Research Article - Der Pharma Chemica ( 2018) Volume 10, Issue 8
Cyanoacrylates: An Overview in Its Application as a Tissue Adhesive
Harsha K and Vasudha P*
Department of Pharmaceutics, Vivekanand Education Society’s College Of Pharmacy, Chembur(E), Mumbai-400074, India
- *Corresponding Author:
- Vasudha P
Department of Pharmaceutics
Vivekanand Education Society’s College Of Pharmacy
Chembur(E), Mumbai-400074, India
Abstract
The wound closure has been a matter of concern for the patients more than the surgery itself. Major reasons include the endless pain and the complications involved in the conventional tissue adhesion methods like sutures, staples, screws, etc. Not only the pain involved in suturing and in removing sutures post healing but also the infections in the sutured wounds along with the cosmetically unacceptable scars are the major reasons for the development of the tissue adhesives and sealants for the wound closure. Tissue adhesives seem to be of great importance since they have the capability of either completely replacing the conventional wound closure technique or at the minimal provide a support for reducing the number of stitches. The ideal dressing should achieve rapid healing at reasonable cost, with minimal inconvenience to the patient. This article offers a review of the emerging technology using cyanoacrylates for achieving improved, painless and patient compliant wound healing. Cyanoacrylates are strong, fast-setting liquid monomers. These upon exposure to moisture start to set due to polymerization reaction. Fast-setting, high tensile strength, good flexibility, durability, waterproof nature and transparent appearance, are few of the properties which make cyanoacrylates ideal for wound closure and skin barrier products. General formation and formulation approach of cyanoacrylates towards achieving optimum physical properties, mechanism of tissue adhesion and evaluation parameters along with applications, safety assessment, their key advantages and shortcomings are also considered briefly.
Keywords
Cyanoacrylates, Polymerization, Hemostat, Tissue adhesive.
Introduction
The first line of defense for a human body is the largest organ itself, the skin. The skin defends the body from the external environment by forming a physical barrier. So it is of utmost importance to keep this barrier intact. The wounds generally cause the tissues beneath to get exposed. The basic mechanism behind healing the separated flaps of skin is bringing it together and securing it in place to avoid its movement. This induces the healing of the wound with reconstruction of the damaged tissues. The conventional wound healing methods like sutures and staples pose a risk of further damage to the skin by causing foreign body response, growth of bacterial infection due to phenomenon of wicking, tissue necrosis due to improper fastening, etc. While tissue adhesives are pain free, safe and biocompatible with no immunogenic response. An ideal tissue adhesive should be of low cost, easy to use, should be required in minimal quantity and yet be effective with fast curing time leaving minimal scar after wound healing with good cosmetic outcome. Cyanoacrylates have a number of advantages over conventional suture like their fast and painless application, rapid setting which reduces the total quality time, their antibacterial properties. Cyanoacrylate itself acts as a water proof dressing and helps in reduction in the number of follow-up visits. As they do not require any needles, accidental needle stick injuries are prevented.
Introduction to cyanoacrylates
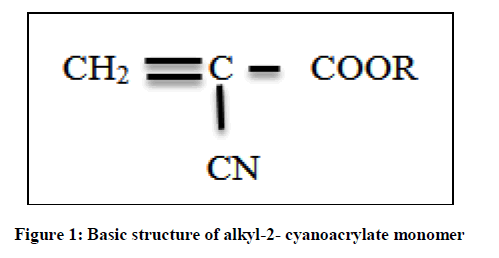
The basic structure of alkyl-2-cyanoacrylate is as shown in Figure 1. The R represents methyl, hexyl or decyl grouping.
