Research Article - Der Pharma Chemica ( 2018) Volume 10, Issue 5
Cytotoxicity Evaluation and In Silico Admet Study of New (E)-1-(4-Chlorobenzyl)-N'-Benzylidene-5-Bromo-1h-Indole-2-Carbohydrazides
Harishkumar S1, Satyanarayan ND1*, Raghavendra R2, Nandini HS3, Prabhudas N3 and Kiranmayee P3
1Department of Pharmaceutical Chemistry, Kuvempu University, Post Graduate Centre, Kadur-577548, Chikkamagaluru Dt. Karnataka State, India
2Department of Microbiology and Cell Biology, Indian Institute of Science, Bangalore-560012. Karnataka State, India
3Sri Devaraj Urs Academy of Higher Education and Research, Tamaka, Kolar-563101, Karnataka, India
- *Corresponding Author:
- Satyanarayan ND
Department of Pharmaceutical Chemistry
Kuvempu University, Post Graduate Centre
Kadur-577548, Chikkamagaluru Dt. Karnataka State, India
Abstract
Cytotoxic evaluation using Peripheral Blood Mononuclear Cells (PBMCs) was achieved by a novel series of (E)-1-(4-chlorobenzyl)-N'- benzylidene-5-bromo-1H-indole-2-carbohydrazide 6(a-j). Among the series, the compounds 6b, 6c, 6f, 6h and 6j were found to be active compared to other analogues. These compounds showed excellent dose dependent cytotoxicity on PBMCs, whereas, 6j showed reduction in activity. The in silico pharmacokinetic parameter study such as ADME and toxicity of the synthesized compounds were evaluated using ACD/Ilab and ADMETSAR@MDDM methods. The synthesized molecules 6a, 6b, 6c, 6f, and 6g are in acceptable range and less toxic in nature.
Keywords
PBMCs, Cancer, Lymphocytes, In silico, ACD/Ilab
Introduction
Cancer is a term for diseases in which abnormal cells divide uncontrolled and can affect nearby tissues [1]. It affects everyone from young to adults, wealthy to poor and represents a great trouble on societies. Cancer is one of the leading causes of death in the world [2]. It continues to be a major health problem all over the globe and causes about 550, 000 deaths a year and is an important cause in both more and less economically developed countries [3]. In 2017, American Society of Cancer has estimated 1, 688, 780 new cancer cases diagnosed and 600, 920 cancer deaths in US [4]. In the present scenario treatment for cancer have become highly versatile and sophisticated, in this regard extensive efforts have been dedicated to the development of novel anticancer drugs which will reduce limitations on cancer therapy [5].
Heterocyclic framework constitutes the basis of an important class of compounds possessing interesting anticancer activities [6]. Indole derivatives have been a topic of substantial research interest in heterocyclic chemistry due to their natural occurrence and pharmacological activities [7]. Substituted indoles have been referred to as ‘‘privileged structures’’ for plenty of pharmacologically active lead compounds in the drug research and development since they are capable of binding to many receptors with high affinity [8]. Indole and its derivatives are one of the most versatile groups for design and development a new anticancer drugs [9]. Vincristine and vinblastines are also indole containing compounds which are effective against cancer in the present time [10]. Some hydrazide-hydrazone (–CONHN=CH–) derivatives possessed a broad range of biological activities in vivo and in vitro including antitumor and other pharmacological activities [11-17]. Previously synthesized indole-carbohydrazides are useful for inhibiting angiogenesis and cell proliferation [18].
