Research Article - Der Pharma Chemica ( 2019) Volume 11, Issue 2
Development and Validation of First Order Derivative Spectrophotometric Method for Simultaneous Estimation of Atorvastatin Calcium and Aspirin in Capsules
Padmavathi Y*, Raghavendra Babu N, Ravi Kumar P, Sowmya D and Rohini KPadmavathi Y, Department of Pharmaceutical Analysis, G. Pulla Reddy College of Pharmacy, Mehdipatnam, Hyderabad, Telangana, India,
Abstract
A simple UV spectrophotometric method using first order derivative technique was developed for the simultaneous estimation of atorvastatin calcium and aspirin in capsules. The atorvastatin calcium and aspirin stock solutions were prepared in (50: 50 v/v) methanol: water and scanned in UV- region for first order derivative spectrum. The zero crossing point for atorvastatin calcium and aspirin was 243 nm and 276 nm respectively. Linearity was established over the concentration range of 6-14 μg/ml and 20-100 μg/ml for atorvastatin calcium and aspirin with correlation coefficient (r2) value 0.998 and 0.999 respectively. The method was validated according to ICH guidelines for validation parameters like accuracy, precision, limit of detection and limit of quantification. They found to be within limits. The method was successfully applied for quantitative estimation of atorvastatin calcium and aspirin in capsules.
Keywords
Atorvastatin calcium, Aspirin, Derivative spectroscopy, Zero crossing point, Correlation coefficient, ICH guidelines.
Introduction
Atorvastatin Calcium (Figure 1) is: [(βR, ds)-2-(4-flouorophenyl)-β, δ-di hydroxy-5- (1-methylethyl) -3- phenyl-4 [(phenyl amino) carbonyl]-1-heptenoic acid calcium salt (ATV). It is statin used for lowering blood cholesterol. It is a selective competitive inhibitor of the enzyme HMG-CoA reductase, which catalyses the conversion of HMG-CoA to mevalonate, an important rate limiting step in cholesterol biosynthesis. Aspirin (ASP) is chemically known as acetyl salicylic acid and is used as non-steroidal anti-inflammatory and analgesic drug [1,2].
Both atorvastatin calcium and aspirin are freely soluble in methanol, slightly soluble in ethanol and very slightly soluble in distilled water.
As per literature HPLC and UV spectrophotometric methods are available for simultaneous estimation of atorvastatin calcium and aspirin in marketed formulations [3-11]. To the best of our knowledge there is no derivative spectrophotometric method reported for determination of ATV and ASP simultaneously. Therefore, an attempt has been made to develop a simple, derivative spectrophotometric method for analysis of these two drugs. Derivative spectroscopy uses first or higher derivatives of absorbance with respect to wavelength for qualitative analysis and for quantification. The spectra obtained in derivative mode give high resolution and differentiation which makes easy for multicomponent analysis of mixtures of components [12,13].
Chemicals and reagents
Atorvastatin calcium and Aspirin are the gift samples from Aurobindo pharmaceuticals, Hyderabad. Methanol (Merck specialities private limited), water (SDFCL), sodium hydroxide (SDFCL) used are of analytical grade.
Instruments
UV-Visible Spectrophotometer (Shimadzu UV-1800 (UV probe 4.0 software), Digital balance (Shimadzu BL-220H), Ultra sonic bath sonicator (PCI Analytics 6.5 li200H), Refrigerated centrifuge (Eltek RC4100F) were used in analytical work.
Marketed formulation
AZTOR ASP capsules containing 10 mg of atorvastatin calcium and 75 mg of aspirin manufactured by Sun pharmaceuticals Ltd. were purchased from local market.
Selection of solvent
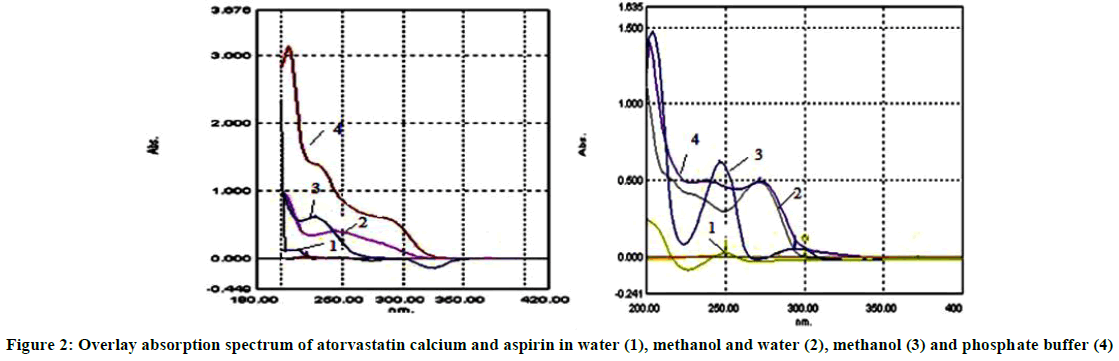
Solvent selection is the first step involved in the method development. Solvent is selected based on the solubility of the drugs. Absorption characteristics of 10 μg/ml standard solution of both drugs were checked in water, methanol, phosphate buffer pH 7.4, 0.1 N NaOH and methanol: water (50: 50). The overlay absorption spectrum of two drugs in all the solvents is shown in Figure 2.
