Research Article - Der Pharma Chemica ( 2024) Volume 16, Issue 2
Determination and Quantification of an N-nitroso-Betaxolol in the Betaxolol Hydrochloride Active Pharmaceutical Ingredient by LC-MS/MS
Vinayak T Vele1*, Kishor More2, Mohan A Chandavarkar2, Shivaji Kadam2, Sushil Singh and Amol Kumbhar22Department of Pharmaceutical Analysis, University of Mumbai, Maharashtra, India
Vinayak T Vele, Department of Analytical Research, FDC Pharma, R and D Centre, Kandivali, Mumbai, Maharashtra, India, Email: vinayak.vele@fdcindia.com
Received: 18-Mar-2024, Manuscript No. Dpc-24-129909; Editor assigned: 21-Mar-2024, Pre QC No. Dpc-24-129909 (PQ); Reviewed: 04-Apr-2024, QC No. Dpc-24-129909; Revised: 05-Apr-2024, Manuscript No. Dpc-24-129909 (R); Published: 26-Apr-2024, DOI: 10.4172/0975- 413X.16.2. 257-262
Abstract
A Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS) method was developed for the quantification of N-nitroso-betaxolol in the betaxolol hydrochloride active pharmaceutical ingredient. Chromatographic separation was achieved using a waters acquity BEH C18 column, with 0.1% formic acid in water and Methanol as mobile phase in gradient elution mode at a 0.25 ml/ min flow rate. Quantification of impurities was carried out using triple quadrupole mass detection with electrospray ionization in the multiple reaction monitoring modes. The method was validated with good linearity over the concentration range of 0.0042 ppm-0.0210 ppm of the betaxolol hydrochloride test concentration for Nnitroso- betaxolol. The correlation coefficient obtained in each case was>0.9990. The recoveries were found to be satisfactory over the range between 80.0 and 120.0 % for N-nitroso-betaxolol. The developed method was able to quantitate N-nitroso-betaxolol at a concentration level of 0.014 ppm with respect to 30 mg mL-1 betaxolol hydrochloride.
Keywords
Nitrosamine; N-nitroso-betaxolol; LCMS/MS; Validation; Betaxolol hydrochloride
Abbreviations
LC-MS: Liquid Chromatography Mass Spectrometry; UPLC: Ultra Performance Liquid Chromatography; NDSRI: Nitrosamine Drug Substances Related Impurities; LOD: Limit of Detection; LOQ: Limit of Quantification; ESI: Electrospray Ionization; MRM: Multiple Reaction Monitoring; RSD: Relative Standard Deviation; ICH: The International Council for Harmonization of technical requirements for pharmaceuticals for human use.
Introduction
N-nitroso compounds have been listed as one of the cohorts of concern as per the ICH M7 guidance1 and are internationally considered as a class of strong carcinogens as per the international agency for research on cancer [1,2]. The issue of nitrosamine impurities first reported in the industry in 2018 by FDA and EMA in certain class of drugs specifically sartan [3,4] series of active pharmaceutical ingredients. From the initial phase of nitrosamine impurities assessment formed due to reagent and solvents, in recent years regulatory agencies has shifted the focused from common nitrosamine impurities to nitrosamine impurities of drug products. Thus n-nitrosamine risk assessment of pharmaceuticals moved from small molecules N-nitrosoamine to Nitrosamine Drug Substances Related Impurities (NDSRI).
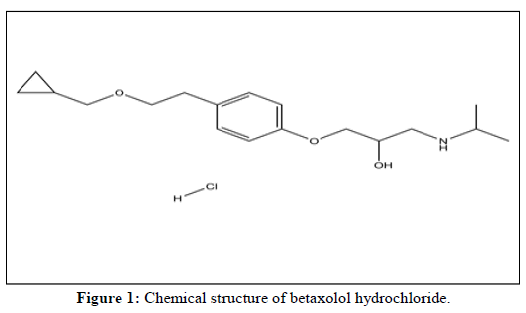
Betaxolol hydrochloride [5] (trade name Kerlone) is a selective beta receptor blocker used in the treatment of hypertension and angina. It is an active pharmaceutical ingredient of an approved drug by EMA [6] and USFDA [7]. It is usually used in finished formulation as ophthalmic solution and tablet.
