Research Article - Der Pharma Chemica ( 2021) Volume 13, Issue 5
Development of Visible Spectrophotometric Methods for the Determination of Ranolazine in Bulk and Pharmaceutical Dosage Form
Magesh AR*, Kavitha D and Dhanaraju MDMagesh AR, Department of Pharmaceutical Analysis, GIET School of Pharmacy, Rajahmundry, Andhra Pradesh-533294, India, Email: armagesh09@gmail.com
Received: 24-Dec-2020 Accepted Date: May 20, 2021 ; Published: 28-May-2021
Abstract
Two precise, accurate and simple visible spectrophotometric methods were developed for the determination of Ranolazine in bulk drug and in pharmaceutical dosage form. The proposed methods were based on determination of ranolazine after its reaction with 3-Methyl-2-benzothiazolinone hydrazine hydrochloride and potassium dichromate and measuring the chromogen at the λmax at 661 and 432, respectively. Beer’s law obeyed in the concentration range of 50-150 μg/ml for Method A and 25-125 μg/ml for Method B. The accuracy of the methods was calculated by performing recovery studies. The methods were found to be simple, economical, accurate and reproducible and can be used for routine analysis of Ranolazine in bulk drug and in pharmaceutical formulations.
Keywords
Ranolazine, UV-Vis Spectrophotometry, MBTH
Introduction
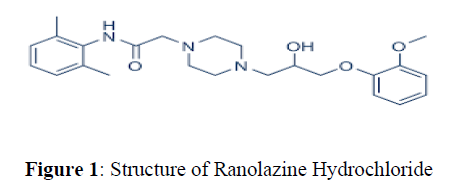
Ranolazineis an antianginal drug and chemically it is (±)-N-(2,6-dimethylphenyl)-4- [2-hydroxy-3-(2-methoxyphenoxy) propyl]-1-piperazine acetamide or Its enantiomers (R)-(+)-N-(2,6-dimethylphenyl)- 4- [2-hydroxy-3-(2- methoxyphenoxy)-propyl]-1 –piperazineacetamide [1-4] (Figure 1)
Very few spectrophotometric methods have been previously reported for the determination of Ranolazine hydrochloride in pharmaceutical dosage forms [5-10]. Other techniques reported for the assay of Ranolazine in pharmaceuticals include HPLC [11-13] and LC-MS [14-18].
Spectrophotometric methods are the most suitable methods because of their inherent simplicity, high sensitivity, low cost and extensive availability in quality control laboratories. Unfortunately, the spectrophotometric methods that have been published for the estimation of Ranolazine in their pharmaceutical formulations were associated with some disadvantages such as lack of sensitivity and also lacking in evaluating the most of the analytical method validation parameters as per ICH guidelines. Also, the reported methods have mainly emphasised on UV methods and only one visible method have been reported which utilised a reagent which has the capability to degrade the drug fast. Hence the present work was aimed in fulfilling the analytical method parameters for the developed method which is an evidence for the selectivity of the proposed method and also concentrated on incorporating statistical data’s wherever necessary which indicates the novelty of the developed method.
Furthermore, utilisation of suitable stable reagent was employed in the present study which makes an efficient analytical method for the selected drug formulation.
Table 1 represents comparison of Ranolazine estimation in the present work and other published methods. The main aim of the present work is to develop simple, rapid, reliable and precise, accurate and economical methods for the estimation of Ranolazine in bulk and in Pharmaceutical dosage form by simple colorimetry using 3-Methyl-2- benzothiazolinone hydrazine and Potassium dichromate.
