Research Article - Der Pharma Chemica ( 2021) Volume 13, Issue 7
Corrosion inhibition studies of Quinoline intermediate on mild steel
Praveen B M1*, Narayana Hebbar2 and Prasanna BM32Department of Chemistry, Sri Dharmasthala Manjunatheshwara College ((Autonomous) Ujire – 574 240, India
3Department of Chemistry, Jain Institute of Technology, Davanagere, Karnataka, India
Praveen B M, Department of Chemistry, College of Engineering and Technology, Srinivas University, Mukka, Mangalore, India, Email: bm.praveen@yahoo.co.in
Received: 23-May-2021 Accepted Date: Jul 21, 2021 ; Published: 28-Jul-2021
Abstract
Corrosion resistance “properties of 4-chloro,8-(trifluoromethyl) quinoline on Mild Steel in 1 M HCl solution by electrochemical methods such as Tafel and Electrochemical Impedance measurement. 1000 ppm of the inhibitor gives highest inhibition efficiency of 92%. All the experiments were conducted at room temperature. Electrochemical results show that it is a mixed type of inhibitor. Polarisation and Impedance measurements results are similar in nature. Scanning Electron microscopic images confirms the formation of passive layer on the” metal surface. Overall results indicates that quinoline derivative is good inhibitor for mild steel in HCl Medium.
Keywords
Quinoline, Inhibitor; SEM; Polarisation
Introduction
Mild steel is an essential iron alloy. It is superior in mechanical and metallurgical properties. It is used in a wide variety of industries. Owing to the aggressive environment, mild steel corrosion is a major problem for industries. To separate the metal from the aggressive environment, many successful methods have been implemented. Using of corrosion inhibitors to control the corrosion of mild steel is simple and effective method and especially in acidic medium [1-6]. Organic compounds [7,8] with multifunctional hetero atoms like Nitrogen, Sulphur and Oxygen showing good inhibition nature. The toxic characteristics of organic compounds leads to introduce the green corrosion inhibitors such as plant extracts [9] and pharmaceutical compounds [10].
The presence of polar N, S, or O atoms, as well as the molecular and electronic configurations of aromatic compounds, play a critical role in the inhibition process. In order to continue our investigation into the corrosion inhibiting role of 4-Chloro,8-(Trifluoro Methyl) Quinoline, a medicine intermediate used in the preparation of analgesic drugs [11-21], we tested mild steel corrosion in hydrochloric acid under various experimental conditions.
The Quinoline derivative was used in these studies and it is a novel molecule. No one was tried as a corrosion inhibitor for mild steel in acidic medium. This molecule is having nitrogen in the ring and fluorine and chloride in the structure helps to donate the lone pair of electrons of nitrogen atom to mild steel by it form the strong bond between metal and inhibitor. In this work very small amount of inhibitor gives around 92% inhibition efficiency. The aim of the work in this paper is development of green corrosion inhibitor for mild steel in acidic medium.
Materials and Methods
Material
The electrochemical experiments were carried out on a freshly prepared mild steel sheet. The metal specimens are cut in grade 80 to 2000, washed in double distilled water and rinsed in acetone. The inhibition effect is measured using a 1cm2 mild steel exposure (The rest is covered with insulator).
Inhibitor
The Inhibitor 4-Chloro, 8-(Trifluoro Methyl) Quinoline. 4-Chloro, 8-(Trifluoro Methyl) Quinoline was procured from Sigma Aldrich with AR grade. All the chemicals in this work were AR grade chemicals and supplied by Sigma Aldrich. Quinoline was prepared as a stock solution, and this stock solution was used for all experimental purposes. The molecular structure is shown in Figure 1. In 1M HCl, different concentrations of the 4 Quinoline inhibitor were prepared.
Methods
In the potentiodynamic polarization experiments, mild steel strips with a 1 cm2 exposed area were used. Regular three electrode assembly with mild steel surface of 1cm2 exposed area acts as working electrode, Silver/silver chloride electrode was reference electrode and platinum foil was counter electrode. CH analyser was used to conduct the electrochemical measurements. Polarisation measurements were conducted 2 mVs-1 with respect to open circuit potential value. Corrosion parameters were calculated by using the software associated with CH instrument. Impedance measurements were conducted to calculate polarization resistance, solution resistance and double layer capacitance values.
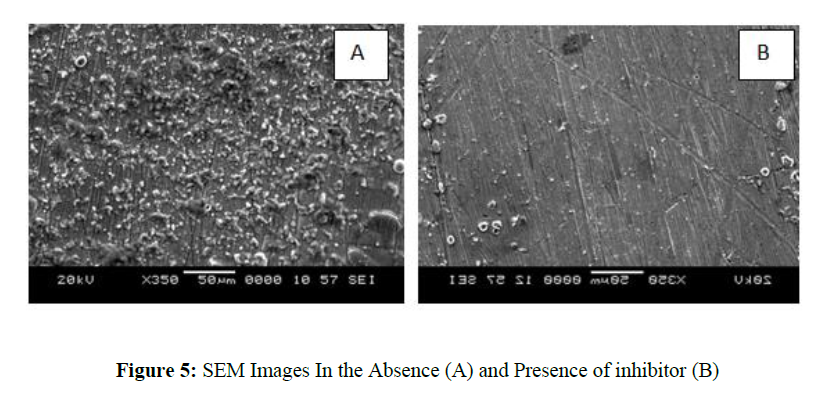
Scanning electron microscope was used to anlayse the surface morphology of mild steel in 1M HCl before and after corrosion experiments. The accelerating beam used had a voltage of 20 kV.
