Research Article - Der Pharma Chemica ( 2021) Volume 13, Issue 9
Design, Synthesis And Characterization of Indolyl-6-Amino 3,4-Dihydro-3 Methylpyrano[2,3-C]Pyrazol-5-Carbonitrile Derivatives as Antioxidant Agents
Prabhaker Walmik*Prabhaker Walmik, Department of Post-Graduate Studies & Research in Chemistry, Sahyadri Science College, Kuvempu University, Shivamogga- 585 106, India, Email: prabhakarchavan7@gmail.com
Received: 18-Jul-2021 Accepted Date: Sep 22, 2021 ; Published: 30-Sep-2021
Abstract
The fused pyrazolo[2,3-c]pyrano and indole derivative are well known for their therapeutic properties. The present paper describes about the synthesis of pyrazolo[2,3-c]pyrano derivative linked to the indole nucleus (4a-c) were prepared using multi-component reaction (MCRs) . All the newly synthesized compounds were characterized by spectral studies and also by C, H, and N analyses. Anti-oxidant activities were assessed by different methods. The analogs (4a) showed promising antioxidant properties.
Keywords
Indole, pyrazolo[2,3-c]pyrano, DPPH, FRAP activities
Introduction
The multicomponent reaction (MCRs) are one-pot process in which at least three or more compound react together to form target product without separation and purification of intermediates, the main advantages of MCRs, in comparison with traditional multistep protocols, are high efficiency, experimental simplicity, low cost, avoidance of large quantity of waste, reducing labor cost, reaction times, and waste production [1]. As MCRs is one-pot one pot reaction, they are easier to perform than the multistep reactions. In addition, MCRs are eco-friendly, and often preceded with excellent chemo selectivities [2-3]. The design and development of multi-component synthetic strategies based on green principles and renewable and recyclable materials, using solvent free condition, green solvents and green or renewable catalyst, using conventional techniques to achieve an ideal synthesis. In addition, the combination of MCRs process with environmentally benign protocols, such performing organic reactions in water is a protocol that has becoming highly interesting for synthetic chemist [4]. Water emerged as a versatile solvent for organic reactions recently, because of special effects of water such as abundant, eco-friendly benign, hydrogen bonding in the transition state and high cohesive energy and also negative activation volume. The use of water as a natural and green solvent is emphasized in green chemical process [5,6]. For these reasons, we synthesis the compounds using MCRs in water are of prominent value in green chemistry and organic chemistry [7-9].
Heterocyclic compounds containing the 4H-pyran ring display significant roles in medicinal and synthetic chemistry, 4-H-pyran has attracted the attention of many researcher involved drug discovery process [10-12], on the other hand pyranopyrazole compounds, oxygen and nitrogen ring fused heterocycles, are important group of heterocyclic compounds with natural and synthetic molecules [13]. They showed numerous biological activities such as antibacterial [14], antimicrobial [15,16], anticancer [17], anti-inflammatory and analgesic [18], fungicidal, bactericidal and herbicidal properties [19, 20]. Indole and its analogs constitute the active class of compounds possessing wide spectrum of biological activities. A number of indole derivatives have been reported to exhibit anti-inflammatory [21], Antihistaminic [22], Anticancer [23], antibacterial [24], antifungal [25], Anticonvulsant [26]. In view of these observations and in continuation of our research on the synthesis of biologically active molecules [27-31], the objective of this study to synthesize title compounds in which pyrano[2,3-c]pyrazole derivatives linked to indole nucleus at position-3, the molecule which may exhibit enhanced biological activities.