| S. No | Reagents/Solvents Used | Method | λ max (nm) |
Linearity Range (µg/ml) |
LOD | LOQ | Ref |
|---|---|---|---|---|---|---|---|
| 1. | Methanol | UV | 272 | 10-100 | 0.27 | 0.82 | [5] |
| 2. | Methanol: Water | Nanodrop UV |
272 | 12.5-2000 (ppm) |
--- | --- | [6] |
| 3. | Water: Methanol | UV | 272 | 20-100 | 0.899 | 2.725 | [7] |
| 4. | Methanol | UV | 272 | 10-100 | 2.806 | 8.503 | [8] |
| 5. | Methanol: Water | UV (AUC) |
265-279 | 10-100 | 0.65 | 1.98 | [9] |
| 6. | Potassium permanganate &Sulphuric acid | I | 525 | 5-15 | --- | --- | [10] |
| Ferric Phenanthroline | II | 510 | 5-15 | --- | --- | ||
| 7. | MBTH (0.5%) & CAS (1%) | I | 661 | 50-150 | 0.970 | 3.20 | Present Work |
| Potassium Dichromate (0.3%) &Sulphuric acid | II | 432 | 25-125 | 0.81 | 3.23 | ||
| 8. | Methanol | UV | 273 | 20-150 | 1 | 4 | [11] |
| Method | λ max (nm) |
Linearity Range (µg/ml) |
Flow Rate |
Rt | Ref | ||
| M. Phase Buffer: Acetonitrile (60;40) pH-6 |
HPLC | 224 | 20-150 | 1.0 | 5.09 | [11] | |
| 9. | Isopropanol:PO4 Buffer, pH-7 |
HPLC | 227 | ---- | 0.25 | 32.6 | [12] |
| 10. | Mix A Buffer/Acetonitrile (90:10) |
HPLC | 210 | 50-150 | 1.0 | 11.94 | [13] |
| Mix B Acetonitrile/Water (90:10) pH-4.5 |
Materials and Methods
Perkin Elmer (LAMBDA 25) Double beam UV-Vis spectrophotometer with 1 cm matched quartz cells was used for spectral and absorbance measurements.
Reagents and standards
All chemicals used in the study were of analytical purity grade and all solutions were prepared in double distilled water.
Method A
Reagents and chemicals used
3-Methyl-2-Benzothiazoline Hydrazine (MBTH, 0.5%).
Ceric Ammonium Sulphate(1%).
Sulphuric acid (0.1N).
Preparation of MBTH (0.5 %)
Weigh accurately about 500 mg of MBTH and dissolve it in 100 ml of distilled water.
Preparation of Ceric ammonium sulphate (1%)
Weigh accurately about 1g of Ceric Ammonium Sulphate and dissolve in 20 ml of 0.1N Sulphuric acid and made up to the mark with distilled water.
Method B
Materials and Reagents used
Potassium dichromate. (0.3%)
Concentrated Sulphuric acid.
Preparation of Potassium dichromate (0.3%)
Accurately weigh about 300 mg of Potassium dichromate dissolve it in 100 ml volumetric flask by using distilled water.
Preparation of stock solution
Standard stock solution of Ranolazine HCL was prepared by dissolving 10 mg drug in 10 ml volumetric flask and made up to volume with methanol to get 1 mg/ml.
Procedures
Method A – Varying aliquots (0.5 ml to 1.5 ml) of stock Ranolazine solution were transferred into a series of 10 ml volumetric flasks. To each flask, 0.5 ml of MBTH (0.5%, w/v) and 1 ml of ceric ammonium sulphate (1%, w/v) were added and allowed to stand for 20 min under occasional shaking. The volume was then made up with water and absorbance of each solution was measured at 661 nm.
Method B – Different aliquots (0.25 ml to 1.25 ml) of stock Ranolazine solution were transferred into a series of 10 ml volumetric flasks. To each flask, 1 ml of Potassium dichromate (0.3% w/v) and 1.5 ml of Concentrated Sulphuric acid were added. The volume was then made up with water and absorbance of each solution was measured at 432 nm.
Procedure for Tablets Assay
Method A
20 tablets were accurately weighed and powdered (Ranozex (500 mg) -Sun Pharma Laboratories Ltd). The tablet powder equivalent to 100 mg of Ranolazine was transferred to 100 ml volumetric flask and 60 ml of methanol was added to it. This mixture was sonicated in bath sonicator for 40 minutes and thevolume was made up to 100 ml with methanol. It was then filtered through Whatman filter paper [18].