Standard solutions
Standard stock solutions of atorvastatin calcium and aspirin (1000 μg/ml) were prepared separately in methanol: water (50: 50 v/v). Working standard solutions of these two drugs (100 μg/ml) were obtained by dilution of the respective stock solutions in methanol: water (50: 50 v/v).
Spectral characteristics and wavelength selection
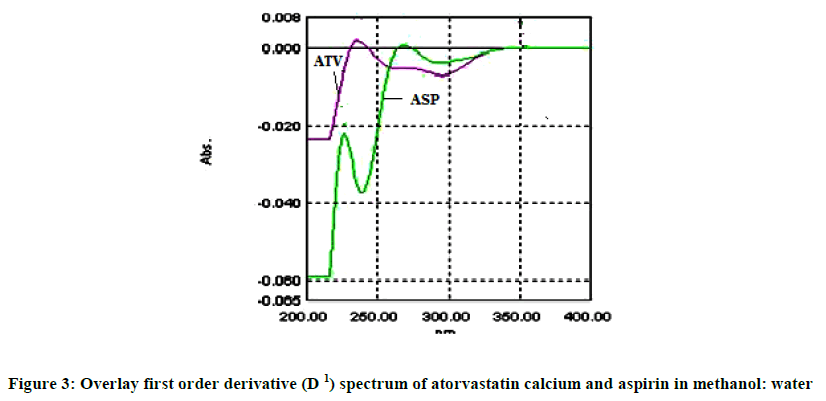
The absorption spectra of 10 μg/ml of atorvastatin calcium and 20 μg/ml in the diluent were recorded by scanning in the UV wave length range 200-400 nm and overlay zero order (D0) spectrum is shown in Figure 1. Zero order spectrums (D0) were converted to first derivative spectra (D1) using delta lambda 1.0 and scaling factor 4.0. The zero crossing point of atorvastatin and aspirin was found to be 246 nm and 276 nm respectively. Overlay first order derivative spectrum was shown in Figure 3.
Selection of analytical concentration ranges
Calibration standards at five levels were prepared by diluting the standard stock solution in the concentration range of 6-14 μg/ml for atorvastatin calcium and 20-100 μg/ml for aspirin respectively. The absorbance of these solutions was measured at their zero crossing point wavelengths.
Method validation
The method developed was validated for linearity, precision, accuracy, specificity, ruggedness, robustness, limit of detection, limit of quantification and robustness according to the ICH Guidelines [14].
Linearity and range
The linearity of analytical method is ability to obtain test results that are directly proportional to the concentration of analyte in the sample. The range of analytical method is the interval between upper and lower concentration (amounts) of analyte that have been demonstrated with suitable level of precision, linearity and accuracy.
Series of calibration standards were prepared in the concentration range 6-14 μg/ml of ATO and 20-100 μg/ml of ASP. The absorbance of these solutions was measured at 246 nm and 276 nm against solvent blank respectively and D1 (first order derivative) absorbance values were recorded. A graph of concentration versus absorbance was plotted and correlation coefficient (r2) was reported.
Limit of detection (LOD) and limit of quantification (LOQ)
The detection limit of an individual analytical procedure is the lowest amount of analyte in a sample which can be detected but not necessarily quantified as an exact value. Quantification limit of an individual analytical procedure is the lowest amount of analyze in a sample which can be quantitatively determined with suitable precision and accuracy.
The sensitivity of proposed method for measurement of atorvastatin and aspirin was estimated in terms of LOD & LOQ determined using the standard deviation of the response and slope method.
Precision
The precision of an analytical procedure defines the degree of closeness of agreement between a series of measurements obtained from multiple samplings of homogenous sample under prescribed conditions. Precision of the method was reported as RSD% at different levels- repeatability, Intra-day precision and Inter-day precision.
