Research Article - Der Pharma Chemica ( 2017) Volume 9, Issue 4
Correlation between IL-6 Concentration and TNF-α with the Stages of Infection for Patients Infected with HSV-1 and the Control Group
Hussein Ali Kadhim1 and Siham Jassim al-Kaabi22Assistant Proffesor in Immunology, University of Kufa, Iraq
Abstract
Objective: It aims to Evaluation the level of interleukin-6 concentration and TNF-α with the stages of infection for patients infected with HSV-1 and the controls. Methodology: The study was conducted at Hakim General Hospital and Al-Sadr Teaching Hospital. was divided the number of samples that have been collected 64 samples from patients with herpes simplex virus type I by 32 blood samples during the primary infection and recurrent and 32 blood samples after recovery from of infection as well as 16 blood samples collected from healthy donors and promised as a control group and carried her diagnosis specific of Immunoglobulin IgM and IgG HSV-1. The statistical analysis, by using (statistical package for social sciences) SPSS and one way ANOVA test. The least significant difference was calculated at the level P<0.05. Results: The results were obtained, and there was a significant increase (P<0.05) the level between concentration of interleukin-6 and TFN-α in patients during primary and latent infection compared with the control group. Conclusion: Increase the level of interleukin-6 and TNF-α plays an important role in the resistance to the virus during primary infection and maintain the Status latent virus. Recommendation: Study detection of herpes simplex virus type I in patients with thyroid and know the level of some cytokines.
Keywords
Herpes simplex virus-1, Interleukin-6 (IL-6), TNF-α , Cold sore infection
Introduction
Considered virus herpes simplex type I (HSV-1) of large viruses as it possesses nuclear acid undiminished oxygen dual chain Double strand DNA which is swirled in a ring [1]. Naming the virus HSV from the Latin term Herpes, which came from the Greek word Herpein meaning creeping Creep came and this reflects the nature of the crawl or spread of pests causing skin mediated many types of viruses herpes [2,3].
The virus belongs to the family of Herpesviridae that cause infection very painful to humans and which are also called Human herpes virus and under the Alpha Herpesvirinae family [4], it is a virus, herpes simplex of the most common viruses that cause many infections in the world and one of these infections cold fever cold sore it also caused infections to the reproductive system and the fingers and toes and eye infections, meningitis and many other infections [5,6]. Eczema herpeticum virus also causes in children who have effective active eczema [5]. As well as gingivitis herpetic gingivostomatitis [7]. The cause herpetic whitlow which is one of the painful infections as it affects people who are manual contact with body secretions containing the virus and oral secretions, especially where it enters through small wounds on the hand [8].
Spreading virus herpes simplex widely among the people and has the ability to replicate in different cells as it spreads quickly and works on cell lysis and is spread through direct contact or indirectly, such as body fluids and lesions formed at the site of infection [9]. Also moving by kissing or sharing eating utensils, towels [10].
Considered virus herpes simplex viruses opportunism where when people get sick cases different preoccupation with the immune system works these viruses to appear in the body are secondary infections [11], as there are many factors that encouraged the re-activation of which exposure to the sun in directly, stress, menstrual period and inhibition of the immune [12,13], as it plays the virus plays a virus an important role in injury to people with weakened immune systems such as people recipients of the kidneys [14].
Are infection by HSV-1 lifelong through the establishment of Latent infection in sensory nerve cells of the host and its ability to replicate in the epithelial cells infection through the primary infection and reactivation [15].
Replication HSV-1 in oral mucosa cells vesicles container on the virus, since the patient's fever is suffering and therefore these vesicles ulcerate and covered peeling around where the virus enters during the initial infection ends of the sensory nerve in the lesion area and becomes a condition latent and that can be reactivated [16].
(IL-6) Produces by many of the cells that stimulate mediated infection microbiological and cellular Pleiotropic is playing a useful role in the immune response by contributing to the activation neutrophil [17], one of these important cells in the body producing interleukin are T cells, B cells, polymorphonuclear cells, eosinophils, macrophages, monocyte, stem cells, mast cells, osteoblasts and chondrocytes, in addition to the endothelial cells muscles smooth , structural , fibroblasts cells, thyroid, keratinocytes, astrocytes and some cancer cells. The estimated molecular weight has about 20 kDa [18,19].
