Research Article - Der Pharma Chemica ( 2018) Volume 10, Issue 10
Corrosion Inhibition of Mild Steel in Hidrochloric Acid Solution by Gnetum gnemon. L Peel Extract as Green Inhibitor
Emriadi*, Velly Yulistia and Hermansyah Aziz
Department of Chemistry, Faculty of Mathematics and Natural Sciences, Andalas University, Limau Manis Campus, Padang, 25163, Indonesia
- *Corresponding Author:
- Emriadi
Department of Chemistry
Faculty of Mathematics and Natural Sciences
Andalas University
Limau Manis Campus, Padang, 25163, Indonesia
Abstract
The inhibition of corrosion of mild steel in hydrochloric acid in the presence of Gnetum gnemon L. peel extract (GGPE) has been studied using the weight loss, potentiodynamic polarization, FTIR and scanning electron microscopy (SEM) analysis. It was found that the inhibition efficiency increased with increasing concentration of GGPE and temperature. The inhibition was assumed to occur via adsorption of inhibitor molecules on the metal surface. The adsorption of the extract on the mild steel is found to obey Langmuir adsorption isotherm and acts as a mixed-type inhibitor. Thermodynamic and kinetic parameter data for the adsorption process were calculated and discussed.
Keywords
Gnetum gnemon. L, Peel extract, Corrosion inhibitor, Isotherm Langmuir.
Introduction
Mid steel is one of materials that are widely used in the field of industry and construction due to its excellent mechanical properties and low cost. However, mild steel also has a weakness such as it tends to be susceptible to corrosion, especially in the acid environment [1,2]. As a result, research continues to be undertaken to develop effective ways of corrosion control. There are several ways to slow down the corrosion rate, namely coating, anodic or cathodic protection and with the addition of inhibitors [1-3].
The addition of an inhibitor is one of effective ways to slow down corrosion rate. A number of synthetic compounds and natural compounds have been studied and are known to be applied as good corrosion inhibitors for metals. Although the synthesis compound has shown the effect of high corrosion inhibition, but its toxic nature can damage the environment as well as the cost of more expensive synthesis becomes one of the problems for its use in industry [4].
The use of natural inhibitor or 'Eco friendly Inhibitor' in recent years has been widely studied, because it shows good inhibition efficiency, natural inhibitors are also non-toxic, biodegradable, environmentally friendly and cost less expensive than synthetic compounds, so this is very interesting to investigate [5]. Several studies have been conducted with the use of environmentally friendly materials as corrosion inhibitors such as leaf extract of Theobromacacao [3], leaf extract of cassava (Manihot esculenta) [6],, leaf extract of Dodonaea viscosa (L.) [7], leaf extract of Ziziphus ma uritiana [8], and Toona sinensis leaf extract [9].
Gnetum gnemon L. is one of the plants that grow in Indonesia. The Gnetum gnemon L. peel is proven to contain phenolic compounds, flavonoids, β-carotene, lycopene, carotenoids, vitamin C, and antioxidant activity [10]. Organic compounds which contained in the Gnetum gnemon L. peel is expected to be adsorbed on the steel surface, so it can act as a corrosion inhibitor.
Material and Methods
Preparation of materials (mild steel specimen)
Mild steel strips of size: 3.0 cm x 2.0 cm x 0.1 cm containing 0.0149% P, 0.0715% Si, 0.001% Ni, 0.141% Mn, 0.189% C, 0.019% Se, 0.0018% Cr and remainder Fe were used for measurement. The surface of the steel is cleaned and smoothed by steel grinder, washed with distilled, rinsed with acetone and dried with an oven temperature of 60°C. After drying, the steel is weighed and the weighing result is expressed as the initial weight (W1).
Preparation of Gnetum gnemon. L peel extract
Fresh GGP was dried up for 1 month, ground until it become powder and weighed 500 g. The sample was extracted with methanol as much as ± 1500 ml for 3 days. The extract was filtered and the solvent was evaporated using a rotary evaporator to obtain a concentrated extract. The concentrated GGPE is stored into a glass to make an inhibition solution with different concentration.