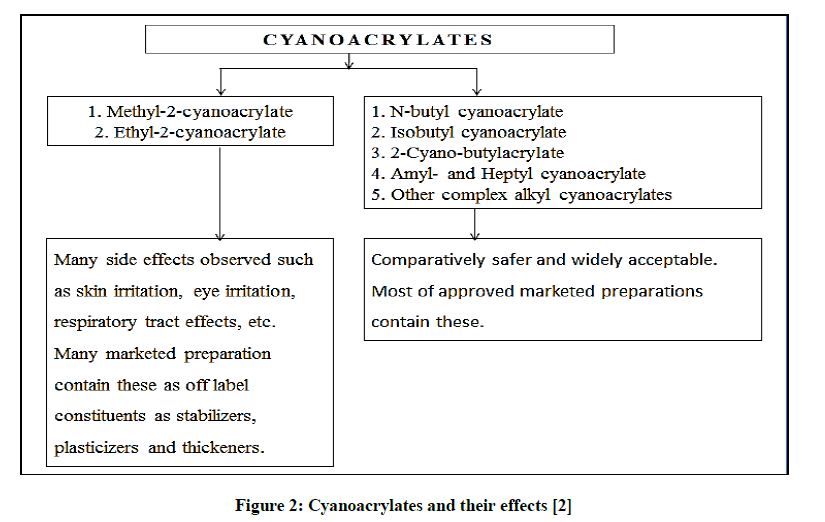
Ardis patented alkyl-2-cyanoacrylates as adhesives in 1949. The adhesive properties of cyanoacrylates were characterized by Coover et al. [1]. Cyanoacrylates better known as super glues were majorly used in automotive and construction industry. After 1960s their application in the field of medicine was identified. The cyanoacrylates were used clinically by Watson and Maguda for tympanic membrane repair. Initially the cyanoacrylates applied for wound closing purposes were Methyl-2-cyanoacrylate and Ethyl-2- cyanoacrylate. These earlier methyl analogues were found to be histotoxic and thus were discontinued. N-butyl cyanoacrylate (NBC) is biocompatible and amongst the widely accepted tissue adhesives. Figure 2 depicts various cyanoacrylates and their acceptability:
Figure 2: Cyanoacrylates and their effects [2]
Cyanoacrylate polymers and its properties
The cyanoacrylate based adhesives are topical glues that bond to outer surface of the skin to form a seal over the edges of incision. These are quick bonding super glues used in conjugation with another liquid which generally acts as a hardener.
The Table 1 given below compares the properties of Octyl cyanoacrylate and Butyl cyanoacrylate tissue adhesives:
| Properties | OCA | BCA |
|---|---|---|
| Degradation | slower | slow |
| Firm flexibility | higher | high |
| Bonding Strength | stronger | strong |
| Heat released | Less | more |
| Setting time | Short | shorter |
Table 1: Comparison of properties of OCA and BCA [3]
Process of formation and formulation of cyanoacrylates
Condensation of formaldehyde and alkyl cyanoacetate in presence of base catalyst for polymerization. The polymerisation reaction takes place at the unsaturated position and then gradual addition of further monomer units to form desired polymer. Depolymerisation at high temperature in presence of polymerization inhibitor like sulphur dioxide and nitric oxide. Distillation of liquid cyanoacrylate adhesive monomer. Purification by consecutive fractional distillation. Stabilization by free radical inhibitor and base scavengers. Formulating final preparation as per specifications using additives to adjust viscosity, bond strength, degradation rate, physical and mechanical properties by adding stabilizers, plasticizers, colorants for easy application etc. [4,5].
Mechanism of tissue adhesion with cyanoacrylates
Adhesion between the glue and the tissue is accomplished via covalent bonds between cyanoacrylates and functional groups in tissue proteins. Cyanoacrylate glue by itself does not polymerize but in presence of slightest amount of moisture it starts to react and forms tight bonds. On initiation of polymerization it generates its own heat which is in turn used for curing. This heat may damage soft tissues. To avoid the risk of this damage, long chains of methyl group are added which prolong the rate of heat generation by prolonging the rate of polymer formation [6].