Indole pharmacophores are found to possess a wide range of pharmacological properties and also indole coupled compounds were exhibited selective cytotoxicities [19]. Molecules containing –NHN=CH- groups constitute an important class of compounds for design and development of drugs, and also acts as bridged or linker between different pharmacophores [20]. The benzoylated indole analogues were showed good pharmacological activities this might be because of lone pair of electrons which are present on heteroatoms and easily interaction between tested compounds and target sites [21]. Halogens in the structure will provide specificity and protection from metabolism, and also enhance the lipophilicity [22]. This deliberation was decided to synthesize of (E)-1-(4-chlorobenzyl)-N'-benzylidene-5-bromo-1H-indole-2-carbohydrazide and its derivatives.
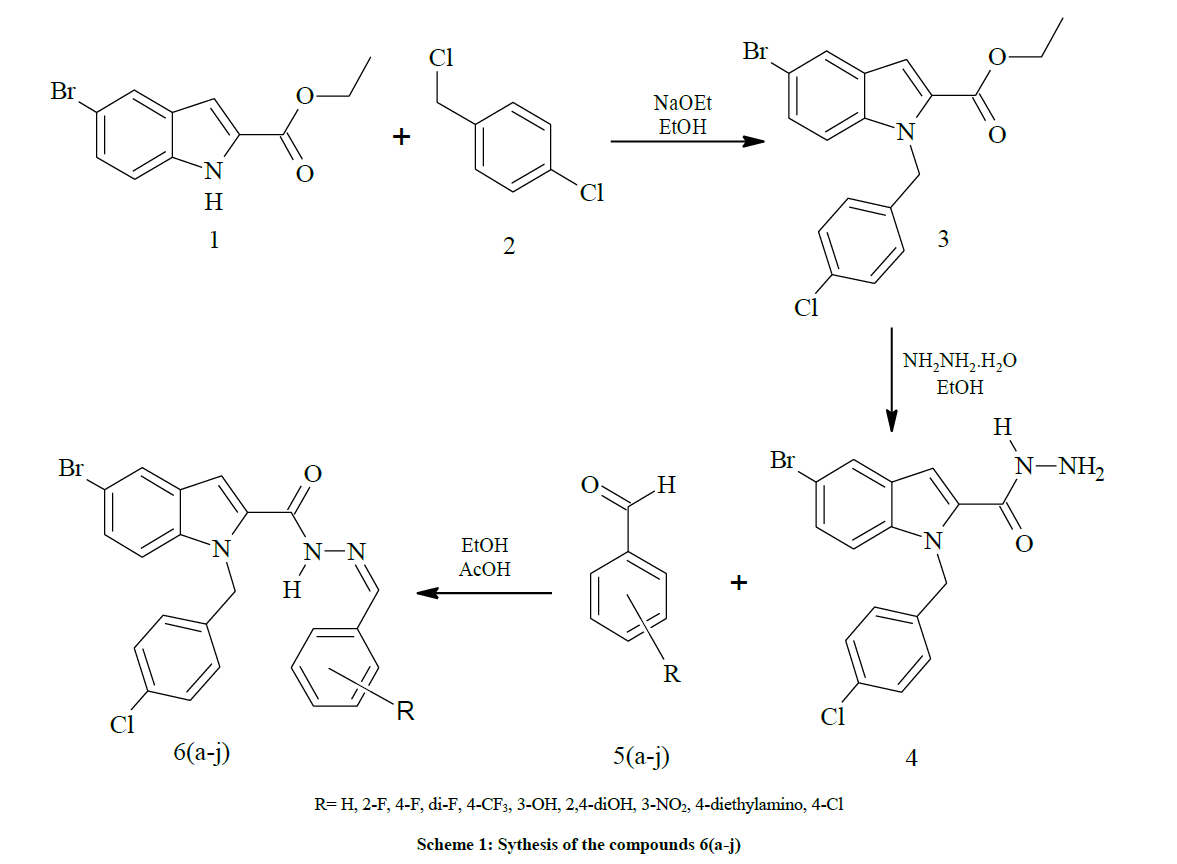
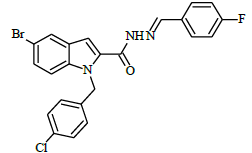
Based on the above facts, in continuation of our research towards identifying the newer anticancer agents, we synthesized (E)-1-(4- chlorobenzyl)-N'-benzylidene-5-bromo-1H-indole-2-carbohydrazide 6(a-j). The synthesis of a novel series of (E)-1-(4-chlorobenzyl)-N'- benzylidene-5-bromo-1H-indole-2-carbohydrazide 6(a-j) was achieved by reacting with methyl 5-bromo-1-(4-chlorobenzyl)-1H-indole-2- carbohydrazide (4) with substituted aromatic aldehydes. The title compounds 6(a-j) were undergone in vitro cytotoxic activity by using Peripheral Blood Mononuclear Cells (PBMCs) or Buffy Coat. Furthermore, we incorporated in vitro absorption, distribution, metabolism, excretion and toxicity (ADMET) study during the early phase of optimization effort which is recognized as a critical step to avoid high attrition rate during the late-stage drug development program.
Materials and Methods
Materials
Chemicals used in the synthesis of compounds were purchased from Alfa aesar and Spectrochem Pvt. Melting points were determined in open capillary and are uncorrected. The purity of the compounds and the progress of the reaction were checked on precoated silica gel TLC plates. IR spectra were recorded with KBr pellet method on Nicolet-Impact-410 FT-IR spectrometer. 1H-NMR spectra were recorded on a JEOL FT-NMR (400 MHz) spectrometer with TMS as an internal standard. The chemical shifts are represented in δ units, and the coupling constant J was measured in Hz. The mass spectra were recorded on a JEOL SX 102/DA-6000 (10 kV) FAB mass spectrometer.
Method
Synthesis of ethyl 5-bromo-1-(4-chlorobenzyl)-1H-indole-2-carboxylate (3) [19]
A mixture of ethyl 5-bromo-1H-indole-2-carboxylate (1 g, 1.0 mol) and NaOEt (3.0 mol) in ethanol (10 ml) was stirred for half an hour then, 1- chloro-4-(chloromethyl)benzene was added and the mixture heated under reflux for 2 h. The completion of the reaction was monitored by thin layer chromatography [ethyl acetate and hexane (2: 8 v/v)]. The solvent was removed, water was added and organic layer was extracted using ethyl acetate. The products were purified by using column chromatography (ethyl acetate/hexane 1: 9 v/v).
Synthesis of 5-bromo-1-(4-chlorobenzyl)-1H-indole-2-carbohydrazide (4)
Ethyl 5-bromo-1-(4-chlorobenzyl)-1H-indole-2-carboxylate (0.1 g, 0.00025 mol), was refluxed with hydrazine hydrate(0.03 g, 0.00075 mol), in (10 ml) ethanol (4 h), The completion of the reaction was monitored by thin layer chromatography [ethyl acetate and hexane (2:8 v/v)]. The formed precipitate was collected and recrystllized from 95% ethanol.
Synthesis of 5-bromo-1-(4-chlorobenzyl)-N'-[(Z)-phenylmethylidene]-1H-indole-2-carbohydrazide 6(a-j) (Scheme 1)
A solution of 5-bromo-1-(4-chlorobenzyl)-1H-indole-2-carbohydrazide 4 (0.1 g, 0.00026 mol), and substituted aromatic benzaldehydes 5(a-j) (0.04 g, 0.00026 mol) in ethanol (10 ml) was refluxed for 8 h. The completion of the reaction was monitored by thin layer chromatography [ethyl acetate and hexane (3:7 v/v)]. After completion of the reaction, the mixture was poured into ice cold water; precipitate formed was thus filtered and dried. The products were purified by using column chromatography (ethyl acetate/hexane 2:8 v/v) in the Table 1.