Characterized interleukin-6 different functions as it works to stimulate B-cell differentiation and production of proteins during the acute phase, fever, affects the functions of T cells [20]. During infection by herpes simplex produces interleukin (IL-6) in many tissues such as the cornea and (TG) as it works IL by special receptors stimulate a series of signaling proteins as stimulating factors Transcription such as NF-IL-6 and STAT3 that block the activation virus herpes simplex [21].
It can be described as a mediator IL-6 candidate for reactivation of the virus because it is an important component of the inflammatory response [20].
Tumor necrosis factor-alpha (TNF-α) is a cellular Pleiotropic is released in response to many diseases and bacterial toxins, such as multi-saccharide adipose LPS and viruses [22], known as TNF-α as a mediator in inflammation and immune, as are genes encoded both (β and TNF-α) is part of a complex histocompatibility class III (MHC class III) and the genes of the major forms of tumor necrosis factor located on the sixth chromosome chromosome 6 [23].
Induction of the inflammatory response occurs through two receptors TNFR1 and TNFR2 as it is activated TNFR1 in most human tissues by TNF-α correlation, on the other hand, the TNFR2 expressed as first in immune cells and is activated by TNF-α and TNF-β [24,25], has a molecular weight of tumor necrosis factor alpha 26 kDa [26].
Tumor necrosis factor secreted mainly by many cells in the body, such as macrophaes, lymphocytes, eosinophiles as well PMNs and secreted in smaller amounts in astrocytes and langerhans cells in addition to kupffer cells and cells fibroblast and tumor cells [27].
Contributes to tumor necrosis factor in the natural immune response against infectious diseases, including the virus herpes simplex type I HSV-1 and works to stimulate the immune acquired respond either his work on natural killer cells may increase in the killing of the target and defend cells against infection viral [28]. In addition, TNF involved in immune diseases Immunopathology of some diseases such as diabetes disease, diabetes, hepatitis, hepatitis and arthritis Aromatidi [29].
The aim of this study
Estimate the level concentration of IL-6 and TNF-α in patients infected by the herpes simplex virus (HSV-1).
Know correlation between the concentration of IL-6 and TNF-α in patients with primary infection and latent than controls properties.
Materials and Method
Patients and study place
This study included 80 of the persons involved and their ages ranged between 60-6 y. The study began in the year-Hakim Hospital and Sadr Teaching Hospital for the period from October 2012 until March 2013. Was divided the number of samples that have been collected 64 blood samples from patients with injury infected by HIV simplex type I herpes (HSV-1) by 32 blood samples during the initial injury and the repeated and 32 after recovery from injury, as well as 16 blood sample collected from donors healthy promised as a group control.
Collecting of blood samples
Pull 5 mL of venous blood using syringes medical sterilized and placed in chapter sterile tubes and left at room temperature 25-20 °C for 30 minut and then discarded in centrifuge at 3000 r/min for 10 minut after the pull the serum separated from the rest the blood components micropipette minutes using a pipette and put in Eppendorf tubes, pipes kept the temperature 20 °C until used in immunological tests.
Estimation of IL-6 and TNF-α in serum
Enzyme immunoassays used method for the quantification of IL-6 and TNF-α in the serum and supplier of processed American RayBio Company.
Statistical analysis
The use of statistical program (Statistical package social sciences) SPSS version 20 in the analysis of the results where the test was used One way ANOVA and when the level of probability (P<0.05).
Results
After that the measurement was conducted level of IL-6 and TNF-α in the sera of patients infected by virus HSV-1 and the control group and the deviation enzyme-linked immunosorbent assay (ELISA), it was to get the results shown in the Table 1.