Results and Discussion
Chemistry
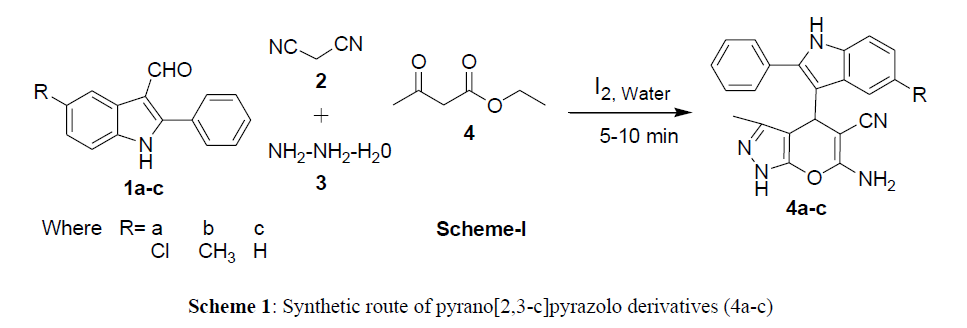
Preparation of starting material 5-substituted 2-phenyl-1H-indol-3-carboxaldehydes using literature method [32]. A mixture of hydrazine hydrate, cyanoacetate, ethylacetoacetate and 5-substituted 2-phenyl-1H-indol-3-carboxaldehydes and iodine in 5 ml water was stirred at 45ºC for about 3hr to afforded 6-amino-4-(5-substituted-2-phenyl-indolin-3-yl)-3-methyl-3,4-dihydropyrano[2,3-c]pyrazol-5-carbonitriles (4a-c) (Sheme-1). IR spectrum, compound 4a exhibited the characteristic absorption peaks at 3342, 3098, 3038 and 2176 cm-1 due to NH/NH/NH2 and CN functions, respectively. In its 1H NMR spectrum the down field singlet resonated at 12.19 ppm integrating for one proton was attributed to indole NH, the one proton of imidazole-NH resonated as singlet at 8.91 ppm, the two protons of amino group attached to pyran ring resonated as singlet at 8.19 ppm. The multiplet extending from 7.17-7.71 ppm accounted for nine aromatic protons, whereas one proton of pyran ring appeared as a singlet at 4.24 ppm, the methyl protons appeared as singlet at 2.27 ppm. The 13C NMR spectrum the down field singlet resonated at 160.3 ppm integrating to C-NH2 function. Methyl carbon resonated a 19.1 ppm, aromatic carbons resonated as 136.8 to 112.5 ppm. The mass spectrum exhibited isotopic molecular ion peaks at m/z 403 (M+), 405 (M++2). Thus, all the above spectral data supports the formation of 4a. Similarly, other compounds in the series were prepared. The physical data and elemental analyses are spectral data are given the experimental section (Scheme 1).
Antioxidant activities
1,1-Diphenyl-2-picryl hydrazyl (DPPH) radical scavenging activity (RSA): Numbers of methods are available for the determination of free radical scavenging activity (RSA) but the assay employing the stable DPPH. Has received much attention owing to its ease of use and convenience. This assay is the most widely used in vitro test to asses’ free radical scavenging capacities of test compounds. The RSA of synthesized compounds was carried out at 25, 50, 75 and 100 μg/mL concentrations in methanol using DPPH method [33]. All the tests analyses were performed on three replicate and the results are averaged. Results are expressed as percentage decrease with respect to the control values. The results are illustrated in the table-1. Compounds 4a showed good RSA (89.58%) at concentration 100 μg/mL. This higher RSA may be attributed to the presence of two amino group and electronegative chlorine group present in it, which may be responsible for stabilization of free radical formed after donating a hydrogen atom to DPPH free radical.
| Comp. No. |
Concentrations | IC50 (µg/ml) | |||
|---|---|---|---|---|---|
| 25 µg/ml (%) |
50 µg/ml (%) |
75 µg/ml (%) |
100 µg/ml (%) |
||
| 4a | 76.05 | 79.80 | 86.73 | 89.58 | < 25 |
| 4b | 62.34 | 65.74 | 70.05 | 74.18 | < 25 |
| 4c | 68.12 | 71.14 | 76.66 | 78.24 | < 25 |
| BHA | 87.98 | 88.98 | 90.90 | 93.28 | < 25 |
| TBHQ | 89.05 | 89.99 | 91.20 | 93.46 | < 25 |
| AA | 89.16 | 90.08 | 92.56 | 94.21 | < 25 |
Ferric ions (Fe3+) reducing antioxidant power (FRAP)
The reductive capacities of synthesized compounds were assessed by the extent of conversion of Fe3+/ferricyanide complex to the Fe2+/ferrous form. The reductive power of the compounds was observed at different concentrations [34] and results were compared with standards BHA, TBHQ and AA. The reducing ability of the synthesized compounds augmented with increasing concentration of test samples with increasing the reductive ability Table-2. Compounds 4a reduced metal ion complexes to their lower oxidation state showed the ability of electron donor to scavenge free radicals. The best result was obtained by compound 4a higher absorbance 0.691nm, at concentration 100 μg/ml when compared to standards BHA, TBHQ and AA. The higher the absorbance of the compounds indicated greater reducing power.