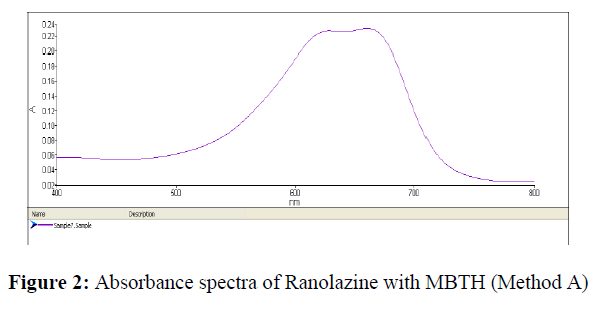
An aliquot of filtrate was transferred to 10 ml volumetric flask, 0.5 ml of MBTH (0.5%, w/v) and 1 ml of ceric ammonium sulphate (1%, w/v) were added and allowed to stand for 20 min for the development of colour under occasional shaking and the volume was made up with water. The absorbance of coloured chromogen was measured at 661 nm against reagent blank.[19,20] (Figure 2).
Method B
20 tablets were accurately weighed and powdered (Ranozex (500 mg) -Sun Pharma Laboratories Ltd). The powder equivalent to 100 mg of Ranolazine was transferred to 100 ml volumetric flask and 60 ml of methanol was added to it. This mixture was sonicated in bath sonicator for 40 minutes and thevolume was made up to 100 ml with methanol. Solution was then filtered through Whatman filter paper.
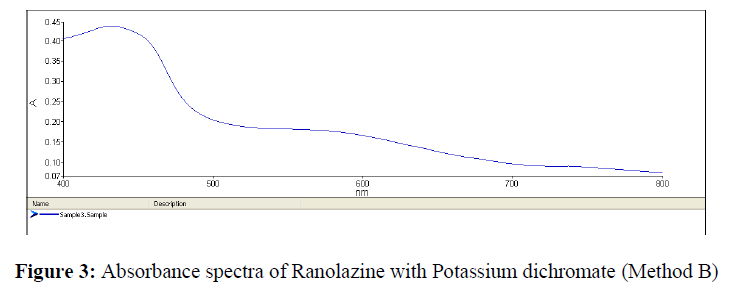
An aliquot of filtrate was transferred to 10 ml volumetric flask, 1 ml of Potassium dichromate (0.3% w/v) and 1.5 ml of Concentrated Sulphuric acid were added. The volume was then made up with water and absorbance of the solution was measured at 432 nm against reagent blank (Figure 3).
| S. No | Concentration (µg) |
Absorbance | Concentration (µg) |
Absorbance |
|---|---|---|---|---|
| 1. | 50 | 0.284 | 25 | 0.199 |
| 2. | 75 | 0.431 | 50 | 0.368 |
| 3. | 100 | 0.568 | 75 | 0.563 |
| 4. | 125 | 0.716 | 100 | 0.759 |
| 5. | 150 | 0.845 | 125 | 0.942 |
Results and Discussions
The method was optimised by conducting preliminary experiments to determine the desired solvent, optimum concentration, volume and the desired order of addition of reagents in order to obtain better response. A volume of 0.5 ml of MBTH (0.5%, w/v) and 1 ml of ceric ammonium sulphate (1%, w/v) for Method A and 1 ml of Potassium dichromate (0.3% w/v) and 1.5 ml of Concentrated Sulphuric acid for Method B were fixed. The optimum time required for the reaction completion in both the methods were studied and was found that the reaction of Ranolazine with MBTH requires 20 min and Ranolazine that of with Potassium dichromate requires 20 min for the development of coloured chromogen.
Method Validation
The developed method was validated as per ICH guidelines. The optical characteristics such as correlation co-efficient, slope, intercept, molar absorptivity, sandell’s sensitivity were calculated. The optical characteristics such as Beer’s law limits and molar absorptivity values, together with other analytical performance characteristics such as LOD, LOQ, regression equation parameters are given in Table 2.
| Parameters | Method A | Method B |
|---|---|---|
| λmax | 661nm | 432 nm |
| Linearity range(µg/ml) | 50-150µg/ml | 25-125µg/ml |
| Correlation Coefficient | 0.9998 | 0.9996 |
| Sandell’s Sensitivity | 0.1 | 0.1 |
| Regression equation | y=0.0057x+0.0021 | y=0.0075x+0.0015 |
| Slope | 0.0057 | 0.0075 |
| Intercept | 0.0021 | 0.0015 |
| %RSD | 0.014 | 0.025 |
| Limit of detection(µg) | 0.970 | 0.81 |
| Limit of quantification(µg) | 3.20 | 2.23 |
Linearity
To determine linearity of the proposed methods, a series of solutions of Ranolazine ranging from 50–150μg/ml for Method A and 25-125 μg/ml for Method B and were prepared from the stock solutions and analysed and tabulated in Table 3.