Phytochemical analysis
Phytochemical analyses of GGPE were screened for several secondary metabolite compounds such as terpenoid, steroid, flavonoid, phenolics, saponins, and alkaloid.
Weigh loss measurement
The pretreated steel is immersed in 50 mL of HCl 1 N solution as corrosive medium with various concentration of GGPE for 7 hours using a water bath, then cleaned, washed, and dried in the oven. After drying, the steel is weighed and the weighing result is expressed as the final weight (W2).
The corrosion rate (CR) was determined using the following equation [1]:
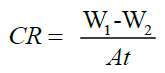 (1)
(1)
Where A is area in cm2 and t is time in h. From corrosion rate (CR), the degree of surface coverage (θ) and inhibitor efficiency (IE%) can determined with equation [2] and [3]:
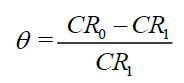 (2)
(2)
And
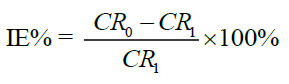 (3)
(3)
Where CR0 and CRI are corrosion rate in the absence and presence of extract, respectively.
Potentiodynamic polarization measurement
Electrochemical measurements were carried out using eDAQ. A conventional three-electrode system was used for this purpose. Mild steel specimen was used as a working electrode. Pt electrode and Ag/AgCl served as auxiliary and reference electrodes, respectively. The three electrodes were immersed in vessels containing corrosive medium (1 N HCl) in the absence and in the presence of inhibitors of various concentrations. The potentiodynamic current potential curves were obtained by changing the electrode potential from -700 to 400 mV. The percentage inhibition efficiency of inhibitors were determined using equation (4):
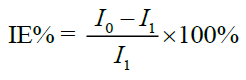 (4)
(4)
where Io and I1 are current density in the absence and presence of extract, respectively.
Fourier Transform Infrared measurement (FTIR)
FT-IR measurements were carried out by taking corrosion products from surface of steel that has been immersed in 1 N HCl in the presence and absence of inhibitor for 7 h at room temperature, then corrosion products were dried and analyzed by FT-IR using KBr pellet. FT-IR measurements were also performed for GGPE.
Analysis of Scanning Electron Microscopy (SEM)
Mild steel was immersed in 1 N HCl without and with the addition of GGPE for 7 days and then dried and analyzed by Scanning Microscopy Electron (SEM) for to record surface morphology of mild steel.
Results and Discussion
Phythochemical analysis of Gnetum gnemon L peel extract
Phytochemical analysis was to identify the secondary metabolite compound that exists in GGPE. Table 1 shows that GGPE consist of several secondary metabolite compounds such as Triterpenoids, Steroids, Flavonoids, Phenolics and Saponins and Alkaloids did not exist in GGPE. Secondary metabolite compounds that consist in GGPE have potential as corrosion inhibitors and indicate can decrease inhibition efficiency.