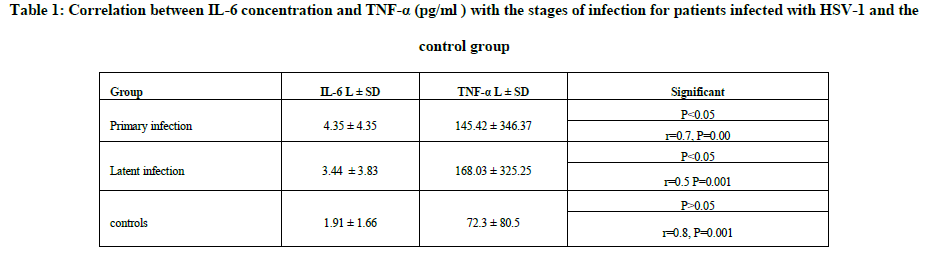
Discussion
Primary infection
The current results have shown in the Table 1 a significant relationship and the level of significant P<0.05 and the direct relationship between the concentration of IL-6 factor TNF-α in patients infected by virus, herpes simplex type I and this is consistent with the findings of the researchers. To the interleukin IL-6 and TNF-α rise Infection by the virus and between the expression of the concentration of TNF-α in serum and cellular farms larger during the infection [30], it agreed with the researchers produce high levels of IL-6 and TNF-α during primary infection by the virus by macrophages and lymphocytes [31] and agreed the current study also with the findings of the researchers the high level of IL-6 and TNF-α during the primary infection by the virus as well as being prevailed during the latent stage before exposure to the re-activation [32] and also agreed with the findings of the researchers who confirmed that microglia cells produced significant levels of IL-6 and TNF-α in response to infection by HSV-1 [32]. The reason to the virus herpes simplex stimulates the immune response and thus natural killer cells, NK cells phagocytic cells play an important role in removing the virus from the body works during the first hours of the attack through the secretion TNF-α and IL-6 during early
infection [34] and also because of the fact that the tumor necrosis factor alpha stimulates the production of IL-6 infection by virus [35].
Latent infection
The results showed that there were significant relationship and the level of significant P<0.05 and the direct relationship between the concentration of interleukin IL-6 and TNF-α during latency. This is consistent with researchers [36], which among that both IL-6 and TNF-α prevalent in white laboratory mice infected are latent by virus HSV-1 more than the rest of other cytokines and also agreed with the researchers who confirmed the high level of IL-6 and TNF-α during the latent stage and level rises after exposure to ultraviolet (UV) [32], also agreed with the researchers the tumor necrosis factor alpha increased when the virus is removed [37]. As well as a study by researchers to the IL-6 and TNF-α produced by various cells to inhibit the reactivation of the virus HSV-1 latent within the ganglia TG [38], the reason for the rise remain in the level of cytokines is weak expression and repeated genes IE and E during the latent stage [39].
As well as an increase in the level of IL-6 and TNF-α in the underlying case, because the virus is subject to periodic re-activate chronic [40]. The results showed a low level of concentration of IL-6 and TNF-α were statistically significant P<0.05 in healthy people, and the reason is the lack of infection would increase the level of cytokines and the fact that people are healthy apparently do not suffer chronic diseases such as diabetes, blood pressure and other diseases immune.
Conclusions
The rise in the level of IL-6 and TNF-α is important in controlling the incidence of primary herpes simplex virus Type I. The high level of interleukin and TNF-α after infection (latent condition) is necessary to access the whole of the infection to heal and control the latency of the virus.
Recommendations
study of the role of cytokines ciliary neurotropic factor (CNTF) and IL-11 and overlap with the patients with HSV-1. Study the effect of some high cytokines due to injury infected by HSV-1 and its effect on pregnant women. Trying to grow the virus and diagnosis of genetically industry.
References
[1] D.J. McGeoch, F.J. Rixon, A.J. Davison. Virus Res., 2006, 117(1), 90-104.
[2] T.J. Taylor, M.A. Brockman, E.E. McNamee, D.M. Knipe. Frontier. Biosci., 2002, 7, 752-764.
[3] W. Irving, Ala’aldeen, B, Boswell, T. Taylor and Francis groups, USA., 2005, 64-67.
[4] K.J Ryan, C.G Ray. Sherries Medical Microbiology, 4th Ryan, ed., McGraw-Hill, USA, 2002, pp. 247.
[5] R.J. Whitley, J.W. Gnann. Lancet., 2002, 359, 507-513.
[6] B.C. Branco, P.A. Gaudio, T.P. Margolis. Br. J. Opthalmol., 2002, 1285-1288.
[8] Y. Avitzur, J. Amir. Infection., 2002, 30(4), 234-6.
[9] J. Akhtar, D. Shukla. FEBS. J., 2009, 276(24), 7228-7236.
[10] G. Boivin, N. Goyette, Y. Sergerie, S. Keays, T. Booth. J. Clin. Virol., 2006, 37(4), 248-251.
[11] Westra, D.F. Verjans, G.M. Osterhaus, A.D. Kooij, A.V. Welling, G.W. Scheffer. J. General. Virol., 2000, (81), 2011-2015.