The calibration curves for Method A and Method B for Ranolazine were represented in Figures 2 & 3.
Precision
Precision of the method was determined by performing Inter-day variation, Intra-day variation and repeatability studies and expressed in the forms of %RSD. In Inter-day variation, the absorbances of working standard solutions of Ranolazine were measured on three consecutive days. In intraday variation the absorbance was measured three times a day. The precision results are tabulated in Tables 4 & 5.
| S. No | Intra day | Inter day | ||
|---|---|---|---|---|
| Concentration (µg) |
Absorbance | Concentration (µg) |
Absorbance | |
| 1 | 100 | 0.561 | 100 | 0.578 |
| 2 | 100 | 0.550 | 100 | 0.569 |
| 3 | 100 | 0.572 | 100 | 0.575 |
| 4 | 100 | 0.568 | 100 | 0.559 |
| 5 | 100 | 0.578 | 100 | 0.562 |
| 6 | 100 | 0.582 | 100 | 0.576 |
| Mean | 0.568 | 0.551 | ||
| % RSD | 0.014 | 0.029 | ||
| S. No | Intra day | Inter day | ||
|---|---|---|---|---|
| Concentration (µg) |
Absorbance | Concentration (µg) |
Absorbance | |
| 1 | 100 | 0.769 | 100 | 0.768 |
| 2 | 100 | 0.765 | 100 | 0.761 |
| 3 | 100 | 0.758 | 100 | 0.769 |
| 4 | 100 | 0.768 | 100 | 0.759 |
| 5 | 100 | 0.759 | 100 | 0.757 |
| 6 | 100 | 0.766 | 100 | 0.766 |
| Mean | 0.731 | 0.763 | ||
| % RSD | 0.025 | 0.013 | ||
Accuracy study
To ensure the accuracy of the method, recovery studies were performed, where a known amount of pure drug was added to the previously analysed formulated samples and these samples were re-analysed by the proposed method and percentage recovery was calculated. The Percentage recoveries thus obtained were given in Table 6.
| Method | Amount Added (µg) |
Amount Found (µg) |
Percentage Recovery |
%RSD |
|---|---|---|---|---|
| A | 50 | 50.05 | 100.11 | 0.624 |
| 100 | 99.88 | 99.88 | ||
| 150 | 149.85 | 99.99 | ||
| B | 50 | 49.65 | 99.30 | 0.415 |
| 100 | 99.89 | 99.89 | ||
| 150 | 149.92 | 99.94 |
Ruggedness
The ruggedness of the developed method was determined by analyst variation (analyst 1 and analyst 2) (Perkin Elmer Lambda 25 double beam UV/Vis spectrophotometer). The results were analysed statistically and the effect of variations were estimated. The results were shown in Table 7. For determining the robustness of the method, parameters like detector wavelength are varied within a realistic range and the quantitative influence of the variables is determined. The results were shown in Table 8.
| Parameters | % RSD | |
|---|---|---|
| Method A | ||
| Analyst | 1 | 0.249 |
| 2 | 0.325 | |
| Method B | ||
| Analyst | 1 | 0.638 |
| 2 | 0.594 | |
| Method | Wavelength ±2 nm |
% RSD |
|---|---|---|
| A | 663 | 0.74 |
| 559 | 0.59 | |
| B | 434 | 0.68 |
| 430 | 0.93 |
Discussion
In Method A, secondary amines undertake oxidation to give hydroxylamine which converts into stable nitroxides, thus the nitroxide type of compound is formed which gives coloured complex with MBTH and is measured at 661 nm. In Method B, Potassium dichromate in the presence of sulphuric acid is commonly used as an oxidizing agent. The oxidizing property of it is applied for the quantification of the compounds having pharmaceutical significance by spectrophotometric method, where oxidation of the drug takes place with dichromate ions (VI) in concentrated sulphuric medium to form coloured chromogen measured at 432 nm. The colour developed for both the methods were stable up to 2 hours. The correlation co-efficient was found to be 0.9998 for method A and 0.9996 for method B, respectively. To determine the precision of the method, the analysis was carried out thrice and the RSD (%) values was found to be the prescribed limit. Hence, the precision of the methods was confirmed. Further, the precision was confirmed by intermediate precision. The % RSD value for interday and intraday analysis of formulation was found to be less than 2% and is shown in Tables 4 & 5. The accuracy of method was determined by performing recovery studies as shown in Table 6. A known amount of standard drug was added to the pre-analysed sample in varying levels.