| Comp. No. | Concentrations | |||
|---|---|---|---|---|
| 25 µg/ml | 50 µg/ml | 75 µg/ml | 100 µg/ml | |
| (nm) | (nm) | (nm) | (nm) | |
| 4a | 0.255 | 0.393 | 0.519 | 0.621 |
| 4b | 0.219 | 0.285 | 0.348 | 0.465 |
| 4c | 0.268 | 0.299 | 0.345 | 0.485 |
| BHA | 0.889 | 0.91 | 1.101 | 1.289 |
| TBHQ | 0.892 | 0.949 | 1.101 | 1.295 |
| AA | 0.894 | 0.951 | 0.109 | 1.249 |
Ferrous (Fe2+) metal ion chelating activity
Among the transition metals, iron is known as the most important lipid oxidation pro-oxidant due to its high reactivity. The effective ferrous ions chelators may also afford protection against oxidative damage by removing iron (Fe2+) that may otherwise participate in hydroxyl radical generating Fenton type reactions [35].
Fe2+ + H2O2 → Fe3+ +OH- + OH.
Ferric (Fe3+) ions also produce radical from peroxides although the rate is tenfold less than that of ferrous (Fe2+) ion [36]. Measurement of color reduction therefore allows estimating the metal chelating activity of the co-existing chelators. Lower absorbance indicates higher metal chelating activity. The chelating effects of ferrous ions (Fe2+) with test compounds were determined using standards BHA, TBHQ and AA. In this assay, synthesized compounds interfered with the formation of ferrous and ferrozine complex. From the Table-3 it was concluded that, the compound 4a exhibited good chelating activity and are able to capture ferrous ions before ferrozine.
| Comp. No. | Concentrations | IC50 (µg/ml) | |||
|---|---|---|---|---|---|
| 25 µg/ml | 50 µg/ml | 75 µg/ml | 100 µg/ml | ||
| (%) | (%) | (%) | (%) | ||
| 4a | 60.12 | 60.85 | 66.15 | 68.42 | < 25 |
| 4b | 58.17 | 54.05 | 51.17 | 58.91 | < 25 |
| 4c | 57.77 | 54.01 | 57.27 | 66.27 | < 25 |
| BHA | 66.46 | 68.42 | 70.65 | 72.38 | < 25 |
| TBHQ | 65.6 | 68.39 | 69.34 | 70.58 | < 25 |
| AA. | 67.15 | 68.63 | 70.58 | 72.48 | < 25 |
Experimental Procedure
Materials and methods
All the reagents were obtained commercially and used by further purification using standard procedures. Melting points were determined by an open capillary method and are uncorrected. Purity of the compounds was checked by thin layer chromatography using silica gel-G coated Al plates (Merck) and spots were visualized by exposing the dry plates in iodine vapors. The IR (KBr pellet) spectra were recorded on a Perkin-Elmer (Spectrum ONE) FT-IR Spectrometer. The 1H and 13C NMR (DMSO-d6) spectra were recorded with a BRUKER NMR 500 and 125 MHz spectrometers, and the chemical shift values are expressed in ppm (δ scale) using tetramethylsilane as an internal standard. The mass spectral measurements were carried out by Electron Impact method on JEOL GC mate spectrometer at 70 eV. Elemental analyses were performed on flash EA 1112 series elemental analyzer.