| Entry | Hydrazides | Benzaldehydes | Product | % of Yield | M. P. °C |
|---|---|---|---|---|---|
| 6a | 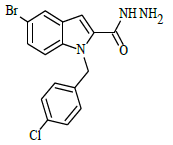 |
 |
 |
95 | 230-232 |
| 6b | 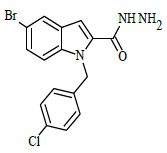 |
 |
 |
85 | 240-242 |
| 6c | 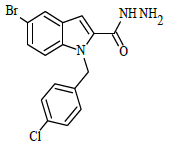 |
 |
 |
90 | 225-227 |
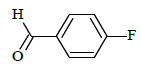
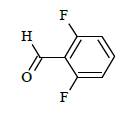
| 6d | 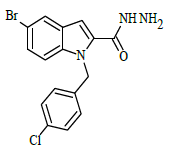 |
 |
 |
88 | 205-207 |
| 6e | 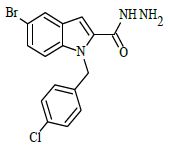 |
 |
 |
77 | 245-247 |
| 6f | 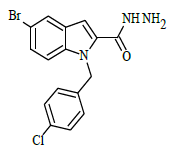 |
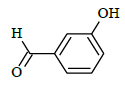 |
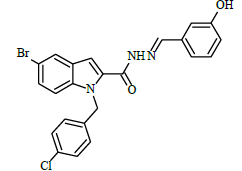 |
73 | 255-257 |
| 6g | 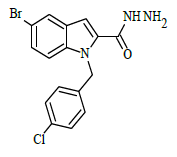 |
 |
 |
78 | 262-264 |
| 6h | 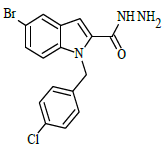 |
 |
 |
86 | 255-257 |
| 6i | 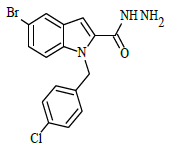 |
 |
 |
90 | 217-219 |
| 6j | 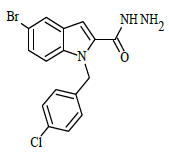 |
 |
 |
92 | 235-237 |
Table 1: Characterization data of 5-bromo-1-(4-chlorobenzyl)-N'-[(Z)-phenylmethylidene]-1H-indole-2-carbohydrazide 6(a-j) derivatives
Spectral Details
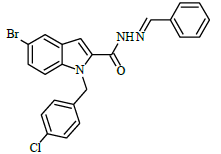
(E)-1-(4-chlorobenzyl)-N'-benzylidene-5-bromo-1H-indole-2-carbohydrazide (6a)
Yield (95%): White amorphous, M. P. 230-232°C. IR (KBr) cm-1: 3250 (Ar C-H), 2250 (C=N), 1690 (C=O), 1200 (C-N), 1090 (N-N), 750 (C-Cl), 690 (C-Br); 1H-NMR(400 MHz, DMSO-d6) δ (ppm) = 5.854 (s, 2H), 7.0785 (d, 2H, J= 8.4Hz), 7.359 (t, 3H, J=2Hz), 7.398 (d, 1H, J=2Hz), 7.442-7.516 (m, 3H), 7.5545 (d, 1H, J= 9.2Hz), 7.71 (d, 2H, J=5.6Hz), 7.978 (s, 1H), 8.423 (s, 1H), 12.044 (s, 1H); 13C-NMR (100 MHz, DMSO-d6) δ (ppm) = 53.501, 105.906, 113.119, 124.128, 126.886, 127.047, 127.468, 128.310, 128.391, 128.802, 130.105, 131.116, 131.669, 134.131, 136.914, 137.299, 147.837, 166.826, 167.656, 167.886, 170.119, 173.802, 173.914; Calculated mass: 466.75 gm/mol. MS (ESI-m/z): 467.80 (M+1).
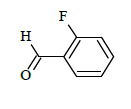
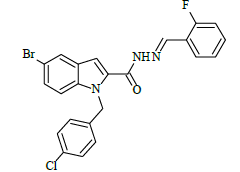
(E)-N'-(2-fluorobenzylidene)-1-(4-chlorobenzyl)-5-bromo-1H-indole-2-carbohydrazide (6b)
Yield (85%): White amorphous, M. P. 240-242°C. IR (KBr) cm-1: 3230 (Ar C-H), 2280 (C=N), 1680 (C=O), 1150 (C-N), 1050 (N-N), 1100 (C-F), 760 (C-Cl), 670 (C-Br). 1H-NMR (400 MHz, DMSO-d6) δ (ppm) = 5.593 (s, 2H), 7.158 (t, 2H, J=3.6Hz), 7.464 (d, 2H, J=3.2Hz), 7.832 (d, 2H, J=8.8Hz), 7.982 (s, 2H, J=3.2Hz), 8.059 (d, 2H, J=7.2Hz), 8.091 (s, 1H), 8.295(s, 1H), 11.684(s, 1H); 13C-NMR (100 MHz, DMSO-d6) δ (ppm) = 55.829, 112.865, 113.162, 116.573, 123.829, 124.136, 127.228, 128.944, 130.889, 131.149, 131.181, 131.446, 131.537, 131.703, 132.604, 133.946, 134.512, 140.001, 145.722, 146.276, 148.270, 152.143, 152.143, 163.235; Calculated mass: 484.74 gm/mol. MS (ESI-m/z): 485.56 (M+1).
(E)-1-(4-chlorobenzyl)-N'-(4-fluorobenzylidene)-5-bromo-1H-indole-2-carbohydrazide (6c)
Yield (70%): White amorphous, M. P. 260-263°C. spectral details same as (E)-N'-(2-fluorobenzylidene)-1-(4-chlorobenzyl)-5-bromo-1H-indole- 2-carbohydrazide (6b).
(E)-N'-(3,5-difluorobenzylidene)-1-(4-chlorobenzyl)-5-bromo-1H-indole-2-carbohydrazide (6d)
Yield (88%): White amorphous, M. P. 205-207°C. IR (KBr) cm-1: 3200 (Ar C-H), 2190(C=N), 1670 (C=O), 1150 (C-N), 1150 (N-N), 1150 (C-F), 780 (C-Cl), 690 (C-Br); 1H-NMR (400 MHz, CDCl3) δ (ppm) = 5.690 (s, 2H), 7.000 (d, 2H, J=8Hz), 7.600 (t, 2H, J=16Hz), 7.790 (t, 1H, J=16Hz), 7.850 (d, 1H, J=8Hz), 7.94 (d, 2H, J=8Hz), 8.05 (d, 2H, J=8Hz). 8.679 (s, 1H), 11.621 (s, 1H); 13C-NMR (100 MHz, DMSO-d6) δ (ppm) = 55.289, 111.959, 112.853, 116.506, 120.297, 124.297, 127.332, 127.474, 131.216, 132.592, 133.666, 135.612, 139.874, 145.732, 146.413, 148.294, 152.140, 157.129, 167.129, 169.576, 172.979, 173.332, 173.338; Calculated mass: 502.73 gm/mol. MS (ESI-m/z): 503.66 (M+1).
