Research Article - Der Pharma Chemica ( 2018) Volume 10, Issue 2
Corrosion Behaviour of Ni-Ti Alloy and SS 18/8 Alloy in Artificial Saliva in the Presence of Ferikind Tablet
Anandan A1, Rajendran S2*, Sathiyabama J3, Sathiyaraj D1 and Abdulhameed Al-Hashem4
1SKV Higher Secondary School, Kandampalayam-637201, Tamil Nadu, India
2Corrosion Research Centre, Department of Chemistry, St Antony’s College of Arts and Sciences for Women, Dindigul–624005, Tamil Nadu, India
3PG and Research Department of Chemistry, GTN Arts College, Dindigul–624005, Tamil Nadu, India
4Petroleum Research Centre, Kuwait Institute for Scientific Research
- *Corresponding Author:
- Rajendran S
Corrosion Research Centre
Department of Chemistry
St Antony’s College of Arts and Sciences for Women
Dindigul–624005, Tamil Nadu, India
Abstract
The purpose of present study two types of orthodontic braces were investigated namely Ni-Ti Super Elastic Shape Memory alloy (Ni-Ti SESM alloy) and SS 18/8 alloy the corrosion behavior of orthodontic braces in the artificial saliva (AS solution) in the absence of tablet and presence of tablet Ferikind by using Potentiodynamic Polarization method and AC Impedance spectra techniques. Potentiodynamic Polarization study to determine cathodic or anodic reaction, corrosion current, linear polarization resistance and AC Impedance spectra techniques double layer capacitances, charge transfer resistances, impedance values calculated. It observed from the studies corrosion resistance of Ni-Ti SESM alloy increases in presence of tablet Ferikind and confirmed the protective layer formed on the Ni-Ti SESM alloy surface. The date showed that people implies with orthodontic braces made of Ni-Ti SESM alloy without hesitation to take Ferikind tablet. But corrosion resistance SS 18/8 alloy decreases in the presence of Ferikind and that people should avoid this tablet.
Keywords
Ferikind, Orthodontic wires, Artificial saliva, Polarization study, AC Impedance
Introduction
Various metal alloys have been used for manufacture of dental implants. These dental implants may undergo corrosion caused by the food items, temperatures, pH and bacteria [1]. Ni-Ti based shape memory alloys have been widely used due to its biocompatibility and ductility [2]. The influences of different concentration of eugenol on corrosion resistance for titanium alloy in saliva solution have investigated by Latifa kinani [3]. Impact of modified acidic soft drink on enamel erosion was evaluated by T. Attin et al. [4]. Corrosion of two gold alloys and pure titanium wire in saliva solution for two month [5]. The pure titanium metal was recently introduced as alternative material for metallic brackets for its low allergenicity, more corrosion resistance [6] Nickel- free alloys or stainless steel alloys with reduced amount of nickel have been tested in the artificial saliva with various acidities [7].
Human saliva which plays a significant role in oral mouth [8] the corrosion rate of orthodontic wires bases on nickel-titanium alloy in artificial saliva investigated decreases in following order: N Ni-Ti > Ni-Ti > Rh Ni-Ti by V. Katic [9]. Influence of desulfotomaculum nigrificans sulfate reducing bacteria on corrosion process of commonly used metallic biomaterials for dental applications [10] comparative study of chemical properties and nickel releases of stainless steel and nickel titanium wires [11]. Motojima et al., investigated corrosion and abrasion resistances of TiN coated stainless steel in sea water, whirled of sea sand [12]. Corrosion behaviour of SS 18/8 alloy and SS 316L alloy with toothpaste vicco [13]. The present study evaluates the corrosion behavior of two orthodontic alloys with artificial saliva (AS solution) in presence and absence of Ferikind tablet by potentiodynamic polarization method and AC impedance techniques used (Table 1).
| Tablet | Compositions | Representation |
|---|---|---|
| Ferikind (ascorbic acid) | 1. Elemental iron-100 mg 2. Folic Acid-1.5 mg |
Tablet-D |
Table 1: Compositions of Tablet-D
Material and Methods
Composition of Artificial saliva (AS) solution
| Compounds | KCl | NaCl | CaCl2. 2H2O | NaH2PO4 | Na2S.9H2O | Urea |
|---|---|---|---|---|---|---|
| g/l | 0.4 | 0.4 | 0.906 | 0.690 | 0.005 | 1.0 |
Polarization techniques
Polarization study was carried out using CHI- electrochemical work station/impedance analyzer 660 A. This analyzer was provided with iR compensation facility. It consist of three electrode cell system were used in Figure 1. The Ni-Ti SESM alloy and SS 18/8 alloy using working electrode. SCE, Platinum were used for reference electrode and counter electrode respectively. The polarisation method, corrosion measurements like Ecorr (potential), Icorr (current), Tafel slopes anodic reaction (ba) and cathodic reaction (bc), LPR (resistance) were calculated.