Properties of adhesives
1. A strong bond is formed within five seconds to three minutes.
2. No need to mix with a curing agent. Neither pressurization nor heating is required.
3. Strong bonds are formed at room temperature.
4. Small amounts are required-due to its low viscosity.
5. Spreads well and also around the corners.
6. The bonded part is clear and colorless, resulting in a neat finish.
7. Since it is solvent-free, virtually no shrinkage occurs during curing.
Evaluation of the adhesives
There are numerous tests which help in evaluation of the tissue adhesives. Since these are used widely in surgical procedures their efficacy cannot be compromised. These tests can be briefly elucidated as below.
Adhesive strength and flexibility and Lap shear tensile strength
Tested by measuring the force required to break two overlapped pieces of pig skin adhered by the adhesive onto the two steel substrates. FDA recommends that you conduct mechanical testing to evaluate the ability of the polymerized adhesive to provide enough bond strength to hold the wound edges together without manual approximation.
The following four test methods are intended to provide a means for comparison of the adhesive strengths of tissue adhesives for use as surgical adhesives or sealants on soft tissue.
ASTM F2255-05: Standard Test Method for Strength Properties of Tissue Adhesives in Lap-Shear by Tension Loading-This test method may be used for comparing adhesives or bonding processes for susceptibility to fatigue and environmental changes.
ASTM F2256-05: Standard Test Method for Strength Properties of Tissue Adhesives in T-Peel by Tension Loading-Two bonded layers that are equally flexible, when pulled apart will form a T shape. The angle of separation is 180 degrees, but this is called a T-test. In all 180 degree tests there needs to be sufficient column height in the universal tester for adequate sample length and peel distance.
ASTM F2258-05: Standard Test Method for Strength Properties of Tissue Adhesives in Tension-a method for testing adhesion by pulling bonded layers apart along the plane of adhesion, where each end of the sample is held by vice grips and pulled apart at a controlled rate, and the force applied is expressed proportional to the total adhesive surface area, or shear area.
ASTM F2458-05: Standard Test Method for Wound Closure Strength in Tissue Adhesives and /Sealants-the specimen placed in a measured area of seam is observed to failure at a constant crosshead speed [7,8].
Assessment of efficacy of wound closure
Wound closure strength
Force measured should be about 2 to 4 lbs/in2 which is required to disrupt the wound closed by test adhesive on pig skin.
Heat release
Heat released during polymerization should be optimum, since polymerization in cyanoacrylates is an exothermic process. Excess heat release may cause soft tissue destruction or discomfort in patients. It is determined using a temperature recording system with a thermistor and a temperature bridge for connection with a recording system.
Setting time
It is positively correlated to polymerization rate. To achieve short setting time, trace amount of accelerator is added.
Surface coverage
This is tested on the pig skin by measuring the length and width of adhesive spread by an applicator.
Permeability
Adhesives with high Moisture Vapor Transmission Rate (MTVR) improve wound care. The adhesives are applied on a 2 inch square collagen film and MTVR determined using water vapor permeability instrument. The cyanoacrylate adhesive films should ideally exhibit a moisture vapor transmission rate of from about 950 to about 3000 g/m2/day. Such transmission rates are important a desirable permeability rate is highly important in maintaining an optimal microenvironment at wound closing site [9]. Higher the permeability better is the wound healing.
Viscosity measurement
Viscosity of the liquid adhesive in the final product is a primary indicator of the stability of the subject device. The viscosity increases due to the transition of the monomer into a polymer [7]. This, in effect, reduces the concentration of monomer and can affect the adhesive bond formed with underlying tissue, thus achieving optimum viscosity becomes inevitable. The viscosity of cyanoacrylate compositions are measured using Brookfield viscometer. Viscosity of 5-30 centipoises at 25ºC is preferable.
Safety assessment of cyanoacrylates
The safety of cyanoacrylate tissue adhesive can be tested by assessing its chemical stability and its toxicity and biocompatibility studies.