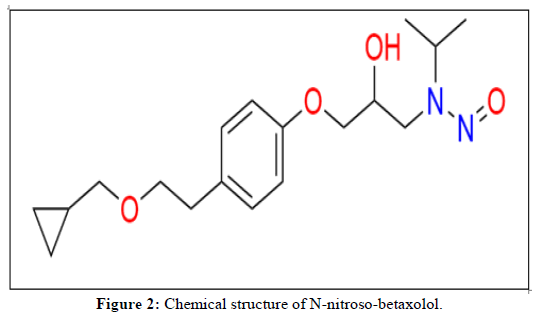
As per the latest guidance by EMA [8] and USFDA [9] the N-nitroso-betaxolol was reported as possible nitrosamine impurity due to betaxolol hydrochloride (Figure 1) active pharmaceutical ingredient itself due to presence of secondary amine. As per the guidance carcinogenic potency categorization approach, N-nitroso-betaxolol impurity falls in category [4] with Acceptable Intake (AI) 1500 ng/day. Based on the maximum daily dose of 40 mg/day, N-nitroso-betaxolol need to control at 37.5 ppm in betaxolol hydrochloride (Figure 2).
We have developed a LC-MS/MS method for the quantification of the N-nitroso-betaxolol as nitrosamine impurity in betaxolol hydrochloride active pharmaceutical ingredient at limit of 0.45 ppm. The method was further validated with respect to the specificity, Limit of Detection (LOD), Limit of Quantification (LOQ), linearity, repeatability, accuracy and robustness, in accordance with ICH guidance [10].
Materials and Methods
Experimental
Reagents and chemicals: LCMS grade formic acid and methanol procured from fischer scientific and J.T. Baker, USA respectively. LCMS grade water used was from J.T. Baker. Sample of betaxolol hydrochloride and standard of N-nitroso-betaxolol were synthesized and analyzed at FDC LTD Pharm., India.
Preparation of sample and standard solutions: The stock solution of the N-nitroso-betaxolol and betaxolol hydrochloride were prepared individually by dissolving an appropriate amount of the substances in diluent. For quantitation of N-nitroso-betaxolol in betaxolol hydrochloride a solution of 14.0 ng mL-1 (0.0140 ppm) concentration was used. The target analyte concentration was fixed as 30 mg mL-1.
Chromatographic conditions of LC-MS/MS: Analysis was performed on Nexa Shimadzu UPLC system equipped with a binary pump and an autosampler and Sciex 5500+ LC-MS/MS Triple Quad with an electrospray ionization interface. The analytical column used in the LCMS/MS study was Acquity, BEH C18 (100 × 2.1 mm, 1.7 μm) (waters Co. Ltd, USA) employed in gradient mode using 0.1% formic acid in water as mobile phase A and methanol as mobile phase B at a flow rate of 0.25 mL min-1. The column oven temperature was maintained at 35°C. The sample injection volume was 10.0 μL. The auto sampler temperature was set at 15°C. The LC gradient program (time/% mobile phase A) was set as follows: 0.00/50, 5.0/10, 15.0/10, 16.0/50 and 20.0/50. The positive electrospray ionization (ESI) probe was operated in MRM mode for the quantification of N-nitroso-betaxolol in the form of protonated ions (M+H)+at m/z 337.10˃307.20.
The different voltage i.e., Declustering Potential (DP), entrance potential and collision exit cell potential was maintained at 50 V, 10 V and 15 V respectively. The ion spray Voltage (V) was maintained at 3500 V. The curtain gas flow, ion Source Gas (GS1) and ion Source Gas (GS2) pressure was maintained at 50 psi, 30 psi and 50 psi. All parameters of LC and MS were controlled using Sciex analyst version 1.7.3.
Method validation
The developed method was successfully validated as per ICH guidance in terms of specificity, repeatability, linearity, accuracy, limit of detection, limit of quantification, robustness and solution stability. The repeatability at the determined limit of detection and Limit of quantification values was verified experimentally by injecting the same solutions six times. Linearity of the method was evaluated from six concentration levels between the LOQ and 150% level. Calculate the slope, intercept and regression coefficient values. The specificity of the developed method was assessed with betaxolol hydrochloride. Accuracy of the method was calculated in triplicate at LOQ to 150% concentration level by the standard addition method. The recoveries and RSD values were calculated for the N-nitroso-betaxolol impurity in betaxolol hydrochloride. The robustness of the method was tested by altering the mobile phase flow rate and column temperature. Further, the analysis of the sample solution at different intervals of time was compared against fresh samples to evaluate the stability of impurity in the sample solution.