Results and Discussions
Tafel polarization method
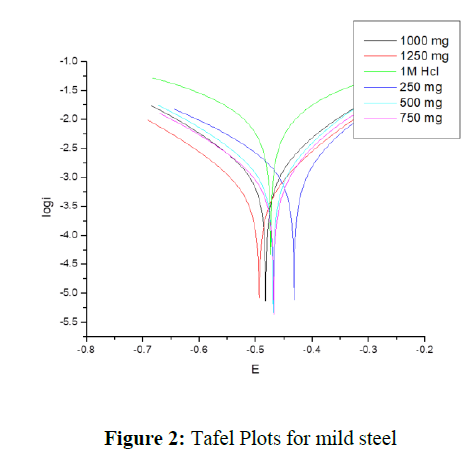
This method can be used to “investigate the corrosion behavior of mild steel in presence of different concentration of inhibitor in 1M HCl medium. Table 1 lists the parameters for corrosion rate and inhibition performance (ηp). The ηp was calculated using the following formula.
| Temperature (0C) | Inhibitor conn (mg/L) |
Ecorr ( V) |
I corr A cm-2 |
Corrosion rate(mpy) | Βc mV/decade |
Βa mV/decade |
% ηp | Rp Ωcm2 |
Cdl ( µF cm-2) |
% ηI |
|---|---|---|---|---|---|---|---|---|---|---|
| 30 | Blank | -0.496 | 0.177 | 34.70 | 5.663 | 6.274 | - | 6.536 | 246 | - |
| 250 | -0.489 | 0.09 | 17.91 | 7.757 | 9.630 | 49 | 23.57 | 230 | 72 | |
| 500 | -0.483 | 0.077 | 15.91 | 7.903 | 9.096 | 56 | 52.24 | 100 | 87 | |
| 750 | -0.499 | 0.050 | 10.09 | 6.667 | 9.951 | 71 | 59.7 | 123 | 89 | |
| 1000 | -0.521 | 0.0192 | 14.04 | 7.743 | 8.532 | 89 | 89.37 | 3500 | 92 | |
| 1250 | -0.500 | 0.023 | 17.32 | 6.835 | 6.795 | 87 | 39.75 | 140 | 83 |
Table 1: Electrochemical Results
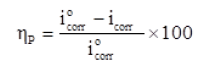
iºcorr and icorr are corrosion current in absence and presence of inhibitor respectively.
Tafel plots in the presence of different concentration of inhibitor at temperatures 303K given in figure 2. Corrosion current and corrosion rate are the most important parameters in this method. As the inhibitor concentration increases, the icorr and vcorr values decrease, indicating the corrosion prevention of metal by the inhibitor.
Corrosion of the metal is associated with anodic and cathodic reactions. Anodic reaction is metal dissolution and cathodic reaction is hydrogen evolution. Corrosion can be stopped/lowered by reducing both the reactions or by hindering any one of the reaction. Corrosion potential values are giving the information on type of inhibitor. In this work corrosion potential values shifted less than 85mV compare to blank solution [6]. The developed inhibitor in this work is mixed type inhibitor and it reduces both anodic and cathodic reactions.
AC-impedence method
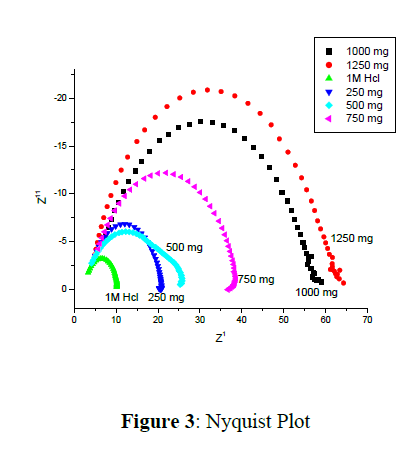
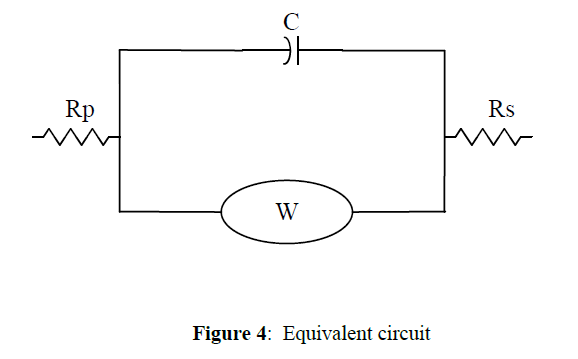
Nyquist data are given in figure 3. It is recorded for Mild Steel in 1 M HCl. In order to calculate the corrosion parameters it is fitted with equivalent circuit and it is given in figure 4. EIS data is given in table 1. Here polariasation resistance, Solution resistance and Double layer capacitance with corrosion inhibition efficiency was given in the table.