[12] R.G. Alan. Altern Med Rev., 2006, 11(2), 93-101.
[13] C. Cernik, K. Gallina, R.T. Brodell. Arch. Intern. Med., 2009, 168(11), 1137-44.
[14] I. Kremer, A. Wanger, D. Shmuel, A. Yassim, Z. Shapira. Br. J. Ophthalmol., 1991, 75, 94-96.
[15] E.E. Heldwein, C. Krummenacher. Cell. Mol. Life. Sci., 2008, 65, 1653-1668.
[16] R.V. Goering, J.M. Dockrell, M. Derek, Z. Mark, C.H. Peter, K. Roi, M. Ivan, C. Mims. Mims Medical Microbiology. 4th ed . , Elsevir limited, UK., 2008, 185-188.
[17] B. Schobitz, E.R. De Kloet, W. Sutanto, F. Holsboer, F. Eur. J. Neurosci., 1993, 5, 1426-1435.
[18] V. Bhandari. Front. Bios., 2007, (7), 624-33.
[19] C. Guzman, C. Hallal-Calleros, L. Lopez-Griego, J. Morales-Montor. Open. Neuroendocrinol. J., 2010, (3), 152-160.
[20] T. Kishimoto, S. Akira, M. Narazaki, T. Taga. Blood. 2002, (4), 1243-54.
[21] J.D. Kriesel, J. Ricigliano, S.L. Spruance, H.H. Garza Jr, J.M. Hill. J. Neuro Virol., 1997, 6, 441-448.
[22] J. Debnath, K.R. Mills, N.L. Collins, M.J. Reginato, S.K. Muthuswamy, J.S. Brugge. Cell., 2005, 111, 29-40.
[23] EL-Harith el-HA. Saudi Med J., 2004, 25(2), 135-40.
[24] V. Baud, M. Karin. Trends. Cell. Biol., 2001, 11, 372-377.
[25] H. Kawasaki, R. Onuki, E. Suyama, K. Taira K. Nat. Biotechnol., 2002, 20(4), 376-80.
[26] Q. Tang, R.L. Hendricks. J. Exp. Med., 1996, 184(4), 1435-47.
[27] J. Debnath, K.R. Mills, N.L. Collins, M.J. Reginato, S,K. Muthuswamy, J.S. Brugge. Cell., 2002, 111, 29-40.
[28] K.E. Sullivan. Ped. Rheumat. Online J., 2004, 2, 7-22.
[29] P. Vassalli. Annu. Rev. Immunol., 1992, 10:411-452.
[30] J. Gosselin, L. Flamand, M. D'Addario, J. Hiscott, J. Menezes. J. Clin. Invest., 1992, (89), 1849-1856.
[31] Z. Mikloska, V.A. Danis, S. Adams, A.R. Lioyd, D.L. Adrian, A.L. Cunningham. J. Infect. Dis., 1998, 177(4), 827-38.
[32] C. Shimeld, D.L. Easty, T.J. Hill. J. Virol., 1999, 73(3), 1767-73.
[33] J.R. Lokensgard, S. Hu, W. Sheng, M. Vanoijen, D. Cox, M.C. Cheeran, MC, P.K. Peterson, PK. J. Neurovirol., 2001, 7(3), 208-19.
[34] S. Ellermann-Eriksen. Virol. J., 2005, 2, 59.
[35] J. Sanceau, T. Kaisho, T. Hirano, J. Wietzerbin. J. Biol .Chem., 1995, 270(46), 27920-31.
[36] D.J. Carr, S. Noisakran, W.P. Halford, N. Lukacs, V. Asensio, V, I.L. Campbell. J. Neuroimmunol., 1998, 85(2), 111-21.
[37] V. Hukkanen, E. Broberg, A. Salmi, J.P. Erälinna. Int. Rev. Immunol., 2002, 21, 355-371.
[38] H. Minagawa, K. Hashimoto, Y. Yanagi. J. Gen. Virol., 2004, 85, 343-7.
[39] M.F. Kramer, S.H. Chen, D.M. Knipe, D.M. Coen. J. Virol., 1998, 72(2), 1177-85.
[40] D.A. Padgett, J.F. Sheridan, J. Dorne, G.G. Berntson, J. Candelora, R. Glaser. Proc. Natl. Acad. Sci. U.S.A., 1998, 95(12), 7231-5.