General procedure for the synthesis of 6-amino 4-(5-substituted-2-phenyl indolin-3-yl)-3,4-dihydro-3-methylpyrano[2,3-c]pyrazol-5-carbonitrile (4a-c)
A mixture of 2-phenyl indole-3-carboxadehydes (1a-c) (0.01 mol), malanonitrile 2 (0.01 mol), hydrazine hydrate 3 (0.01 mol) and ethylacetoacetate (0.01 mol), was stirred in iodine water at room temperature for 5-10 mins. Than the reaction mixture was decomposed into ice-cold water, the separated solid product was filtered, washed with water and dried, recrystalized from ethanol to give pure compounds 4a-c.
6-Amino 4-(5-chloro-2-phenyl indolin-3-yl)-3,4-dihydro-3-methylpyrano[2,3-c]pyrazol-5-carbonitrile (4a): Yield: 89%; M.p.: 213-14 ºC; Rf 0.89 ethyl acetate: pet ether (1:1) mixture; FTIR (KBr) (cm-1): 3342 (NH2), 3198, 3038 (NH), 2176 (CN); 1H NMR (DMSO-d6): δ: 12. 19 (s, 1H, indole NH), 8.91 (s, 1H, imidazole-NH), 8.19 (s, 2H, NH2), 7.17-7.71 (m, 9H, Ar-H), 4.24 (s, 1H, pyran-H), 2.27 (s, 3H, CH3); 13 C NMR (DMSO-d6): 160.3, 126.8, 133.4, 129.4, 128.8, 126.8, 127.5, 123.1, 121.6, 119.3, 117.3, 112.5, 75.6, 58.1, 27.9 & 19.1; MS (EI) m/z 403 (M+), 405 (M++2); Anal. Calcd. for C22H18N5OCl (403), C, 65.43, H, 4.49, N, 17.34. Found: C, 65.40, H, 4.51, N, 17.36%.
6-Amino 4-(5-methyl-2-phenyl indolin-3-yl)-3,4-dihydro-3-methylpyrano[2,3-c]pyrazol-5-carbonitrile (4b): Yield: 74%; M.p.: 247-8 ºC; Rf 0.86 ethyl acetate: pet ether (1:1) mixture; FTIR (KBr) (cm-1): 3348 (NH2), 3193, 3039 (NH), 2171 (CN); 1H NMR (DMSO-d6): δ: 12. 17 (s, 1H, indole NH), 8.90 (s, 1H, imidazole-NH), 8.18 (s, 2H, NH2), 7.15-7.70 (m, 9H, Ar-H), 4.26 (s, 1H, pyran-H), 2.25 (s, 3H, CH3), 2.71 (s, 3H, CH3); 13 C NMR (DMSO-d6): 160.5, 126.9, 133.2, 129.7, 128.9, 126.8, 127.5, 123.4, 121.7, 119.4, 117.4, 112.6, 75.6, 58.8, 27.8, 24.2 & 19.4; MS (EI) m/z 383 (M+); Anal. Calcd. for C23H21N5O (383), C, 72.04, H, 5.52, N, 18.26. Found: C, 72.01, H, 5.47, N, 18.24%.
6-Amino 3,4-dihydro-3-methyl-4-phenyl indolin-3-yl)pyrano[2,3-c]pyrazol-5-carbonitrile (4c): Yield: 91%; M.p.: 250-51 ºC; Rf 0.83 ethyl acetate: pet ether (1:1) mixture; FTIR (KBr) (cm1): 3340 (NH2), 3199, 3038 (NH), 2174 (CN); 1H NMR (DMSO-d6): δ: 12. 20 (s, 1H, indole NH), 8.88 (s, 1H, imidazole-NH), 8.19 (s, 2H, NH2), 7.19-7.74 (m, 10H, Ar-H), 4.23 (s, 1H, pyran-H), 2.23 (s, 3H, CH3); 13 C NMR (DMSO-d6): 160.3, 126.8, 133.4, 129.4, 128.8, 126.8, 127.5, 123.1, 121.6, 119.3, 117.3, 112.5, 75.6, 58.1, 27.9 & 19; MS (EI) m/z 369 (M+); Anal. Calcd. for C22H19N5O (369), C, 71.53, H, 5.18, N, 18.96. Found: C, 71.54, H, 5.19, N, 18.90%.