| Compounds | Results |
|---|---|
| Flavonoids | + |
| Phenolics | + |
| Steroids | + |
| Triterpenoids | + |
| Alkaloids | - |
| Saponins | + |
(+): Present, (-): Absent
Table 1: The Result of Phytochemical Test from GGPE
Weight loss measurements
Corrosion rate and inhibition efficiency
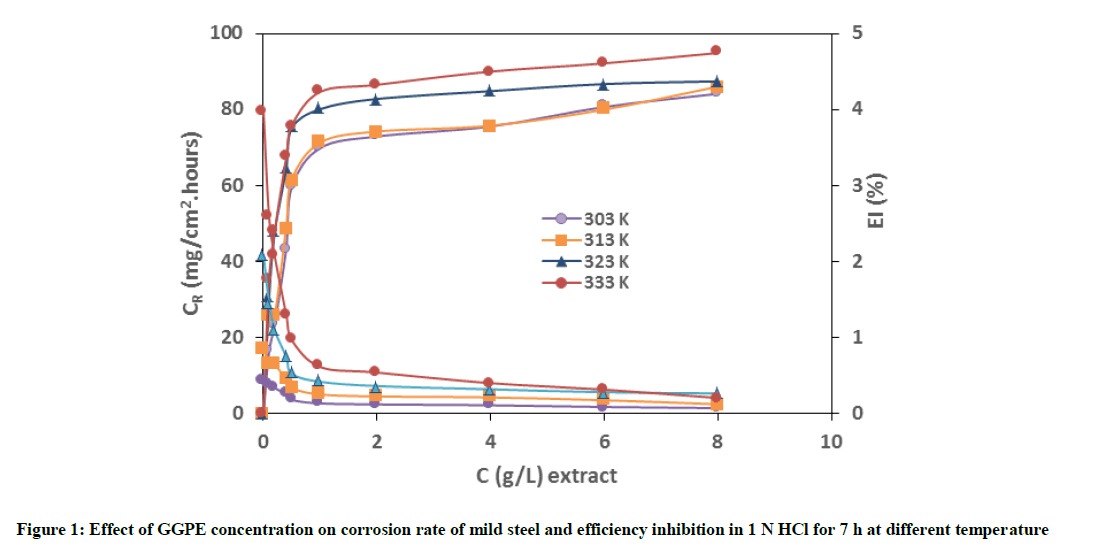
Figure 1 show that corrosion rate decrease and efficiency inhibition increases with increasing concentration of GGPE. This is due to an increase in the amount of adsorption and coverage of compounds contained GGPE on mild steel surface. It can be also seen that corrosion rate and inhibition efficiency increase with increase in temperature. It is noted that at elevated temperature, reacting molecules collide faster leading to more depletion of the reactant and formation of the product.
Adsorption isotherm and thermodynamic parameters
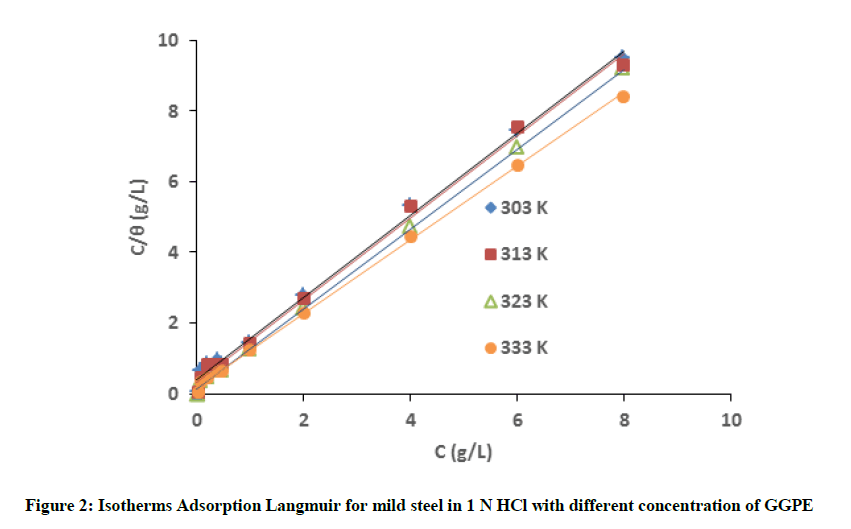
The Adsorption isotherm is one of methods used to explain the mechanism of corrosion inhibition using organic compound (extract) where it block the metal surface to prevent the corrosion. The degree of surface coverage (θ) is very useful to discuss about the adsorption isotherm. It was observed that data fitted the Langmuir, Temkin, and Freundlich adsorption isotherm and the correlation coefficients were used to determine the best fitted isotherm. Based on experimental data obtained this study tits with Langmuir isotherm formulated as [11-14]:
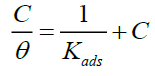 (5)
(5)
Where θ is the degree of surface coverage, C is the concentration of inhibitor and Kads is the adsorption equilibrium constant. The plot of C/θ versus C as shown in Figure 2 with correlation coefficients (R2) equal to 1, which indicated the experimental data obtained GGPE onto metal surface follows Langmuir isotherm.