AC impedance spectra measurements
The impedance CHI-660A electrochemical analyzer system was using to measured impedance parameters like Rct (charge transfer resistance), Impedance and Cdl (double layer capacitance). The impedance set up was same for polarization techniques. The impedance values of Z’ (real part) and Z” (imaginary part) was measured in different frequencies (ohms).
Results and Discussion
Influence of Tablet-D on corrosion behavior of Ni-Ti SESM alloy and SS18/8 alloy with Artificial Saliva (AS solution)
Analysis of potentiodynamic polarization methods
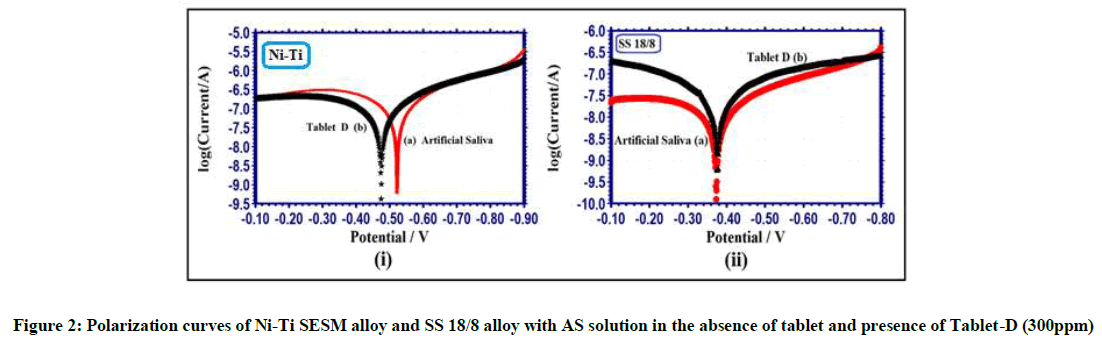
The polarization methods used to confirm the protective film formed on metal alloy surface during corrosion process [14-17]. If the protective layer is formed on the metal alloy surface, increase in LPR (resistance) and decrease in Icorr (current). The polarization curves of Ni-Ti SESM alloy and SS 18/8 alloy with AS solution in the absence of tablet and presence of Tablet-D from polarization methods is shown Figure 2 and Table 2.
| Metals | Systems | Ecorr (mV vs SCE) |
bc (mV/ Decade) |
ba (mV/ Decade) |
LPR (ohm cm2) |
Icorr (A/cm2) |
|---|---|---|---|---|---|---|
| Ni-Ti SESM alloy | AS | -521 | 205 | 249 | 419028 | 11.68 x 10-8 |
| AS+Tablet D | -474 | 187 | 265 | 574015 | 8.322 x 10-8 | |
| SS 18/8 alloy | AS | -373 | 197 | 309 | 3352584 | 1.561 x 10-8 |
| AS+Tablet D | -376 | 216 | 200 | 1435500 | 3.154 x 10-8 |
Table 2: Polarization parameters of Ni-Ti SESM alloy and SS 18/8 alloy with AS solution in the absence tablet and presence of Tablet-D (300ppm)
Ni-Ti SESM alloy immersed in Artificial Saliva, the Ecorr shifted from -521 mV vs SCE to -474 mV vs SCE in Tablet-D Figure 2(i). It indicates the anodic reaction (ba) is controlled. The Tablet-D contains Elemental iron which has been adsorbed on metal alloy surface of the anodic sites. Similarly SS 18/8 alloy with AS solution the Ecorr shifted from -373 mV vs SCE to -376 mV vs SCE in presence of Tablet-D Figure 2(ii). It indicates the cathodic reaction (bc) is controlled. Both metal alloys the Ecorr shift in values is low. Hence that systems function as (cathodic and anodic reaction) mixed type of corrosion inhibitors.
