000 mg/l. The calculated chloride values of surface and ground water samples were compared with the BIS values of Tables 1 & 2 shown the source, effects and treatment measures to reduce the concentration of chloride.
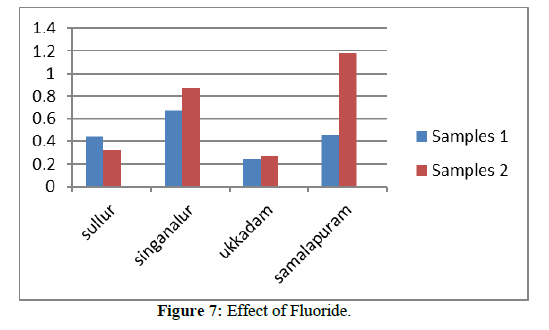
Fluoride
Fluoride ions maybe present either naturally or artificially in drinking water and are absorbed to the some degree in the bone structure of the body and the tooth enamel. Fluoride ions at extremely high level may cause dislocation of teeth. Permissible limit of fluoride ions is 0.6 mg/l-1.5 mg/l for ground water. All the collected samples are well below the desirable limits. Fluoride at lower concentration causes some beneficial effects on teeth [7]. Tables 1 & 2 shows the sources for presence of fluoride in water, effects of high concentration of fluoride on human, treatment required to reduce the concentration of fluoride [8] (Figures 1-7).
Conclusion
From the analysis of the semi-structured interview, it is clear that more than 50% of the people residing around the tanks dispose the waste from their households into the tank, which is the prime reason for water quality deterioration. Since the quality of water is being deteriorated because of anthropogenic activities, the contaminant gets transported and affects the groundwater quality. So this ground water and surface water are not absorbent for drinking purpose.
References
[1] BardeAnupama, S.S. Jain and S.T Mali. International Journal of Research in Advance Technology. 2014, 2: p. 438-453.
[2] D. Balacandar, P. Sundararaj, Rutharve1 Murthy et al., International Journal of Environmental Sciences. 2010, (1)2: p. 176-190.
[3] MA Jin-zhu, LAI Tian-wen, LI ji-juli, Chinese Geographical Science. 2002, 12(1): p. 50-54.
[4] Abdesselemakabour, Heniazedine, Chebbahlyndac et al., Procedia Engineering. 2012, 33: p. 242-247.
[5] S.S. Asadi, Padmaja Vuppala and M. Anji reddy. International Journal of Environmental Research and Public Health. 2007, 4: p. 45-52
[6] A. Pallavi Patil and S. Chaudhari Pravin. International Journal of Innovative Research Advanced Engineering. 2014, 1: p. 188-192.
[7] Loai Aljerf. EC Dental Science. 2017, (1): p. 36-38.
[8] Loai Aljerf and Nuha Al Masri. EC Dental Science. 2018, 17(6): p. 892-899.
[9] Maren Brehme, Traugott Scheytt, Mehmet Çelik et al., Environmental Earth Sciences. 2011, 63(6): p. 1395-1408.
[10] Bodrud-Doza Md., A.R.M. Towfiqul Islamb, Fahad Ahmed et al., Water Science. 2016, 30(1): p. 19-40.
[11] Fabíola Geovanna Piga. Multi-criteria potential groundwater contamination and human activities: Araras watershed, Brazil. RBRH 22. 2017.
[12] Nada Sasakova. Frontiers in Sustainable Food Systems 2018.
[13] SalahZereg. Sustainable Environment Research. 2018, 28(6): p. 340-349.
[14] Rakesh Kumar, Sudesh Yadav and Sudesh Chaudhary. Proceedings of the Indian National Science Academy, 2019.
[15] Ana Luiza Cunha Soares. Environmental Science and Pollution Research. 2020, 27(12): p. 14085-14099.
[16] Sanjay Kumar Goyal, B. S. Chaudhary, Omvir Singh et al., Environmental Earth Science. 2010, 61: p. 1587-1597.