From Table 2, it was found that the value of Kads increases with increasing of temperature. It shows that the higher the value of Kads, the adsorption of extracts on the steel surface is more stable, as evidenced by the increasing percentage of corrosion inhibition efficiency with the rising of temperature. The value of free Gibbs (ΔG0 ads) obtained by a negative value indicates a spontaneous and stable adsorption reaction between the extract and the steel surface [11].
| Temperature (K) | Kads (kJ / mol) | ΔG0ads (kJ / mol) |
|---|---|---|
| 303 | 2.62 | -16.75 |
| 313 | 3.16 | -20.7 |
| 323 | 7.13 | -36.44 |
| 333 | 6.36 | -35.39 |
Table 2: Thermodynamic parameters for adsorption of GGPE on mild steels in 1 N HCl at different temperature
Kinetic-thermodynamic corrosion parameters
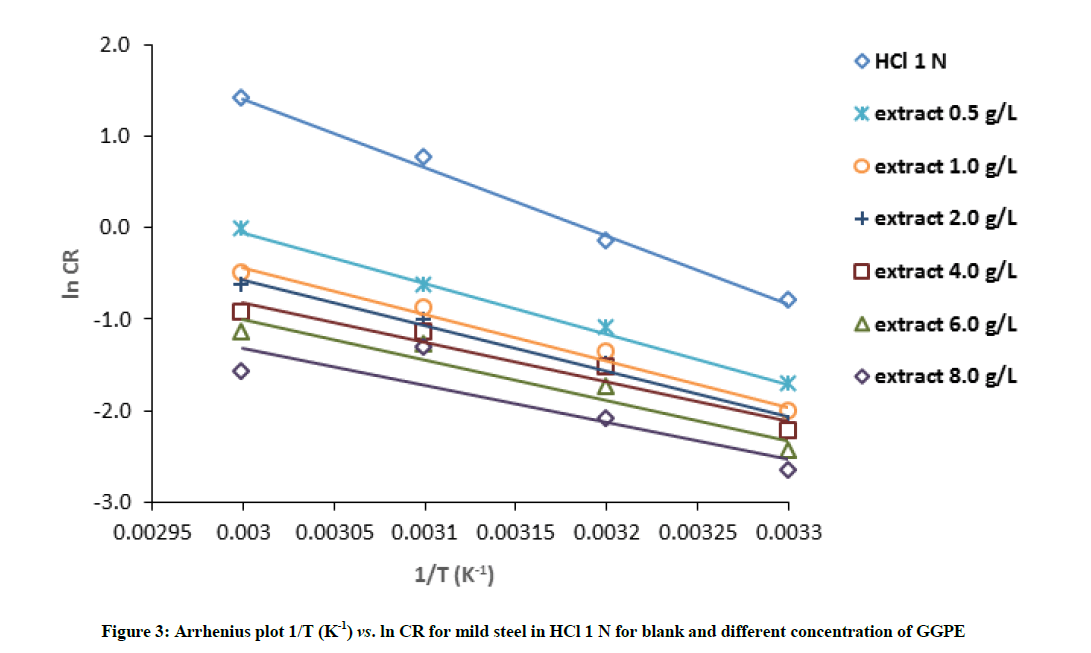
An Arrhenius plot (corrosion rate (ln v) versus temperature (1/T) for mild steel in 1 N HCl solution in the absence and presence of different GGPE concentrations shown in Figure 3. The Arrhenius equation can be used to determine activation energies (Ea) for mild steel corrosion. The values of activation energy of corrosion of mild steel in 1 N HCl solution were obtained from the slopes of the linear plots (Arrhenius plot) and are given in Table 3. Results shown in the table indicate that Ea of extract is lower than in their absence. The activation energy Ea was found to be 62.46 kJmol-1 for absence of extract and decrease to 55.53 kJmol-1 with added 0.1 gl-1 of extract. It suggested that the more GGPE were added, the smaller corrosion reactions energy were required. It will reduce the corrosion reaction of the steel in HCl solution and the more difficult the corrosion reaction occurs.