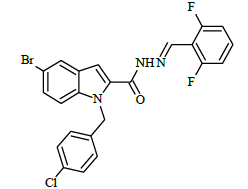
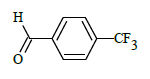
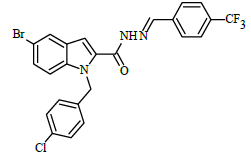
(E)-N'-(4-(trifluoromethyl)benzylidene)-1-(4-chlorobenzyl)-5-bromo-1H-indole-2-carbohydrazide (6e)
Yield (77%): White amorphous, M.P. 245-247°C. IR (KBr) cm-1: 3267 (Ar C-H), 2448(C=N), 1706(C=O), 1151(C-N), 1089(N-N), 1011(C-F), 765(C-Cl), 660(C-Br); 1H-NMR (400 MHz, DMSO-d6) δ (ppm) = 5.714(s, 2H), 7.06(d, 2H, J=4Hz), 7.46(d, 2H, J=4Hz), 7.84(d, 2H, J=8Hz), 7.977-8.003(m,4H), 8.07 (d, 2H, J=8Hz), 8.338(s, 1H), 11.770(s, 1H); 13C-NMR (100 MHz, DMSO-d6) δ (ppm) = 54.839, 107.946, 112.049, 112.828, 114.646, 114.829, 116.308, 121.386, 121.429, 124.839, 126.946, 127.024, 137.716, 137.831, 141.428, 145.743, 147.945, 152.159, 155.761, 158.298, 165.102, 170.509, 173.492, 179.839; Calculated mass: 534.75 gm/mol. MS (ESI-m/z): 535.82 (M+1).
(E)-1-(4-chlorobenzyl)-N'-(4-hydroxybenzylidene)-5-bromo-1H-indole-2-carbohydrazide (6f)
Yield (73%): White amorphous, M.P. 255-257°C. IR (KBr) cm-1: 3090 (Ar C-H), 2210(C=N), 1700(C=O), 1159(C-N), 1081(N-N), 784(C-Cl), 686(C-Br); 1H-NMR (400 MHz, DMSO-d6) δ (ppm) = 4.557 (s, 1H), 5.769 (s, 2H), 7.07 (d, 2H, J=4Hz), 7.48 (d, 2H, J=4Hz), 7.592-7.686 (m, 2H), 8.01 (d, 4H, J=8Hz), 8.08(d, 2H, J=8Hz), 8.513(s, 1H), 12.619(s, 1H); 13C-NMR (100 MHz, DMSO-d6) δ (ppm) = 54.426, 116.492, 120.080, 120.346, 124.534, 124.619, 127.565, 127.606, 128.367, 129.426, 130.014, 130.219, 131.170, 136.975, 143.759, 143.075, 146.020, 146.047, 150.887, 152.073, 156.163, 162.012, 164.851; Calculated mass: 482.75 gm/mol. MS (ESI-m/z): 483.80 (M+1).
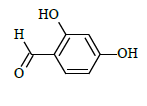
(E)-N'-(2,4-dihydroxybenzylidene)-1-(4-chlorobenzyl)-5-bromo-1H-indole-2-carbohydrazide (6g)
Yield (78%): White amorphous, M. P. 262-264°C. IR (KBr) cm-1: 3250 (Ar C-H), 3030(C-OH), 2250(C=N), 1590(C=O), 1170(C-N), 1010(N-N), 780(C-Cl), 690(C-Br); 1H-NMR(400 MHz, CDCl3) δ (ppm) ==5.402 (s, 2H), 7.471 (t, 2H, J=10Hz), 7.529 (t, 2H, J=7.6), 7.633 (d, 1H, J=9.6Hz), 8.033 (s, 2H), 8.105(d, 2H, J=9.2Hz), 8.156-8.180 (m, 2H), 8.249(s, 1H), 10.647(s, 1H), 11.771(s, 1H), 13.544(s, 1H); 13C-NMR (100 MHz, DMSO-d6) δ (ppm) = 55.020, 112.688, 114.789, 115.973, 116.548, 121.357, 121.401, 125.096, 126.844, 127.049, 127.130, 132.805, 136.759, 138.075, 141.020, 141.047, 145.687, 146.973, 152.163, 156.812, 159.051, 163.662, 164.011; Calculated mass: 498.75 gm/mol. MS (ESI-m/z): 499.82 (M+1).
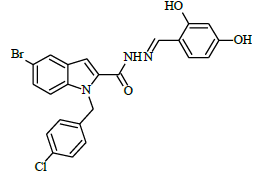
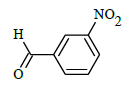
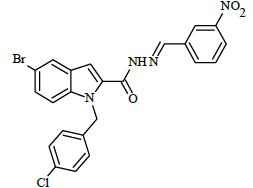
(E)-N'-(3-nitrobenzylidene)-1-(4-chlorobenzyl)-5-bromo-1H-indole-2-carbohydrazide (6h)
Yield (86%): White amorphous, M. P. 255-257°C. IR (KBr) cm-1: IR (KBr) cm-1: 3220 (Ar C-H), 2250(C=N), 1700(C=O), 1560(N-O), 1125(C-N), 990(N-N), 750(C-Cl), 670(C-Br); 1H-NMR (400 MHz, DMSO-d6) δ (ppm) = 5.394 (s, 2H), 6.892-7.009 (m, 2H), 7.04 (d, 1H, J=8Hz), 7.415 (d, 2H, J=10.6Hz), 7.498-7.587 (m, 3H), 7.891 (t, 2H, J=8Hz), 7.976 (d, 2H, J=7.2Hz), 8.603 (s, 1H), 10.967 (s, 1H); 13C-NMR (100 MHz, DMSO-d6) δ (ppm): 55.818, 110.002, 112.049, 112.828, 114.646, 114.829, 116.308, 121.386, 121.429, 124.839, 126.946, 127.024, 137.716, 137.831, 141.428, 145.743, 147.945, 152.549, 155.761, 158.298, 165.102, 168.509; Calculated mass: 511.75 gm/mol. MS (ESI-m/z): 512.90 (M+1).