The amount of drug recovered was calculated and the average percentage recovery was found to be in the range of 99.88 -100.1 % for method A and 99.30–99.94 % for method B. The percentage recovery values indicated the method was accurate. The LOD and LOQ for Method A was found to be 0.970 μg/ml and 3.20 μg/ml, respectively and for Method B was found to be 0.81μg/ml and 2.23 μg/ml, respectively. The method was also found to be rugged and robust as indicated by the % RSD values which are less than 2% as shown in Table 7 & 8.
Conclusion
Two simple and sensitive colorimetric methods for the determination of Ranolazine have been developed and validated as per ICH guidelines. The developed methods are found simple and rapid taking not more than 30 min for the assay. These developed methods are more sensitive than the existing UV and HPLC methods, and are free from such experimental variables as heating or extraction step. The reagents employed in the proposed study are easily available, less expensive and the analytical procedures does not involve any critical reaction conditions or tedious sample preparation. Besides, the methods are free from interference from common additives and excipients. The described methods depend on the use of simple and inexpensive chemicals and techniques but provide better sensitivity comparable to that achieved by sophisticated and expensive technique like HPLC. Thus, they can be used as alternatives for rapid and routine quality control determination of bulk sample and tablets.
Acknowledgment
The authors are thankful to the management for providing the facilities for the successful completion of the work.
References
- Cheng JWM, Clin Ther, 2006. 28(12): p. 1996-2007.
- http://www.drugbank.com/Ranolazine Hydrochloride
- http://www.rxlist.com/Ranozex
- Sean C Sweetman, Martindale: The Complete Drug Reference, Pharmaceutical Press, London, 200.
- Sharma A, PrakashD and Sachin Kumar S. Int J Chem Tech Res. 2010, 2(4): p. 1945-1948.
- Rakesh Kumar S, Pankaj Singh P, Malik P et al., Int J Pharm Sci Res., 2011. 2(4): p. 905-998.
- Vanparia DJ, Rajanikant MC, Prashant BP et al., Int. J. ChemAnal.Sci. 2011. 2(9): p. 1150-1152.
- Jagdish PB, Indrajit DG, Chandrakant SR et al., Der Pharma Chem. 2011. 3(2): p. 1-4.
- Kalyan Kumar B, Santosh Kumar T, Venkateshwarlu P et al., Int J Pharm Res Dev. 2011. 3(5): p. 15-23.
- Samba Siva Rao V and Phani RSCh. Int J Res Pharm. 2011. 1(1): p. 66-72.
- Patel RC, Rathod DK, Patel PR et al., Int J Pharm Appl Sci., 2010. 1(2): p. 79-83.
- DeleeE, Le GarrecL, Jullien I et al., Chromatographia., 1987. 24: p. 357-359.
- Madhavi A, Subba Rao DV, Srinivasu P et al., Chromatographia.,2009. 70: p. 333-338.
- Zhong J, Liu XQ, Chen Y et al., Chromatographia.,2006. 63: p. 123-127.
- Bhaumik U, Ghosh A, KantiSarkar A. J Pharm Biomed Anal. 2008. 48: p. 1404–1410.
- Herron WJ, Eadie J and Penman AD. J Chromatogr A. 1995, 712 (1): p. 55-60.
- Penman AD, Eadie J, Herron WJ et al., Rapid Commun Mass Spectrom.,1995. 9(14): p. 1418-1430.
- Tian L, Jiang J, Huang Y et al., J Chromatogr B.,2007.846 :p. 346-350.
- Laghari GN, Nafady A, Al SI et al., Nanomaterials (Basel). 2019, 9(1): p. 83.
- Alijerf L and Mashlah A, et al, Microchem J, 2017. 132(1): p. 411-421.
Select your language of interest to view the total content in your interested language
Google Scholar citation report
Citations : 15261
Der Pharma Chemica received 15261 citations as per Google Scholar report
Der Pharma Chemica peer review process verified at publons