The polarization methods used to confirm the protective film formed on metal alloy surface. The protective film is formed on metal alloy surface, the LPR value from 419028 ohmcm2 to 574015 ohmcm2 increases and the Icorr values from 11.68 x 10-8 A/cm2 to 8.322 x 10-8 A/cm2 decreases Table 2. It observed from the corrosion parameters conclusion in presence of Tablet-D corrosion resistance of Ni-Ti SESM alloy increases and that people have orthodontic braces made of Ni-Ti SESM alloy without hesitation to take Tablet-D. But the LPR value decreases from 3352584 ohmcm2 to 1435500 ohmcm2 and the Icorr increases from 1.561 x 10-8 A/cm2 to 3.154 x 10-8 A/cm2 Table 2. These parameters indicate in presence of Tablet-D corrosion resistances of SS 18/8 alloy decreases and that people have orthodontic braces made of SS 18/8 alloy should avoid the Tablet-D.
Analysis of AC impedance methods
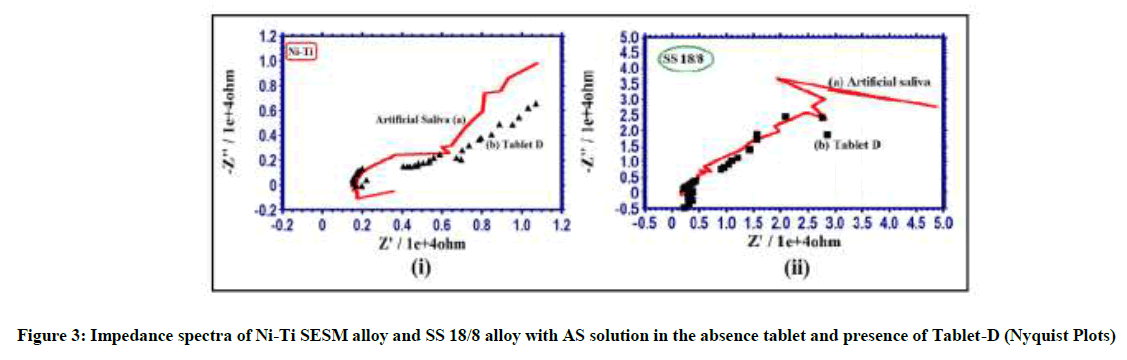
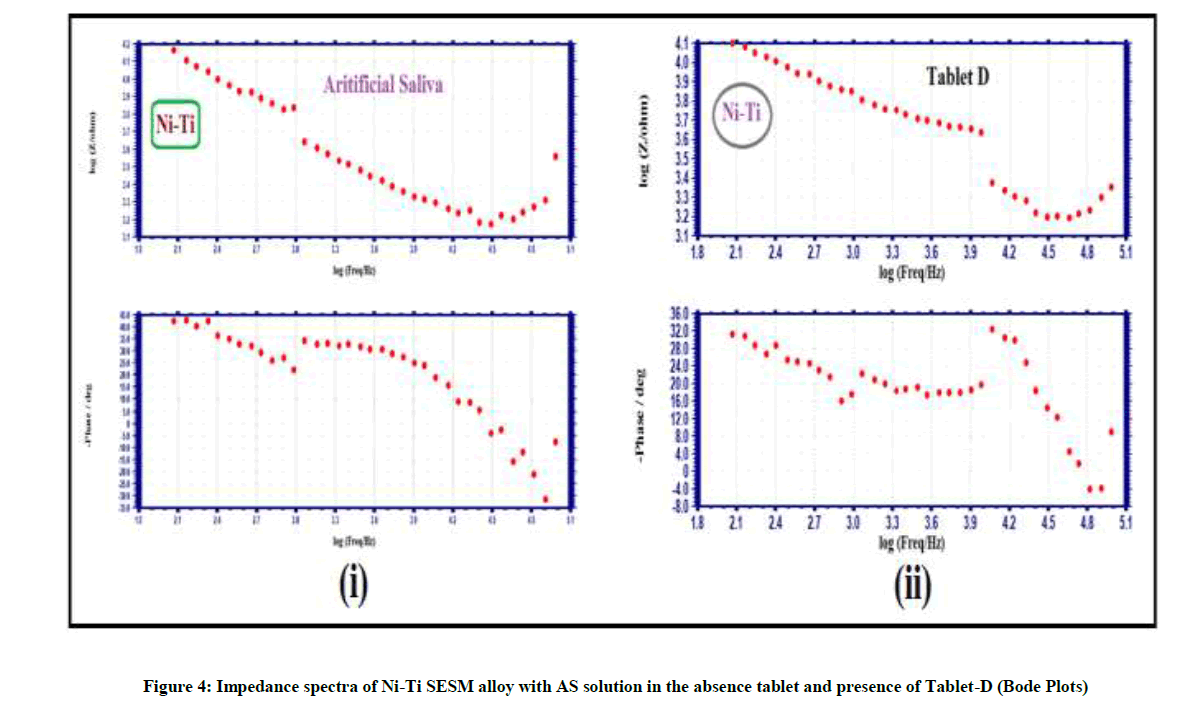
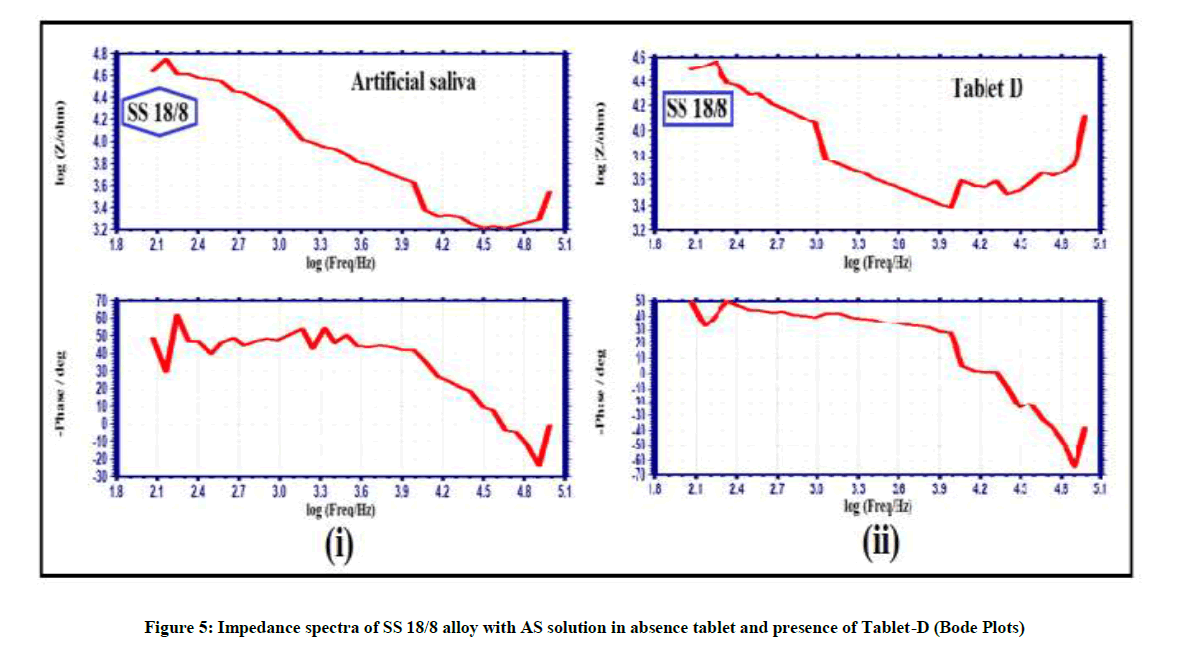
Electro chemical impedance spectra used to confirm the protective layer on metal alloys surface [18-20]. If a protective layer formed on the metal alloy surface, increase in Rct (charge transfer resistance), Impedance and decrease Cdl (double layer capacitance). The impedance spectra of Ni-Ti SESM alloy and SS18/8 alloy with AS solution in absence tablet and presence of Tablet-D is shown Figures 3-5 & Table 3.
| Metals | Systems | Rct (Ohm cm2) |
Cdl (F/cm2) |
Impedance Log (Z/ohm) |
|---|---|---|---|---|
| Ni-Ti SESM alloy | AS | 7281 | 7.004 x 10-10 | 4.167 |