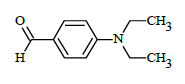
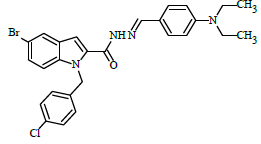
(E)-N'-(4-(diethylamino)benzylidene)-1-(4-chlorobenzyl)-5-bromo-1H-indole-2-carbohydrazide (6i)
Yield (90%): White amorphous, M. P. 217-219°C. IR (KBr) cm-1: IR (KBr) cm-1: 3190 (Ar C-H), 2100(C=N), 1675(C=O), 1150(C-N), 1000(N-N), 765(C-Cl), 640(C-Br); 1H NMR (400 MHz, DMSO-d6) δ (ppm) = 1.323 (t, 6H, J=5.6Hz), 3.764 (d, 4H, J=3.2Hz), 5.672 (s, 2H), 7.464 (d, 2H, J=16Hz), 7.590-7.631 (m, 1H), 7.685 (d, 2H, J=2.8Hz), 7.851 (d, 1H, J=7.6Hz), 7.887-7.943 (m, 1H), 7.985 (d, 3H, J=12Hz), 8.032(d, 2H, J=7.2Hz), 8.532(s, 1H), 10.974(s, 1H); 13C-NMR (100 MHz, DMSO-d6) δ (ppm) = 16.556, 16.606, 49.703, 49.921, 55.289, 111.959, 112.853, 116.506, 120.297, 124.297, 127.332, 127.474, 131.216, 132.592, 133.666, 135.612, 139.874, 145.732, 146.413, 148.294, 152.140, 157.129, 167.129, 168.876, 171.979, 172.832, 172.838;. Calculated mass: 537.87 gm/mol. MS (ESI-m/z): 538.77 (M+1).
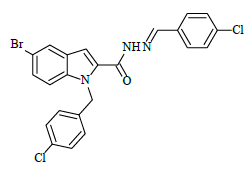
(E)-1-(4-chlorobenzyl)-N'-(4-chlorobenzylidene)-5-bromo-1H-indole-2-carbohydrazide (6j)
Yield (92%): White amorphous, M. P. 235-237°C. IR (KBr) cm-1: IR (KBr) cm-1: 3210 (Ar C-H), 2260(C=N), 1700(C=O), 1170(C-N), 1150(N-N), 790(C-Cl), 640(C-Br). 1H-NMR (400 MHz, DMSO-d6) δ (ppm) = 5.693 (s, 2H), 7.058 (t, 2H, J=3.6Hz), 7.465 (d, 2H, J=3.2Hz), 7.838 (d, 2H, J=8.8Hz), 7.982 (d, 2H, J=1.2Hz), 8.050 (s, 2H), 8.079 (d, 2H, J=9.2Hz), 8.305(s, 1H), 12.384 (s, 1H). 13C-NMR (100 MHz, DMSO-d6) δ (ppm) = 56.029, 113.965, 114.062, 117.573, 124.029, 124.136, 127.028, 129.044, 131.089, 131.249, 131.281, 131.546, 131.637, 131.703, 132.604, 134.046, 134.612, 140.001, 145.622, 146.476, 148.470, 152.143, 164.035; Calculated mass: 501.20 gm/mol. MS (ESI-m/z): 502.10 (M+1).
ADME-Toxicity prediction
The pharmacokinetic properties are essential to predict and to identify the nature of toxicity, poor physicochemical properties, pharmacokinetics, and toxicity of the synthesized molecules which are the principal causes of late-stage failure in drug development [20]. The molecular descriptors of synthesized compounds 6(a-j) are optimized using QSAR properties. Aqueous solubility (PlogS), blood-brain barrier penetration (QPlogBB), intestinal absorption (logHIA) [21], hepatotoxicity, Caco-2 cell permeability (QPPCaco) also helps to understand drug metabolism for the synthesized molecules. Further, to predict the toxicity of lead molecules with intraperitoneal, oral, intravenous and subcutaneous toxic effects of blood, cardiovascular system, gastrointestinal, kidney, liver and lungs are also examined. The in silico study enables to decide the safety and efficacy of active molecules to take up for in-depth studies.
Cytotoxic activity of newly synthesized 6(a-j) derivatives
Preparation of Peripheral Blood Mononuclear Cells (PBMCs) or Buffy coat [22]
Blood samples from healthy volunteers were collected by vein puncture and transferred into 2 ml heparin coated vacutainers. It was diluted to 1:1 ratio with PBS (Phosphate Buffer Saline) layered onto 4 ml Ficol without getting mixed up. It was further separated by centrifuging at 1000 rpm for 30-45 min at room temperature. During the centrifugation the PBMCs move from plasma and suspend in the density gradient. Removed plasma down to about 1 cm above buffy coat, discarded, then aspirated buffy coat (the white layer lying on top of the red cells, it should come as one layer). The PBMC/buffy coat layer was washed twice with PBS. RPMI (Gibco, Life Technologies) medium was prepared by mixing, 10 ml of FBS (Invitrogen) and 200 μl Antibiotic/antimycotic (Antibiotic antimycotic solution with Streptomycin 10 mg/20 ml, 10, 000 U Penicillin, Amphotericin B and 0.9% normal saline). About 4 ml of this mixer was dispensed in falcon tubes, 30 μl of Phytohemagglutin (Invitrogen) and 150-200 μl of PBMCs was incubated at atmosphere of 95% air and 5% CO2 at 37 °C for 4 h [22].
From (6a-j) synthesized compounds sample stock of 1 mg/ml was prepared and from this stock 10 μg, 50 μg and 100 μg samples were added to the respectively labeled PBMC tubes and incubated for 72 h at the earlier mentioned conditions. Controls were maintained without the sample. Percent viability was calculated by applying the following formula.
Percent Viability= (Number of viable cells/Total number of cells) x 100
Results and Discussions
Chemistry
According to the reported method [19], the compound ethyl 5-bromo-1-(4-chlorobenzyl)-1H-indole-2-carboxylate (3) synthesized by using the mixture of ethyl 5-bromo-1H-indole-2-carboxylate (1) and 1-chloro-4-(chloromethyl)benzene (2) in ethanol by using catalytic amount of NaOEt. The compound (3) was refluxed with hydrazine hydrate and ethanol to afford 5-bromo-1-(4-chlorobenzyl)-1H-indole-2-carbohydrazide (4). Finally the carbohydrazides (4) was condensed with aromatic benzaldehydes 5(a-j) in ethanol by using catalytic amount of glacial acetic acid at 90°C for 8 h afforded 5-bromo-1-(4-chlorobenzyl)-N'-[(Z)-phenylmethylidene]-1H-indole-2-carbohydrazide 6(a-j) in good yields. The detailed synthetic view is given in scheme 1. The entitled compounds are purified by column chromatography (using ethyl acetate/hexane as eluent). The compounds 6(a-j) was confirmed on the basis of IR, 1H-NMR, 13C-NMR and mass spectral data.