The inhibition efficiency (ηz) was calculated using the equation below.
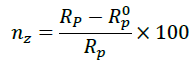
Where, Rp and Rp0 are in presence and absence of inhibitor respectively.
The Nyquist plot's depressed semicircle distinguishes metal corrosion in the presence of inhibitor from metal corrosion without inhibitor. This phenomenon is regulated by a charge transfer mechanism caused by a capacitance values [7]. In other words, the reduction mechanism induced by the inductive loop formed by FeSO4 or inhibitor species [8] adsorption on the electrode surface. The presence of an inhibitor increases Rp levels while lowering Cdl levels, as can be seen.
Decrease in double layer capacitance values with increase in polarization resistance values indicating that, adsorption of the inhibitor takes place on the metal surface [9,10].
This inhibitor gave around 92% inhibition efficiency with 1000 ppm concentration of the inhibitor. In this work inhibition efficiency obtained by tafel and Impedance methods are similar and it shows the reproducibility. This molecule is soluble in HCl medium and it is a green inhibitor. Developed inhibitor is ecofriendly inhibitor compare to available inhibitors in the market and developing inhibitors by other researches.
SEM analysis
SEM images were recorded for the mild steel sample in presence and absence of Quinoline derivative and it is given in figure 5. Mild steel sample has lot of dissolution sites with corrosion debris o the sample in absence of inhibitor. Mild steel sample is completely covered by Quinoline derivative and it reduces the metal dissolution. So corrosion rate is retarded in presence of inhibitor. Metal surfaces have pits and corrosion products, according to SEM images, but these are minimised on the metal surface when an inhibitor is present. Corrosion rate is controlled in presence of inhibitor.
Conclusions
Corrosion Studies confirms Quinoline derivative is a good inhibitor. Very small quantity of 1000 ppm inhibitor shows around 92% inhibition efficiency. SEM confirms the formation of passive layer on the steel surface. It is an ecofriendly inhibitor with mixed type Inhibition nature. This inhibitor could find industrial applications and opens the new domain of research in corrosion field.
References
- Praveen BM, Prasanna BM and Narayana H et al., J. Bio- Tribo-Corros., 2018, 4(2): p. 1-11.
- Mallikarjuna NM, Keshavayya J, Prasanna BM et al., J. Bio- Tribo-Corros, 2020, 6(1): p. 1-17.
- Narayana H, Praveen BM, Prasanna B et al., J Adhes Sci Technol, 2015, 29(24): p. 2692-2708.
- Narayana H, Praveen BM, Prasanna B et al., Int. J. Ind. Chem., 2015, 6(3): p. 221-231.
- Prabhu RA, Venkatesha VT, Praveen BM et al., T Indian I Metals, 2014, 67(5): p. 675-679.
- Mohan R Reddy, Praveen BM and Kumar CMP. Surf. Eng. Appl. Electrochem. 2017. 53(2): p. 179-185.
- Prabhu RA, Venkatesha VT and Praveen BM., Int. Sch. Res. Notices. 2012,
- Rajappa SK, Praveen BM and Venkatesha VT. Int. Res. J. Chem, 2014, 1(2): p. 010-017.
- Shylesha BS, Venkatesha VT, Praveen BM et al., Int. Sch. Res. Notices. 2012.
- Shylesha BS, Venkatesha VT, Praveen BM et al., Anal. Bioanal. Electrochem. 2011, 3(3): p. 249-260.
- Praveen BM and Venkatesha. Int. J. Electrochem. Sci. 2011.
- Shylesha BS, Venkatesha VT, Harshini GK et al., J Chem & Chem Eng, 4(8): p. 35-41.
- Prasanna BM, Praveen BM, Narayana H et al., Moroc. J. Chem. 2015, 3(4): p. 824-837.
- Vani R, Praveen BM and Kumar G et al., Int. J. Mech. Eng. Robot. Res.2015, 4(1): p. 128.
- Shanbhag AV, Venkatesha VT, Praveen BM et al., J. Iron Steel Res. Int. 2014, 21(8): p. 804-808.
- Rashmi D, Pavithra GP, Praveen BM et al., Analysis. J. Fail. Anal. Prev. 2020. p.1-10.
- Narayana H, Praveen BM, Prasanna BM. Et al., Int Res J Chem, 2019, 2: p. 018-020.
- Prasanna BM, Praveen BM, Narayan Hebbar et al., J. Assoc. Arab Univ. Basic Appl. Sci. 2017,22: p. 62–69
- Prasanna BM, Praveen BM, Narayan Hebbar et al., Int. J. Ind. Chem. 2016, 7: p. 9–19
- Prasanna BM, Praveen BM, Narayan Hebbar et al., Anti-Corros Method M, 2016, 63: p. 47-55.
- Praveen BM, Prasanna BM, Mallikarjuna NM et al., Heliyon. 2021, 7: p. 1-9.