| Medium HCl 1 N + Extract (g/l) | Activation Energy (kJ / mol) | ΔH0 (kJ / mol) |
ΔS0 (J / mol) |
|---|---|---|---|
| (Blank) | 62.46 | 59.83 | 142.15 |
| 0.5 | 46.05 | 43.43 | 80.79 |
| 1 | 42.55 | 39.94 | 67.15 |
| 2 | 41.69 | 39.08 | 63.42 |
| 4 | 36.26 | 33.64 | 45.07 |
| 6 | 36.55 | 33.93 | 44.33 |
| 8 | 33.35 | 30.73 | 32.08 |
Table 3: The value of activation energy (Ea), enthalpy (ΔH0) and entropy (ΔS0)
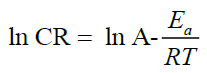 (6)
(6)
Where CR is corrosion rate, Ea is apparent activation energy, R is molar gas constant, T is absolute temperature and A is the frequency factor.
An alternative formulation of Arrhenius Equation is [12-14]:
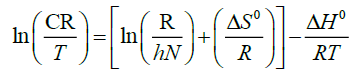 (7)
(7)
Where h is the Plank’s constant, N is the Avogadro’s number, T is the absolute temperature, R is the universal gas constant, ΔS0 is the entropy of activation, and ΔH0 is the enthalpy of activation. The values ΔS0 and ΔH0 (Table 4) can determined from plot of ln CR/T versus 1/T. Straight line were obtained with a slope of -ΔH0/R and intercept of lnR/Nh + ΔS0/R, from which the values of ΔH0 and ΔS0 were calculated.
| The concentration of extract (g/l) | Ecorr (V) | Icorr x 10 -3 (μA) | EI (%) |
|---|---|---|---|
| 0 | -0.53 | 7.41 | 0 |
| 0.2 | -0.56 | 6.01 | 18.93 |
| 0.5 | -0.57 | 4.11 | 44.53 |
| 1 | -0.59 | 3.08 | 58.43 |
| 2 | -0.6 | 2.39 | 67.74 |
Table 4: The potential value of corrosion, corrosion current and efficiency of corrosion inhibition of steel without and with the addition of GGPE
The positive value of ΔH0 (Table 3) reflects the endothermic nature of the mild steel dissolution process. ΔS0 can be interpreted positive values of ΔH0 indicated that the immersion of GGPE produced an endothermic reaction and the value of ΔH0 of blank was higher than ΔH0 by adding GGPE shown that energy which is needed to require the corrosion was higher. As well as the value of ΔS0 with the addition of GGPE was smaller than ΔS0 of blank described the ability of GGPE to slow down corrosion rate [14].