All synthesized compounds showed absorption bands ranging from 3030-2897 cm-1 for C-H aromatic stretching, 1670-1640 cm-1 for C=O stretching, 1651-1633 cm-1 for C=N stretching, 1207-1155 cm-1 for N-N stretching, 829-794 cm-1 for C-Cl stretching, 540-580 cm-1 C-Br stretching. 1286-1253 cm-1 for C-F stretching of compounds 6b, 6c and 6d. In 1H-NMR, the presence of singlet peak between δ=5.394-5.854 ppm for -N-CH2, δ=8.249-8.679 ppm for -N=CH and δ=10.967-12.619 ppm for -HN-C=O of all synthesized compounds 6(a-j). The peaks which are appeared at δ=4.557 ppm corresponding to Ar-OH in 6f. The triplet Peak at 1.323 and doublet at 3.764 ppm for CH3-CH2-N-CH2-CH3 protons of compound 6i.
The 13C-NMR spectra showed a peak at δ=53.501-56.029 ppm corresponds to N-CH2 carbons, the peaks at 163.235-179.839 ppm corresponds to C=O carbon, the peaks between 105.95- 170.14 ppm correspond to aromatic carbons of 6(a-j). The peaks at 16.556, 16.606, 49.703 and 49.921 ppm corresponding CH3-CH2-N-CH2-CH3 four carbons of compound 6i. Mass analysis of 6(a-j) displayed the molecular ion peak confirming their molecular weight.
Pharmacokinetic properties
The experimental evaluation of ADMET profiles is expensive, and the work load cannot meet the demands of drug screening and lead optimization. In conjunction with high throughput in vitro screening, computational techniques that can filter/predict ADMET profiles have become an alternative approach [23]. Hence using computer based methods like ADMET and SAR tools the molecular descriptors and drug likeliness properties were studied.
The pharmacokinetic properties are represented in Table 2. The coefficient of blood/brain barrier penetration (log B/B) was computed and accessed with central nervous system (CNS) [24]. The CNS activity was computed on -2 (inactive) to +2 (active) scales which show all the molecules are displayed within acceptable range [25].
| Ligand | PlogBBa | logHIAc | PCacob | logpGI (substrate)d | logPGI (non-inhibitor)e | PlogSf | logpappg |
|---|---|---|---|---|---|---|---|
| 6a | 0.9753 | 0.9969 | 0.5608 | 0.6591 | 0.7887 | -4.1994 | 0.947 |
| 6b | 0.983 | 1 | 0.5775 | 0.6577 | 0.7109 | -4.1307 | 0.8693 |
| 6c | 0.983 | 1 | 0.5775 | 0.6577 | 0.7109 | -4.1307 | 0.8693 |
| 6d | 0.983 | 1 | 0.5775 | 0.6577 | 0.7109 | -4.1307 | 0.8693 |
| 6e | 0.9781 | 1 | 0.6086 | 0.6478 | 0.7069 | -4.4634 | 0.7841 |
| 6f | 0.9554 | 0.9967 | 0.5779 | 0.5811 | 0.8898 | -3.8415 | 0.8601 |
| 6g | 0.8724 | 0.9938 | 0.5965 | 0.5727 | 0.8988 | -3.8201 | 0.8105 |
| 6h | 0.9636 | 0.9802 | 0.5709 | 0.6575 | 0.8686 | -3.9862 | 0.9966 |
| 6i | 0.9408 | 1 | 0.5211 | 0.6315 | 0.5702 | -4.0451 | 0.9643 |
| 6j | 0.9753 | 0.9969 | 0.5608 | 0.6591 | 0.7887 | -4.1994 | 0.947 |
| Doxorubicin | 0.9898 | 0.8257 | 0.6345 | 0.8824 | 0.9306 | -2.9266 | -0.6751 |
aPredicted blood/brain barrier partition coefficient (1-high penetration, 2- medium penetration and 3- Low penetration);
bPredicted Caco-2 cell permeability in nm/s (acceptable range: -1 is poor, 1 is great);
cPredicted Human intestinal absorption in nm/s (acceptable range: 0 poor, >1 great);
dPredicted P-glycoprotein substrate in nm/s (acceptable range of -5 is poor, 1 is great);
ePredicted P-glycoprotein inhibitor in nm/s (accepted range: 0 to 1);
fPredicted aqueous solubility, (Concern value is 0-2 highly soluble);
gPredicted probability of Caco-2 cell permeability in cm/s (Concern value is -1 to 1)
Table 2: ADME and pharmacological parameters prediction for the ligands 5-bromo-1-(4-chlorobenzyl)-N'-[(Z)-phenylmethylidene]-1H-indole-2- carbohydrazide 6(a-j) using admet SAR toolbox
The interpretation of test compounds with reference (Doxorubicin) showed that compounds were in acceptable range and hence, can be used to make an oral dosage for better absorption, transport, metabolism, and maintain homeostatic condition. The intestinal absorption (logHIA) and Caco-2 cell permeability (PCaco-2) within the range of -2 poor absorption and +2 more absorption show that the compounds are more permeable in intestine and helps for good transport of drug metabolic compounds.
The logPGI (substrate) and non-inhibitors has drug-drug interaction within tissue that transforms xenobiotics of vigorous reduction of drug absorption and released more bile (liver) and urine (kidney) [26].
The reference range of -5 (poor) to +1 (good) and substrate inhibitor from 0 to 1 in which the reference and test compounds 6(a-j) shows good activity with human intestinal absorption and metabolism. The aqueous solubility of compounds lies with range of 0 (poor) to 2 (good) showed that all the molecules had good solubility. While the reference compound as well as tested compounds 6a, 6b, 6c, 6f, and 6g came within acceptable range as represented in Table 2.