Antioxidant activity assay
1, 1-Diphenyl-2-Picryl Hydrazyl (DPPH) Radical Scavenging Activity (RSA): The free radical scavenging activity (RSA) of compounds (4a-c) at concentration (25, 50, 75 and 100 μg/mL) was carried out in the presence of freshly prepared solution of stable free radical DPPH (0.04% w/v) following Hatano’s method [33] using 2-tert-butyl-4-methoxyphenol (butylatedhydroxy anisole, BHA), 2-(1,1-dimethylethyl)-1,4-benzenediol (2-tert-butyl hydroquinone, TBHQ) and Ascorbic acid (AA) as standards. All the test analyses were performed on three replicates and results are averaged. The results in percentage are expressed as the ratio of absorption decrease of DPPH in the presence test compounds and absorption of DPPH in the absence of test compounds at λ 517 nm on ELICO SL 171 Mini Spec spectrophotometer. The percentage scavenging activity of the DPPH free radical was measured using the following equation:

The results are shown in the (Table-1).
Ferric ions (Fe3+) reducing antioxidant power (FRAP)
The Ferric ions (Fe3+) reducing antioxidant power (FRAP) of the synthesized compounds (4a-c) was determined according to the literature method [34]. Different concentration of samples (25, 50, 75 and 100 μg/ml) in DMSO (1 mL) were mixed with phosphate buffer (2.5 mL, 0.2 M, pH=6.6) and potassium ferricyanide (2.5 mL 1%). The mixture was incubated at 50ºC for 20 min. After which a portion of trichloroacetic acid (2.5 mL, 10%) was added to the mixture and centrifuged for 10 min, at 1000 Xg. The upper layer of solution (2.5 mL) was mixed with distilled water (2.5 mL) and ferric chloride (0.5 mL, 0.1 %). Then absorbance at λ 700 nm was measured in spectrophotometer. Higher absorbance of the reaction mixture indicated greater reducing power. The results are shown in the (Table-2).
Ferrous (Fe2+) metal ion chelating activity
The chelating activity of ferrous ion by synthesized compounds (4a-c) was estimated by following reported method [35]. The test samples (25, 50, 75 and 100 μg/mL) in ethanolic solution (0.4 mL) were added to a solution of FeCl2 (0.05 mL, 2 mM). The reaction was initiated by the addition of ferrozine (0.2 mL, 5 mM) and the total volume was adjusted to 4 mL with ethanol. Ferozine reacted with the divalent iron form stable magenta complex species that were very soluble in water. The mixture was shaken vigorously and kept at room temperature for 10 min. Then the absorbance of the solution was measured spectrophotometrically at λ 562 nm. All test analyses were run in triplicate and averaged. The percentage of inhibition of the ferrozine Fe2+ complex formations was calculated using the following formula:
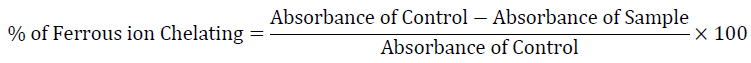
The control contains FeCl2 and ferrozine, complex formation molecule. The results are shown in the (Table-3).
Conclusion
In the present study, a novel, rapid, improved and eco-friendly synthesis of 1,2,3,4-tetrahydro-4-oxo-6-(5-substituted 2-phenyl-1H-indol-3-yl)-2-thioxopyrimidine-5-carbonitrile (4a-c) by using conventional method via one-pot mutlticomponent reaction. The biological screening studies have demonstrated that the newly synthesized compound 4a exhibited promising antimicrobial and anti-antioxidant properties. The presence of chlorine electron-withdrawing group on the indole system favors the activity. The compound 4a have an electron or hydrogen radical donating ability to DPPH radical, so that they become stable diamagnetic molecules. Therefore, it was concluded that there exists better scope for further study on this class of compounds.
Acknowledgements/Funding
This work supported by UGC-BSR-Start Up Grant (F.No.30-478/2019).The author are thankful to the Principal, Sahyadri Science College, Shivamogga, for providing laboratory facilities, to the SAIF, University of Mysore, Mysuru, for providing 1H-NMR & 13C NMR and Mass spectra.