Chemical stability
Chemical stability can be measured by hydrolytic analysis and also by evaluating its physical, chemical and mechanical properties.
Hydrolytic analysis
This is used to detect any degradation of the polymers. Once polymerized, CA can be hydrolyzed at a 1: 10 weight ratio in saline at 50ºC. The solutions are hydrolyzed for 0, 5, 10 and 15 days, respectively, and can be analyzed using HPLC and UV-VIS spectroscopy. Formaldehyde, cyanoacetate or colorants must be checked for passing the detection limits.
Shelf-life determination
Determined by real time/shelf life stability and by accelerated aging test at 80ºC for 12 days, as well as accelerated aging test at 40ºC for 6 months. Stability is then checked against various parameters.
Toxicity and biocompatibilities
Intracutaneous reactivity
This test is required for tissue reactions such as erythema, edema and eschar at 24, 48 and 72 h time interval by injecting the test sample subcutaneously in Albino New Zealand white rabbits.
Ocular irritation
One drop (~0.1 ml) of test sample is to be applied to one eye of each of nine New Zealand White rabbits. Discharge to be observed at all-time points during the 72 h observation period. Also conjunctival reactions, redness, corneal opacity, and iridial reactions is to be checked over a period of 24, 48 and 72 h period.
Skin irritation
Test sample can be applied under occlusive dressing to 6 rabbits for a period of 24 h and observed for erythema and edema.
Local effect after implantation
A test using three Albino New Zealand rabbits is conducted to assess the local effect of adhesive on living tissue. Four strips of test and four strips of controls are to be planted in every rabbit. The implants are to be excised with surrounding tissue after four weeks. The tissue is macroscopically examined for hemorrhage, necrosis, discolorations and infections.
In vitro cytotoxicity
In vitro cytotoxicity is to be tested by determining the biological reactivity of mammalian cell cultures following direct contact with test sample. It is considered to have toxic effect if there are malformed or degenerated cells under observed specimen [3,10,11].
Applications of cyanoacrylates as tissue adhesives
1. The use of cyanoacrylates as a new agent in wound closure can be tried as an alternative to sutures in closure of head and neck incisions is described in a prospective, non-randomized study with reduced pain [1]. The incisions used for the various surgical procedures in these patients were assigned to 2 groups, group 1 incision closed with sutures and group 2 incision closed with 2-octyl cyanoacrylate. Wounds were evaluated. Statistically speaking there was significant difference only in parameter of pain which proved that Group 2 was significant in controlling pain and the scar and surface texture approached significance (z=1.56). The study thus demonstrated the effectiveness and advantages of this new relatively painless wound closure using cyanoacrylates in producing superior cosmetic outcome.
2. The application of cyanoacrylates adhesives in closure of long surgical incisions has been found equivalent to commercially available devices contiguous with decreased incidence of wound infection. Cyanoacrylate tissue adhesives have been witnessed of acting as a barrier against wound colonization by gram-positive organisms. It has also been observed that suture material increases the risk of wound sepsis by serving as an adherent foreign body. The study [12] supports an excellent cosmetic outcome with 2-octylcyanoacrylate tissue adhesives for long wounds. It shows reduced incidence of wound infection also mentioning possibility of 10-fold lesser mean wound closure time when cyanoacrylate adhesives are compared to sutures.
3. Cyanoacrylate tissue adhesive, viz. 2-Octylcyanoacrylate (2-OCA), was studied for laceration repair in children [13]. This adhesive has a bonding strength three to four times that of Histoacryl Blue while at the same time has more flexibility, which allows its use on larger lacerations and incisions. There is also added convenience of not having to return (for suture/staple removal) with this method of repair. About 95% parents of the child receiving cyanoacrylate method would prefer this closure method during their next emergency department visit. The adhesive gives promising evidence in relation to savings of time and resources for laceration repair.