The toxicity of the 5-bromo-1-(4-chlorobenzyl)-N'-[(Z)-phenylmethylidene]-1H-indole-2-carbohydrazide 6(a-j) were predicted based on lethal dosages and functional ranges in different tissues. The LD50 mouse and probability of health effects were predicted using ACD/ ILab 2.0 (guest). The toxicity of selected compounds was listed in Table 3. The LD50 potential compounds detect the cumulative potential of acute toxicity that administered through oral, intraperitonial, intravenous and subcutaneous on mouse models. The comparative analysis of reference compounds with test compounds on oral, subcutaneous, intraperitonial and intravenous is lower when compared to reference molecule. The toxicity results suggests that the compounds 6(a-j) have less toxic effect in internal tissues and with no side effect. The probability of health effects revealed that the synthesized molecules 6a, 6b, 6c, 6f, and 6g had very less toxic in nature.
| ADME-TOX Parameters | Intraperitoneal a | Oral A | Intravenous a | Subcutaneous a |
|---|---|---|---|---|
| 6a | 920 (0.55) | 580 (0.28) | 62 (0.38) | 200 (0.22) |
| 6b | 290 (0.53) | 830 (0.31) | 81 (0.47) | 160 (0.47) |
| 6c | 290 (0.53) | 830 (0.31) | 81 (0.47) | 160 (0.47) |
| 6d | 490 (0.3) | 770 (0.31) | 88 (0.37) | 290 (0.27) |
| 6e | 1100 (0.29) | 490 (0.31) | 48 (0.41) | 130 (0.19) |
| 6f | 1200 (0.51) | 500 (0.3) | 58 (0.38) | 250 (0.21) |
| 6g | 1100 (0.51) | 500 (0.29) | 56 (0.39) | 230 (0.21) |
| 6h | 1300 (0.44) | 450 (0.33) | 61 (0.39) | 240 (0.21) |
| 6i | 190 (0.57) | 1100 (0.28) | 55 (0.51) | 110 (0.48) |
| 6j | 240 (0.58) | 990 (0.3) | 78 (0.51) | 110 (0.46) |
| Doxorubicin | 57 (0.5) | 1800 (0.22) | 98 (0.29) | 200 (0.23) |
aEstimated LD50 - mouse value in mg/kg after intra peritoneal, oral, intravenous and subcutaneous administration;
bEstimated probability of blood, gastrointestinal system, kidney, liver and lung effect at therapeutic dose range of compounds, The drugs with moderate effect on reliability index (>0.5). The drugs with border line effect on reliability index (>0.3, <0.5)
Table 3: LD50 and probability of health effects of 5-bromo-1-(4-chlorobenzyl)-N'-[(Z)-phenylmethylidene]-1H-indole-2-carbohydrazide 6(a-j) using ACD/ I-Lab 2.0
Cytotoxic activity
From (6a-j) synthesized sample stock (1 mg/mL); 10 μg, 50 μg and 100 μg was added to the respectively labeled PBMCs tubes and incubated for 72 h at the earlier mentioned conditions. After 72 h, cell viability was determined by the tryphan-blue dye exclusion method [27]. The cells were clarified by centrifuging at 1000 rpm for 30-45 min at room temperature, supernatant was discarded and to the 10 μl of PBMCs; 10 μl of tryphan blue was added and incubated for 10 min at room temperature. About 10 μl of incubated sample was loaded on previously cleaned Haemocytometer and counted the number of live, total cells and dead cells at four corners under Trinocular microscope, The percentage of cell viability and non-viability was observed in Table 4.
| Sample | Number of cells | Total cells | Percent viable Cells | Percent Nonviable Cells | |
|---|---|---|---|---|---|
| Live cells | Dead cells | ||||
| Control | 125 | 52 | 177 | 70.62 | 29.37 |
| 6a-10 µg | 50 | 18 | 68 | 73.5 | 26.47 |
| 6a-50 µg | 29 | 18 | 47 | 61.7 | 38.3 |
| 6a-100 µg | 38 | 21 | 59 | 64.4 | 35.6 |
| 6b-10 µg | 62 | 51 | 113 | 54.86 | 45.13 |
| 6b-50 µg | 83 | 95 | 178 | 46.62 | 53.37 |
| 6b-100 µg | 16 | 73 | 89 | 17.97 | 82.02 |
| 6c-10 µg | 32 | 98 | 130 | 24.61 | 78.38 |
| 6c-50 µg | 22 | 93 | 115 | 19.1 | 80.87 |
| 6c-100 µg | 18 | 83 | 101 | 17.8 | 82.17 |
| 6d-10 µg | 181 | 87 | 268 | 67.5 | 32.5 |
| 6d-50 µg | 141 | 73 | 214 | 65.9 | 34.1 |
| 6d-100 µg | 136 | 99 | 235 | 57.9 | 42.1 |
| 6e-10 µg | 18 | 9 | 27 | 66.7 | 33.3 |
| 6e-50 µg | 12 | 13 | 25 | 48 | 52 |
| 6e-100 µg | 15 | 16 | 31 | 48.38 | 51.6 |
| 6f-10 µg | 86 | 59 | 145 | 59 | 40.7 |
| 6f-50 µg | 12 | 83 | 95 | 12.6 | 87.3 |
| 6f-100 µg | 3 | 77 | 80 | 3.75 | 96.25 |
| 6g-10 µg | 15 | 36 | 51 | 29.4 | 70.6 |
| 6g-50 µg | 21 | 35 | 56 | 37.5 | 62.5 |
| 6h-10 µg | 71 | 139 | 210 | 33.8 | 66.1 |
| 6h-50 µg | 56 | 172 | 228 | 24.56 | 75.43 |
| 6h-100 µg | 16 | 68 | 84 | 19.04 | 81 |
| 6i-10 µg | 95 | 116 | 211 | 45 | 55 |
| 6i-50 µg | 54 | 92 | 146 | 37 | 63 |
| 6i-100 µg | 32 | 79 | 111 | 28.8 | 71 |
| 6j-10 µg | 73 | 54 | 127 | 57.5 | 42.5 |
| 6j-50 µg | 8 | 62 | 70 | 11.4 | 88.6 |
| 6j-100 µg | 25 | 43 | 68 | 36.8 | 63.2 |
Table 4: Cytotoxic activity of newly synthesized (6a-j) derivatives against PBMC
When compared to control, wherein, only PBMCs are present, all the treatments showed higher per cent non-viability. This indicates, the compounds are showing cytotoxic effect on PBMCs. In all the treatments, there observed concentration dependent increase in cell viability and non-viability.