Potential polarization measurement
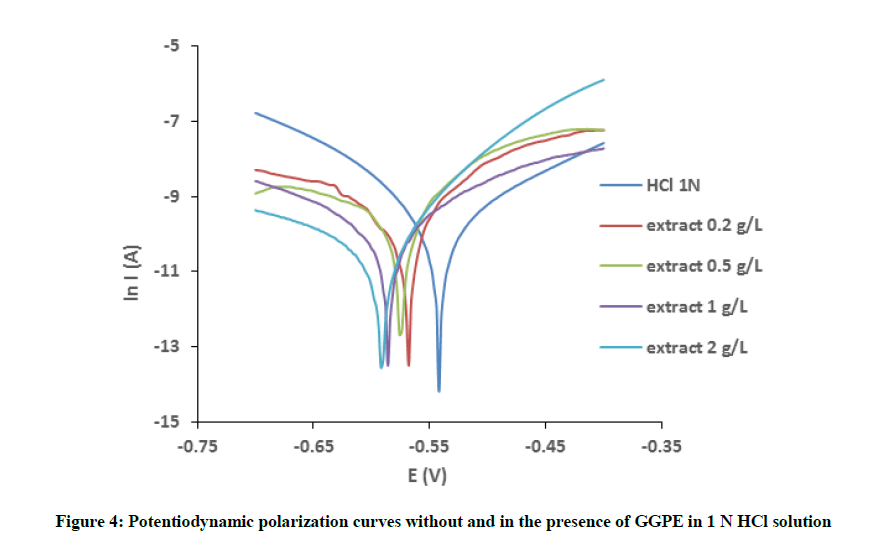
The effect of GGPE concentration on the corrosion behavior of mild steel in 1 N HCl solution has been studied by polarization measurements and Tafel plots are shown in Figure 4. From polarization curves, the electrochemical parameters obtained are presented in Table 4. It can be seen that current densities decrease considerable and inhibition efficiency increase in the present of GGPE. This phenomenon indicates that the inhibitor could reduce anodic reaction of the mild steel dissolution as well as cathodic hydrogen evolution. These results are comparable with weight loss measurement.
In the presence of inhibitor, the Ecorr of mild steel shifted toward cathodic region compared to without inhibitor in solution. However, a compound is usually classified as anodic or cathodic type inhibitor when the change in Ecorr value is larger than 85 mV [15]. Since the largest displacement of the corrosion potential was about 70 mV (Table 4) in the present of GGPE, suggesting that inhibitor acted as mixed-type inhibitor.
FT-IR analysis
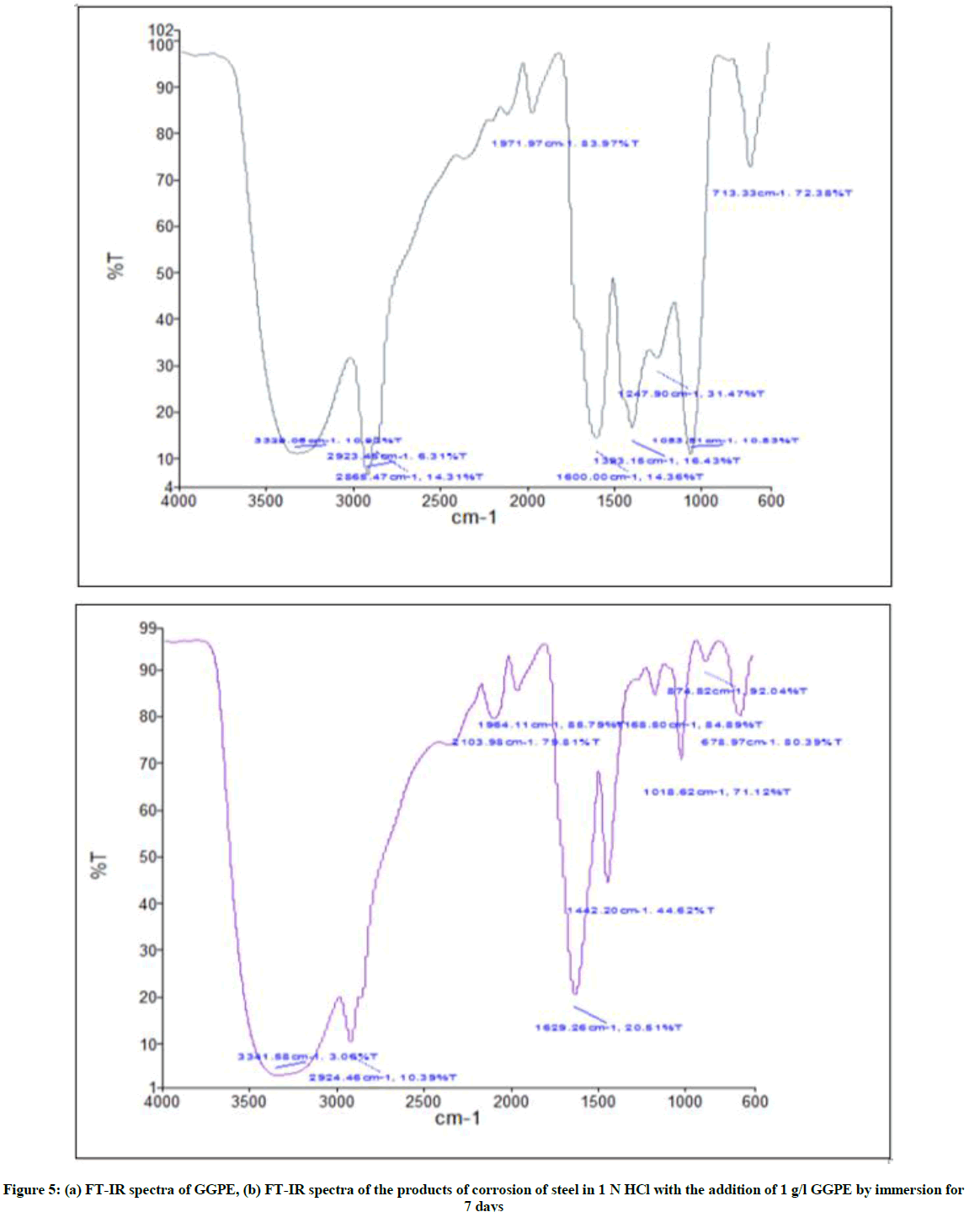
The analysis by using FT-IR was done to identify functional groups of organic compounds, which contained in GGPE that play a role as corrosion inhibitors of secondary metabolite compounds in GGPE and determine the type of bonding that occurred from inorganic adsorbents that adsorbed on the steel surface.