A Table 2 depicting the drug for the type of wound is given below. The applications vary depending on the manufacturer and the excipients added.
| n-Butyl-2-cyanoacrylate |
|
| Butyl lactoyl-2-cyano acrylate |
|
| N-Hexyl-2-cyanoacrylate |
|
| 2-Octyl-2-cyanoacrylate |
|
Table 2: Cyanoacrylates and type of wound they are used with [14].
Advantages and limitations of cyanoacrylates as tissue adhesives
The main advantages of the cyanoacrylates include:
1. Less time requirement (few seconds) for adhesive plug formation.
2. Available in easy to use packs for single or refill use as per the need for a wide variety of application.
3. Since it is a painless procedure it is beneficial in pediatrics.
4. Easily sloughs off by itself so no extra procedure is required post healing.
5. Patient compliance is observed in spite of the marginal cost difference between the adhesives and conventional wound healing procedures.
6. Asset in situations where mechanical fastening is undesirable.
6. Deliver better cosmetic outcome [15,16].
The limitations mainly include:
1. Has a lower mechanical strength in presence of high blood flow.
2. Found to generate histotoxic reactions in case of low molecular weight homologues whereas high molecular weight homologues are found to have generated foreign body granuloma response in some studies [14].
3. Costlier than the conventional techniques reasons being lack of embracement of the concept, cost approval by the institute, regulatory clearance.
4. The fast degradation leads to accumulation of the degradation products like formaldehyde and Cyanoacetate via hydrolysis.
Conclusion
Thus cyanoacrylates provide an extremely beneficial alternative to the conventional wound care techniques in terms of pain during the procedure, good cosmetic outcome, no extra visit and anxiety for removal of the suture or staples post the healing and less time requirement in clinical setting. The newer cyanoacrylate homologues with fewer toxicity issues and appropriate adhesive properties need to be developed. Also the properties like bacteriostatic activity on polymerization can be dwelled upon. Development of these adhesives would definitely be a major achievement in the field of wound healing.
References
- S.V. Kumaraswamy, Evaluation of 2-Octyl Cyanoacrylate tissue adhesive as an acceptable alternative to sutures in head and neck incisions, thesis, 2010, 2, 33-40.
- http://www.who.int/iris/handle/10665/42408
- http://www.lugimed.com/studien/Surgiseal_Whitepaper.pdf
- Quinn J.V. (Edi.), Tissue adhesives in clinical medicine, 2nd (Edn.), Ontario, Canada: BC Decker, 2005.
- http://www.madehow.com/Volume-1/Super-Glue.html
- A.D. Joshi, H. Saluja, U. Mahindra, R. Halli, J. Maxillofac. Oral Surg., 2011, 10, 310-315.
- G.J. Mattamal, Guidance for Industry and FDA Staff: Class II Special Controls Guidance Document: Tissue Adhesive for the Topical Approximation of Skin. Guidance Documents (Medical Devices and Radiation-Emitting Products), 2016.
- http://www.freepatentsonline.com/y2015/0196681.html
- http://www.mecmesin.com/knowledge-centre-test-types
- S.C. Woodward, Ann. Surg., 1965, 162, 113-22.
- http://www.google.com/patents/US20140186289.
- P.N. Blondeel, J.W. Murphy, D. Debrosse, J.C. Nix 3rd, L.E. Puls, N. Theodore, P. Coulthard, Am. J. Surg., 2004, 188, 307-313.
- T.B. Bruns, J. Pediatr., 1998, 132, 1067-1070.
- P.J.M. Bouten, Prog. Polym. Sci., 2014, 39, 1375–1405 (2014).
- https://www.aesculapusa.com/products/wound-closure/topical-skin-adhesive
- A.P. Duarte, J.F. Coelho, J.C. Bordado, M.T. Cidade, M.H. Gil, Prog. Polym. Sci., 2012, 37, 1031-1050.