Repeatability was evaluated by the analysis six replicates of 10 μg/ml of atorvastatin calcium and 30 μg/ml of aspirin for checking the variation of results on the same day. The intra-day and inter-day precision of the proposed method was determined by analyzing samples 32 μg/ml, 40 μg/ml and 48 μg/ml for aspirin and 6 μg/ml, 8 μg/ml, 10 μg/ml for atorvastatin, three replicates of each sample as a batch in a single assay on the same day and three consecutive days for inter-day precision.
Accuracy
Recovery studies were carried out by using standard addition method at three levels, 80%, 100% and 120% for ATV calcium (8 μg/ml, 10 μg/ml and 12 μg/ml) and ASP (60 μg/ml, 75 μg/ml and 90 μg/ml). At each level, the determination was done in triplicate and the amount of drug recovered was calculated and reported.
Analysis of fixed dose combination capsules
Twenty capsules of fixed dose combination capsules were weighed and emptied. The powder quantity equivalent to one capsule was transferred to 100 ml volumetric flask containing 25 ml of solvent and sonicated for 5 min. The solution was centrifuged at 2500 rpm for 15 min and filtered using whattman filter paper. Sample stock solution was further diluted to get a solution containing 10 μg/ml of atorvastatin calcium and 75 μg/ml of aspirin. The absorbance of resulting solution was measured at 246 nm and 276 nm against solvent blank and %purity was calculated.
Conclusion
Both atorvastatin calcium and aspirin have shown similar spectra which cannot be resolved in absorbance mode for their simultaneous analysis. Increased resolution and differentiation of spectra was observed in derivative mode. The developed derivative spectrophotometric method can be employed for routine analysis of atorvastatin calcium and aspirin in marketed formulations. Fixed dose combined capsules were analysed using the developed method and assay results were within the limits for atorvastatin calcium and aspirin.
Acknowledgment
The authors are grateful to the management of G. Pulla Reddy College of pharmacy, for providing the necessary facilities to carry out the research work. Authors are thankful to Aurobindo pharmaceuticals, Hyderabad, India for providing the gift sample of the pure drugs.
References
- Indian pharmacopeia, 7th (Edn.), The Indian Pharmacopoeia Commission, Ghaziabad, 2014, 230-231.
- Martindale, the Complete Drug Reference, 34th (Edn.), London: Pharmaceutical Press, 2005, pp. 15-19.
- M. Sasikala, A. Pravalika, V. Udaya bhaskar, P. Harsha Teja, Int. Bullet. Drug Res., 2013, 3(5), 39-48.
- R. Hirave Rupali, D. BengudeRavindra, G. Maniyar Mithun, S. Kondawar Manish, Patil Sandeep, Int. J. Drug Development & Res.,2013, 5(1), 1-10.
- C.H. Solanki, N.K. Patel, V. Patel, D. Patel, R. Vaishy, Scholars Research Library, 2012, 4(3), 947-953.
- S.O. Havele, S.S. Havele., Int. J. Pharm. Pharmaceut. Sci. Res., 2012, 1(2), 75-79.
- T. Kumbhar Smita, D. Jadhav Swapnil, M. Neela, Bhatia, M.S. Bhatia, Int. J. Pharm. Pharmaceut. Sci.,2011, 3(4), 195-197.
- S.V. Londhe, R.S. Deshmukh, S.V. Mulgund, K.S. Jain, Indian J. Pharmaceut. Sci., 2011, 73, 1, 23-29.
- H.O. Kaila, M.A. Ambasana, A.K. Shah, Int. J. Chem. Tech. Res., 2011, 3, 1, 459-465.
- N.D.Pandurang, V. jadhav, C. Raut, Asian J. Res. Chem., 2010, 3, 2, 1-3.
- S. Singh, N. Dubey, D.K. Jain, Asian J. Res. Chem., 2010, 3, 4, 885-887.
- A.H. Beckett, J.B. Stenlake, Practical pharmaceutical chemistry, CBS Publishers & Distributors, New Delhi, 2004, 2(4).
- J. Karpinska, J. Uddin (In Edi.), Basic Principles and Analytical Application of Derivative Spectrophotometry, Macro to Nano Spectroscopy, In Tech, China, 2012, 253-268.
- ICH Harmonized Tripartite Guidelines, Validation of analytical procedures: Text and Methodology, Q2 (R1), 2005, Geneva.