References
- Maggi R, Ballini R, Sartori G et al., Tetrahedron Letters 45. 2004, p. 2297–9.
- Tietze LF. Chemical Reviews. 1996, p. 115–36.
- Tietze LF and Haunert F. Wiley: Weinheim, 2000, p. 39–64.
- Enders D, Huttl MRM, Grondal C et al., Nature 441. 2006, p. 861–3.
- Sheldon R. J Mol Cat. 1996, p. 75–83.
- Li C and Chan T. \Wiley: New York, NY, 1997.
- Grieco P. Organic Synthesis in Water, Blackie Academic and Professional: London, 1998.
- Kandhasamy K and Gnanasambandam V. Current Organic Chemistry. 2009, 13: p. 11820–41.
- Andrade KZ and Alves LM. Current Organic Chemistry. 2005, p. 195–218.
- Stachulski A, Berry N, Lilian A et al., J Med Chem. 2006, p. 1450–4.
- Sun W, Cama LD, Birzin ET et al., Bioorg. Med. Chem. Lett. 2006, p. 1468–72.
- Elinson M, Dorofeev A, Feducovich S et al., Tetrahedron Letters. 2006, p. 7629–32.
- -H X. Yang P, Zhang –H et al., Ind Crops Prod. 2014, 52: p. 413–19.
- Mandha S, Siliveri S, Alla M et al., Bioorg Med Chem Lett. 2012, 16: p. 5272–8.
- Smith PW, Sollis SL, Howes PD et al., J Med Chem. 1998, 41(6): 787–97.
- Kidwai M, Saxena S, Rahman M et al., Bioorg Med Chem Lett. 2005, p. 4295–8.
- Patil S, Wang J, Li X et al., Bioorg Med Chem Lett. 2012, p. 4458–61.
- Kumar A, Lohan P, Aneja DK et al., Eur J Med Chem. 2012, 50: p. 81–9.
- Nicolaou K, Snyder S, Montagnon T et al., Angewandte Chemie. 2002, p. 1668–98.
- Huang L, Hour M, Teng C et al., Chemical & Pharmaceutical Bulletin. 1992, p. 2547–51.
- Zhao LM, Jin HS, Sun LP et al., Bioorg Med Chem Lett. 2005, 15: p. 5027-5029.
- Leze MP, Palusczak A, Rolf W et al., Bioorg Med Chem Lett. 2008, 18: p. 4713-4715.
- Pirisi MA, Murineddu G, Mussinu GM et al., IL Farmaco. 2000, 57: p. 2331-2342.
- Sondhi SM, Jain S, Rani P et al., Indian J. Chem. 2007, 46: p. 1848-1854.
- Sinha D, Tiwari AK, Singh S et al., Eur J Med Chem. 2008, 43: p. 160-165.
- Falco JL, Pique M., Gonzalez M et al., Eur. J. Med. Chem. 2006, 41: p. 985-990.
- Prabhaker Walmik, Basavaraj S Naraboli, Swathi B et al., Der Pharma Chemica, 2017, 9(22): p. 8-12.
- Prabhaker Walmik and Saundane AR. Der Pharma Chemica, 2014, 6(4): p. 70-79.
- Saundane AR and Prabhaker W. J Chemistry.
- Prabhaker Walmik and Saundane AR. Der Pharma Chemica, 2015, 7(6): p. 131-140.
- Saundane R, Yarlakatti M, Prabhaker W et al., J. Het. Chem, 2014, 51(2): p. 301.
- Hiremath SP, Biradar JS and Purohit MG. Indian J Chem, 1982, 21B: p. 249.
- Hatano T, Kangawa H, Yasuhara T et al., Chem Pharm Bull, 1988, 36: p. 2090.
- Oyaizu M. Jpn. J. Nutri. 1986. 44: p. 307-315.
- Calis I, Hosny M, Khalifa T et al., Phytochem. 1993, 33: p. 1453-1456.
- Miller DD. Food Chemistry. 1996, p. 618-649.