| AS+Tablet D | 8540 | 5.971 x 10-10 | 4.170 | |
| SS 18/8 alloy | AS | 44851 | 1.137 x 10-10 | 4.634 |
| AS+Tablet D | 18410 | 2.77 x 10-10 | 4.508 |
Table 3: Impedance parameters of Ni-Ti SESM alloy and SS 18/8 alloy with AS solution in the absence tablet and presence of Tablet-D (300ppm)
AC impedance spectra of Ni-Ti SESM alloy with AS solution in presence of Tablet-D the Rct increases from 7281 ohm cm2 to 8540 ohm cm2 and the Cdl value decreases from 7.004 x 10-10 F/cm2 to 5.971 x 10-10 F/cm2. The AC impedance value increases from 4.167 to 4.170 Table 3 and Figure 3(i), 4(i); the impedance spectra suggest that protective layer is formed on Ni-Ti SESM alloy surface in Tablet-D and corrosion resistance increases. That people have implanted with orthodontic braces made of Ni-Ti SESM alloy without hesitation to take Tablet-D. The elemental irons of the Tablet-D have not corroded orthodontic braces made of Ni-Ti SESM alloy. But impedance spectra of SS 18/8 alloy with AS solution in Tablet-D the Rct decreases from 44851 ohm cm2 to 18410 ohmcm2 and the Cdl value increases from 1.137 x 10-10 F/cm2 to 2.77 x 10-10 F/cm2. The impedance parameters decreases from 4.634 to 4.508 as shown in Table 3 and Figure 3(ii), 4(ii); these parameters indicates the protective layer formed on metal alloy surface is not stable. It is broken by ingredients of the Tablet-D and the corrosion resistances decreases. Hence impedance spectra suggest that people have orthodontic braces made of SS 18/8 alloy avoid the Tablet-D (Figure 5(i) and 5(ii)).