The FT-IR spectrum of Figures 5a, 5b shows no significant difference as some dominant peaks are in the range of wavelengths despite the shift of wave numbers. The shift of wave numbers indicates the occurrence of bonds between compounds contained in GGPE with steel surfaces. Figures 5a and 5b there are hydroxy groups (-OH) (3500-3000 cm-1), CH-stretching groups (3000-2500 cm-1), -C = C- stretching (1650-1450 cm-1). From the above data it can be concluded that these two spectra have a shift of wave numbers and GGPE has a role as a corrosion inhibitor on steel. However, there is little difference in the strength or weakness of the resulting absorption peaks, as well as the missing absorption peaks and the newly emerging absorption peaks.
SEM analysis
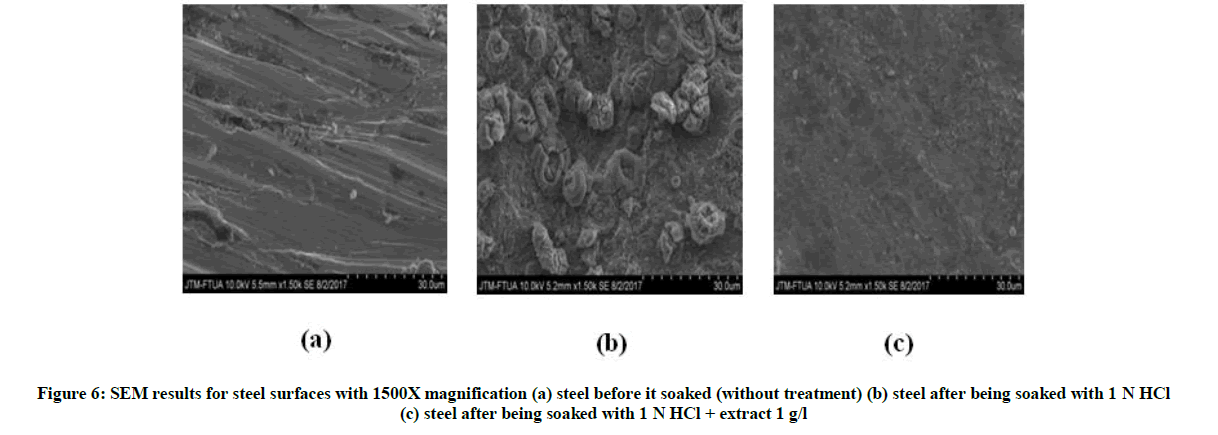
Figure 6a is a steel surface without treated. The steel still looked good, flat and non-corroded because there was no interaction of steel with corrosive medium. Figure 6b shows the steel which has been immersed for 7 days in corrosive medium of 1 N HCl for 7 days which shown its surface. There are holes and rust in the surface. This hole and rust occurs due to the interaction between the steel with corrosive medium 1 N HCl. While in Figure 6c shown the surface of the steel has been soaked for 7 days in 1 N HCl solution and GGPE of 1 g/l smoother and more flat than immersion with 1 N HCl. This due to the presence of a protective layer of GGPE adsorbed on a steel surface which can protect the steel from 1 N HCl [16].
Conclusion