Results and Discussion
Method development
The aim of the study was to develop selective LC-MS/MS method that can able to quantitate N-nitroso-betaxolol in the betaxolol hydrochloride. Columns were tested to obtain the most appropriate peak shape and separation. By using typical CSH C18 column the separation was not desire between the N-nitroso-Betaxolol and Betaxolol Hydrochloride. While on HSS T3 and kinetex peak shape was not up to the marked for the impurity. A Waters Acquity, BEH, C18 (100 × 2.1 mm, 1.7 μm) column was found to be the most suitable regarding both peak shape and separation, as well as the response of analytes. The mobile phase was operated in gradient mode using 0.1% formic acid in water as mobile phase A and methanol as mobile phase B. The flow rate of the mobile phase was maintained at 0.25 mL min-1, with the column temperature set at 35°C. The autosampler temperature was set at 15°C. The retention times of N-nitroso-betaxolol were observed to be 7.67 min and the peak corresponding to betaxolol hydrochloride was eluted at 1.75 min (Figure 3). The chromatogram of standard solution of N-nitroso-betaxolol is given in the Figure 4.
Operating conditions of LC-MS/MS
Initial optimization of the mass parameters for the detection of the N-nitroso-betaxolol was performed at concentration level of 1 μg mL-1. The intensity obtained with Electro Spray Ionization (ESI) in the positive mode was on higher side compare to that of negative mode for the impurity. As a part of optimization in the ESI conditions for N-nitroso-betaxolol, fragmentation was carried out using different collision energy (10, 15, 20, 25 and 30 ev). The ion source parameters such as ion spray and collision gas were optimized to obtain a good response for the ions.
Method validation parameters
The optimized LC-MS/MS method was successfully validated in accordance with the ICH guidelines. Method validation was carried out in terms of its adequate selectivity, linearity, LOD and LOQ, accuracy, repeatability, recovery and robustness.
Specificity
A single N-nitroso-Betaxolol solution was prepared at the specification level in the diluent. The spiked betaxolol hydrochloride solution was then subjected to LC-MS/MS analysis and the results revealed that there was no interference of the betaxolol hydrochloride peak with N-nitrosobetaxolol peak and hence the specificity of the developed method was proven (Table 1).
| Component | Retention time (min) | Relative retention time (min) |
|---|---|---|
| Betaxolol hydrochloride | 1.75 | 1 |
| N-nitroso-betaxolol | 7.67 | 4.38 |
Table 1: System suitability criteria.
Determination of LOD and LOQ values
The Limit of Detection (LOD) and Limit of Quantification (LOQ) determined the sensitivity of the method. The LOD and LOQ values of N-nitrosobetaxolol was determined based on S/N ratios of 3.0 and 10 by injecting standard solutions of known concentrations. The repeatability at the LOD and LOQ value was calculated by analyzing six replicate injections of N-nitroso-Betaxolol and calculating their RSD% values. The chromatograms of solutions of N-nitroso-betaxolol with concentrations of LOD and LOQ shown in Figures 5,6 and Table 2.
| Validation parameter | Results |
|---|---|
| LOD (ng mL-1) | 2.03 |
| LOQ (ng mL-1) | 4.06 |
| Precision at LOQ (%RSD) | 3.63 |
| Regression (r) | 0.999 |
| Calibration range (ng/mL-1) | 0.70-3.70 |
| Slope | 17680388 |
| Intercept | 7627.12 |
| % Intercept | 3 |
Table 2: System suitability criteria.
Linearity
Linearity of the method was studied by using the standard solution of N-nitroso-betaxolol at different concentration level from the Limit of Quantification (LOQ) to 150% of the impurity. The slope, intercept and correlation coefficient values were derived from the linear regression analysis of the average peak area versus the concentration of analytes. A good correlation between the peak area and concentration of analytes was obtained, as can be seen in Table 2.
Accuracy and recovery
The standard addition and recovery experiments were conducted for the N-nitroso-betaxolol in bulk samples of betaxolol hydrochloride in triplicate at LOQ (0.14 ppm), 50% (0.23 ppm), 100% (0.45 ppm) and 150% (0.68 ppm) with respect to test concentration. The acceptance criterion for recovery was set at 80-120%. The percentage recoveries for N-nitroso-Betaxolol are presented in Table 3.
| Accuracy level | Mean recovery (%) | % RSD |
|---|---|---|
| LOQ% | 111.01 | 4.93 |
| 50% | 97.29 | 3.27 |
| 100% | 104.57 | 2.12 |
| 150% | 106.48 | 4.12 |
Table 3: Accuracy (recovery) results of N-nitroso-betaxolol in bulk sample.