Conclusion
Polarisation parameters show the Ferikind tablet act as mixed type of corrosion inhibitor. Impedance spectra suggest that protective layer is formed on Ni-Ti SESM alloy surface and corrosion resistance increases. That people have implanted with orthodontic braces made of Ni-Ti SESM alloy without hesitation to take Ferikind tablet. But corrosion resistance of SS 18/8 alloy with AS solution decreases and that people have orthodontic braces made of SS 18/8 alloy avoid the Ferikind tablet.
References
- Milosev, B. Kapun, V.S. Selih., Acta. Chimica. Slovenica. Open Access, 2013, 60(3), 543-555.
- N. Pandis, C.P. Bourauel, S. Tomobe, Seminars in Orthodontics., 2010, 16, 4, 249-257.
- L. Kinani, A. Chtaini., Leonardo J Sciences., 2007, 11, 3340.
- T. Attin, K. Weiss, K. Becker, W. Buchalla, A Wiegand., Oral Diseases.,2005, 11, 7-12.
- D. Brune, D. Evje, S. Melsom., Eur J Oral Sci.,1982, 90, 168-171.
- C. Gioka, C. Bourauel, S. Zinelis, T. Eliades, N. Silikas, G. Eliades, Dent. Mater., 2004, 20(7), 693-700.
- H.H. Huang, Y.H Chiu, T.H. Lee, S.H. Wu, H.W. Yang, K.H. Su, C.C. Hsu, Biomaterials., 2003; 24(20), 3585-92.
- M.W.J. Dodds, D.A. Jhonson, C.K. Yeh, J. Dent., 2005, 33, 223-233.
- V. Katic̈, L. C̈urkovic̈, M. Ujevic̈ Bošnjak, S. Špalj, Materialwissenschaft und Werkstofftechnik, 2014,45(2), 99-105.
- J. Mystkowska, Acta of Bioengineering and Biomechanics., 2016, 18(4), 87-96.
- W. Wichai, N. Anuwongnukroh, S. Dechkunakorn, Advanced Materials Res., 2014, 560-565.
- S. Motojima, M. Kohno, Thin Solid Films., 1986, 137, 59-63.
- R. D Souza, A. Chattree, S. Rajendran, Der Pharm. Chem., 2017, 9(8), 25-31.
- R. Saranya, S. Rajendran, Der Pharm. Chem., 2017, 9(8), 128-132.
- R. Nagalakshmi, L. Nagarajan, R.J. Rathish, S.S. Prabha, N. Vijaya, J. Jeyasundari, S. Rajendran, Int. J. Nano. Corr. Sci. Engg., 2014, 1, 39-49.
- J.A. Thangakani, S. Rajendran, J. Sathiabama, R.M. Joany, R.J. Rathis, S.S. Prabha, Int. J. Nano. Corr. Sci. Engg. 2014, 1, 50.
- M. Boudalia, A. Guenbour, A. Bellaouchou, A. Zarrouk, Der Pharm. Chem., 2015, 7(2), 301-306.
- A. Nithya, P. Shanthy, N. Vijaya, R.J. Rathish, S.S. Prabha, R.M. Joany, S. Rajendran, Int. J. Nano Corr. Sci. Engg., 2015, 2(1), 1-11.
- A.C.C. Mary, S. Rajendran, H. Al-Hashem, R.J. Rathish, T. Umasankareswari, J. Jeyasundari, Int. J. Nano Corr. Sci. Engg., 2015, 1, 42.
- S. Rajendran, P. Chitra Devi, S. John Mary, A. Krishnaveni, S. Kanchana, R. Nagalakshmi, B. Narayanasamy, Zastita Materijala., 2010, 51, 149-158.