In the research that has been done, it can be concluded that GGPE can be used as corrosion inhibitor. It acts as a mixed-type inhibitor by inhibiting both anodic and cathodic reaction. The inhibition efficiency increases with the increases the concentration of GGPE and rise in temperature. The Adsorption of GGPE follows the Langmuir adsorption isotherm model and the adsorption is spontaneous. The kinetic and thermodynamic parameters of corrosion.References
- R.T. Loto, C.A. Loto., A.P.I. Popoola. J. Mater, Environ. Sci., 2013, 3(5), 885-894.
- A.A.H. Kadhum, A.B Mohamad, L.A Hammed, A.A. Al-Amiery, Ng H. San, A.Y. Moses, Materials, 2014, 7, 4335-4348.
- ]Y. Yetri, Emriadi, N. Jamarun and Gunawarman, Environment Asia, 2016, 9 (1), 45-59.
- M.S. Al-Otaibi, A.M. Al-Mayouf, M. Khan., A.A. Mousa, S.A. Al-Mazroa, H.Z Alkhathlan, Arabian J. Chem.2014, 7, 340-346.
- E.E. Oguzie, Corr. Sci.,2008, 43, 168-178.
- D.R. Gusti, Emriadi, A. Alif, M. Efdi, Int. J. Chem. Tech. Res., 2017, 10(2), 163-171.
- S. Leelavathi, R. Rajalakshmi, J. Mater. Environ. Sci., 2013, 4(5), 625-638.
- S.S. Shivakumar, K.N.S. Mohanna. Eur. J. Chem.,2012, 3(4), 426-432.
- Emriadi, A. Santoni., Y. Stiadi. Der Pharma Chemica, 2016, 8(18), 266-273.
- T.M. Siregar, M. Cornelia, Ermiziar, Assoc. Indonesian Food Technol. Experts (PATPI)., 2010.
- V.G. Vasudha and P.K. Shanmuga, Res. J. Chem. Sci., 2013, 3(1), 21-26.
- A.I. Ali and Y. Sh. Mahrous, Royal Soc. Chem., 2017, 7, 23687-23698.
- L. Xianghong, D. Shuduan, X. Xiaoguang, Corr. Sci., 2014, 81, 162-175.
- E.I. Ating, S.A. Umoren, I.I. Udousoro, E.E. Ebenso, A.P. Udoh, Green Chem. Lett. Rev., 2010, 3(2), 61-68.
- N. Soltani, N. Tavakkoli, M. Khayakashani, M.R Jalali, A. Mosavizade, Corros. Sci., 2012, 62, 122-135.
- B.E.R. Amitha, B.J.B.Barathi, Int. J. Corr., 2012, 12, 1-15.