Precision (Repeatability)
The precision of an analytical procedure expresses the closeness of agreement among a series of measurements obtained from multiple samplings of the same homogenous sample under prescribed conditions. The system and method precision for the N-nitroso-betaxolol were checked at its specification level (i.e., 0.45 ppm with respect to analyte concentration, 30.0 mg mL-1). The percentage RSD of method repeatability and system repeatability for the N-nitroso-betaxolol were reported (Table 4) confirms good precision of the method.
| Precision | % RSD |
|---|---|
| System precision | 1.82 |
| Method precision | 3.05 |
| Intermediate precision | 4.81 |
Table 4: Precision results of N-nitroso- Betaxolol.
Robustness
The robustness of an analytical procedure is measured by its capability to remain unaffected through small, but deliberate, variations in method parameters and provide an indication of its reliability during normal usage. The optimized flow rate of the mobile phase was 0.25 mL min-1 and the column oven temperature was 35°C. The both parameters are altered from 0.22 to 0.28 mL min-1 and 32°C and 38°C receptively. The data obtained confirms that these deliberately changed chromatographic conditions did not impact the chromatographic performance for N-nitroso-betaxolol in spiked samples showing the robustness of the method.
Solution stability
The solution stability of betaxolol hydrochloride and N-nitroso-betaxolol was carried out by leaving spiked and unspiked sample solutions in firmly capped LC vials at 15°C for about 24 h in an auto sampler. The concentration of N-nitroso-betaxolol was determined against freshly prepared standard solutions and no significant changes were observed in the concentration for N-nitroso-betaxolol. The data confirmed the stability of impurity in the sample solution for at least 24 h.
Conclusion
In this study, we have developed a LC-MS/MS method that is capable of quantifying N-nitroso-betaxolol in betaxolol hydrochloride using the positive ionization mode with Multiple Reaction Monitoring (MRM). The method was validated as per ICH recommendations and it was found to be specific and linear over the specified concentration range. The determined LOD and LOQ values for N-nitroso-betaxolol were set very low and well below that of acceptable limit. The sample prepared in the analytical solution was found to be stable for at least 24 h. The method was fully validated and presents good linearity, accuracy, repeatability and robustness. This method could be very useful for the determination of N-nitrosobetaxolol in betaxolol hydrochloride during its manufacture and product release.
Conflicts of Interest
There are no conflicts to declare.
Acknowledgment
We acknowledge FDC Ltd. management for support during the entire work of method development and method validation.
References
- Al-Wadei MJ, Bakheit AH, Alaa AM, et al. Profiles Drug Subst Excip Relat Methodol. 2021; 46: p. 91-136.
[Crossref] [Google Scholar] [PubMed]
- Kadam RS, Kompella UB. J Chromatogr B. 2009; 877(3): p. 253-260.
[Crossref] [Google Scholar] [PubMed]
- Khedr A, Khayyat AN, El-Shorbagi AN, et al. J Chromatogr B. 2020; 1160: p. 122-383.
[Crossref] [Google Scholar] [PubMed]
- Bushee JL, Dunne CE, Argikar UA. Xenobiotica. 2014; 45(5): p. 396-405.
[Crossref] [Google Scholar] [PubMed]
- Zhou J, Liu Q, Su N, et al. J Liq Chromatogr Relat Technol. 2012; 35(13): p. 1767-1778.
- Pujos E, Cren-Olive C, Paisse O, et al. J Chromatogr B. 2009; 877(31): p. 4007-4014.
[Crossref] [Google Scholar] [PubMed]
- Fayyaz A, Ranta VP, Toropainen E, et al. Eur J Pharm Sci. 2020; 155: p. 105-553.
[Crossref] [Google Scholar] [PubMed]
- Galera MM, Vázquez PP, Vázquez MD, et al. J Sep Sci. 2011; 34(15): p. 1796-1804.
[Crossref] [Google Scholar] [PubMed]
- Maurer HH, Tenberken O, Kratzsch C, et al. J Chromatogr A. 2004; 1058(1-2): p. 169-181.
[Google Scholar] [PubMed]
- Ren L, Wang Z, Lou Y, et al. J Chromatogr B Analyt Technol Biomed Life Sci. 2014; 959: p. 16-21.
[Crossref] [Google Scholar] [PubMed]