The dose-dependent activity of the compounds 6 (a-j) exhibited induced activity at high concentration and reduce the activity at low concentrations. Hence, the compounds 6b, 6c, 6f and 6h were observed as dose-dependent agents. In this, the compound 6b induced the inhibition of the growth 82.02% at high concentration 100 μg and reduced the inhibition of the growth 45.13% at low concentration 10 μg. In the compound 6c induced the inhibition of the growth 82.17% at high concentration 100 μg and reduced the inhibition of the growth 78.38% at low concentration 10 μg. The compound 6f exhibit 96.25% of inhibition of the growth at high concentration 100 μg and at low concentration 10 μg shows 40.7% inhibition of the growth of cells. In the compound 6h induced the inhibition of the growth 81% at high concentration 100 μg and reduced the inhibition of the growth 66.1% at low concentration 10 μg. However, the compound 6j reduced the activity 63.2% at high concentration 100 μg but induced the activity 88.6% at low concentration 50 μg.
The results revealed that the compounds 6(a-j) exhibited good to moderate activity, this might be because of a potent indole nucleus is coupled with substituted benzaldehydes with the help of Schiff’s base bridge. Among all the synthesized compounds 6(a-j), the molecule 6f has showed an excellent cytotoxicity effect on PBMCs. This might be the presence of heterocyclic ring system like indole and amide Schiff’s base coupled with m-hydroxy phenyl ring. Here the m-hydroxy acts as an electron donor, hence it stabilizes the coupled ring system, due to this it donates electrons towards the Schiff base functionality and having more chances to interact with target site, by these hypothesis we can assume, in the entitled compounds 6(a-j), 6f is more efficient cell growth suppressor.
Conclusion
Among all the treatments studied, it was noted that 6f with 100 μg showed highest percent cytotoxic effect on PBMCs. Further studies like mode of action of the chemical, its DNA fragmentation pattern have to be studied. The synthesized compounds were evaluated for ADMET studies and found that the molecules 6a, 6b, 6c, 6f and 6g are in acceptable range.
Acknowledgement
The authors are thankful to Kuvempu University, for providing necessary facilities to carry out the present work, one of the authors (Mr. Harishkumar S) is thankful to SC/ST Cell, Kuvempu University for financial support.
Contribution of Author
Harishkumar S involved in the synthesis, characterization and in silico studies of designed molecules. Dr. N D Satyanarayan was responsible for experimental design, intellectual concept, manuscript preparation, editing, review and guarantor. Dr. Kiranmayee P designed the protocol for biological evaluation of the samples and analysis of the biological data generated and Dr. Raghavendra R analyzed the biological data generated. Nandini H S and Prabhudas N performed the experiment with PBMCs.
References
- National Cancer Institute 2017.
- World Health Organization, 10 facts about Cancer, February 2017.
- Global Cancer Statistics 2012.
- American Cancer Society. Cancer Facts & Figures 2017.
- F.T. Unger, I. Witte, K.A. David, Cell. Mol. Life Sci., 2015, 72, 729.
- N. Kuntala, J.R. Telu, V. Banoth, N.S. Babu, J. Anireddy, S Pal, Med. Chem. Commun., 2015, 9, 1.
- C. Sandro, F. Giancarlo, Chem. Rev., 2005, 105, 2873.
- B.E. Evans, K.E. Rittle, M.G. Bock, R.M. Di Pardo, R.M. Freidinger, W.L. Whitter, G.F. Lundell, D.F. Veber, P.S. Anderson, J. Med. Chem., 1988, 31, 2235.
- E. Vitaku, D.T. Smith, J.T. Njardarson, J. Med. Chem., 2014, 57, 10257.
- M.C. Castle, Springer, Dordrecht, 1989, 10, 147.
- J.V. Ragavendran, D. Sriram, S.K. Patel, Eur. J. Med. Chem., 2007, 42(2), 146.
- N. Ergenc, N.S. Gunay, Eur. J. Med. Chem., 1998, 33(2), 143.
- P. Vicini, F. Zani, P. Cozzini, I. Doytchinova, Eur. J. Med. Chem., 2002, 37(7), 553.
- J. Jayabharathi, A. Thangamani, M. Padmavathy, B. Krishnakumar, Med. Chem. Res., 2007, 15, 431.
- H.Z. Zhang, J. Drewe, B. Tseng, S. Kasibhatla, S.X. Cai, Bio. Org. and Med. Chem., 2004, 12(13), 3649.
- S.A.M. El-Hawash, A.E. Abdel Wahab, M.A. El-Demellawy, Archiv der Pharmazie., 2006, 339(1), 14.
- A.R. Todeschini, A.L.P. de Miranda, K.C.M. da Silva, S.C. Parrini, E.J. Barreiro, Eur. J. Med. Chem., 1998, 33(3), 189.
- N.Y. Bamaung, R.A. Craig, M. Kawai, J. Wang, Y. Dai, Y. Guo, G. Sheppard, M.K. Verzal, A. Vasudevan, M. Michaelides, U. S. Pat. US 2002/0091148A1, 2002.
- A.T.A. Boraei, E.S.H. El Ashry, A. Barakat, H.A. Ghabbour, Molecules., 2016, 21, 333.
- M.J. Waring, J. Arrowsmith, A.R. Leach, P.D. Leeson, S. Mandrell, R.M. Owen, G. Pairaudeau, W.D. Pennie, S.D. Pickett, J. Wang, O. Wallace, A. Weir, Nat. Rev. Drug. Discov., 2015, 14, 475.
- C. Feixiong, L. Weihua, Z. Yadi, S. Jie, W. Zengrui, L. Guixia, J. Chem. Inf. Model.,2012. 52, 3099.
- C. Robert, X. Lu, A. Law, T.C. Freeman, D.A. Hume, Immunobiology., 2011, 11, 1203.
- S. Santoshkumar, K. Manjulatha, N.D. Satyanarayan, R. Anantacharya, S. Harishkumar, H.N. Harishkumar, S. Yallappa, B.L. Dhananjaya, Int. J. Pharm. Pharm. Sci., 2016, 8, 313.
- K.S. Manjunatha, N.D. Satyanarayan, S.Harishkumar, Int. J. Pharm. Pharm. Sci., 2016, 8, 251.
- R. Anantacharya, K. Manjulatha, N.D. Satyanarayana, S. Santoshkumar, M.Y. Kaviraj, Cogent Chemistry., 2016, 2, 1158382.
- J. Vitali, D. Carroll, R.G. Choudhury, M.L. Hackert, Acta. Crystallogr. Sect. D., 1999, 55, 1978.
- W. Strober, Curr. Protoc. Immunol. 1997, A.3B.1-A.3B.2.