Research Article - Der Pharma Chemica ( 2022) Volume 14, Issue 12
Direct, 2nd and 3rd Derivative Spectrophotometric Determination of Indium (III) Using 2-Hydroxy-1-Naphthaldehyde-P-Hydroxybenzoichydrazone in CTAB Micellar Medium
Saleem Basha V1* and Govinda Chowdary P22Department of Chemistry, Vignan Institute of Technology and Sciences, Deshmuki, Yadadri, Bhuvanagiri, Telangana, India
Saleem Basha V, Government Degree College, Baruva, Srikakulam, A.P, India, Email: drvsbchemistry@gmail.com
Received: 05-Dec-2022, Manuscript No. dpc-22-83067; Editor assigned: 07-Dec-2022, Pre QC No. dpc-22-83067; Reviewed: 21-Dec-2022, QC No. dpc-22-83067; Revised: 23-Dec-2022, Manuscript No. dpc-22-83067; Published: 30-Dec-2022, DOI: 10.4172/0975-413X.14.12.29-35
Abstract
A new chromophore, 2-hydroxy-1-naphthaldehyde-p-hydroxybenzoichydrazone (HNAHBH) is used first time for the direct and derivative spectrophotometric investigations of indium (III). The metal ion reacts with the reagent in aqueous dimethyl formamide (DMF) in wide pH range forming greenish yellow coloured 1:2 (M: L) complex with λmax at 450 nm. The colour intensity further increases in the presence of cityl tri methyl ammonium bromide (CTAB) micellar medium at room temperature. Obeying of Beer’s law is taken place in the wide range 0.057-2.869 μg mL-1 of In (III). The molar absorptivity, Sandell’s sensitivity, detection limit, determination limit and relative standard deviation are calculated as 8.8 x 104 L mol-1cm-1, 0.0013 μg cm-2, 0.004 μg mL-1, 0.012 μg mL-1 and 1.66 % respectively. The second and third order derivative spectrophotometric methods are also developed for the determination of indium (III) which showed greater sensitivity and selectivity. The proposed direct and derivative methods are applied for the determination of indium in zinc effluents and alloys, in synthetic mixtures and in reference materials.
Keywords
Determination of indium (III); Direct and derivative spectrophotometry; HNAHBH; Alloys and synthetic mixtures
INTRODUCTION
The main application of indium is to form thin-films used in fusible alloys to form lubricated layers. Transparent electrodes from indium tin oxide are used in liquid crystal displays (LCDs) in televisions. It is also used in solders as a doping for semiconductors. Indium in very small amounts is used in aluminum alloy sacrificial anodes to prevent passivation of aluminum. Pure indium in metal form is considered to be non-toxic. But all the indium compounds should be regarded as highly toxic and damages the heart, kidney and liver and may be teratogenic. Anhydrous indium trichloride is quite toxic and indium phosphate is both toxic and a suspected carcinogen. Recently, Koichiet et.al. [1] has reported the comparative study of indium exposed and non-exposed workers for their urine, blood and serum samples some of the conventional reagents reported for the spectrophotometric determination of indium (III) such as PAN [2], PAR [3], pyrocatechol violet [4], xylenol orange [5] and Erichrome cyanine R [6] are non-selective reagents as they form coloured complexes with number of metal ions. To improve the selectivity of these methods, prior separation of the analyte by solvent extraction methods was proposed [7-11]. Singh et.al and Agnihotri et.al reported recently 1-(2-pyridylazo)-2-naphthol [12] and 2-(5-bromo-2-pyridylazo)-5-diethylaminophenol [13] respectively for the simultaneous derivative spectrophotometric determination of gallium (III) and indium (III). In the present paper simple, rapid and highly sensitive and selective zero order and second and third derivative spectrophotometric methods are proposed for the determination of Indium(III) based on its reaction with 2-Hydroxy-1-naphthaldehyde-p-hydroxybezoichydrazone (HNAHBH) in almost neutral buffers forming an intense and stable greenish yellow coloured solution. The methods are robust and are applicable for the analysis of natural complex materials.
EXPERIMENTAL
The absorbance and pH measurements were made on a Shimadzu UV-visible spectrophotometer (Model UV -160A) fitted with 1 cm Quartz cells and ELICO digital pH meter model LI – 120 respectively. The pH meter has temperature compensate arrangement and has reproducibility of measurement within ± 0.01 pH.
Reagents
2-Hydroxy-1-naphthaldehyde-p-hydroxybenzoichydrazone (HNAHBH)
It is prepared by condensing a mixture of equimolar solutions of 2-hydroxy-1-naphthaldehyde in methanol and para hydroxyl benzoic hydrazide in ethanol using the general procedure [14]. The structure of the resultant hydrazone is shown below (Figure 1).
1 x 10-2 M solution of the reagent was prepared by dissolving 0.3100gms in 100 ml of di methyl formamide (DMF). Working solutions were prepared by diluting the stock solution with DMF. Indium (III) solution Stock solution (1 x 10-2 M) of indium sulphate was prepared by dissolving 0.5178gms of In2(SO4)3.xH2O (Himedia) in 2 ml of 2 M H2SO4 and diluting to 100 ml with distilled water. This solution was standardized spectrophotometrically using PAR as reagent [15]. Working solutions were prepared daily by diluting the appropriate volume of this stock solution with distilled water.
Buffer solutions
Buffer solutions of various pH values were prepared by mixing 1 M hydrochloric acid and 1 M of sodium acetate (pH 1.0-3.0), 0.2 M acetic acid and 0.2 M sodium acetate (pH 3.5-7.0), 0.2 M acetic acid and 0.1 M sodium acetate (pH 7.0) and 2M ammonium chloride and 2 M ammonium hydroxide (pH 8.0-10.0) solutions in appropriate ratios. The pH of the solutions was checked with pH meter.
Cityl tri methyl ammonium bromide solution
A 1% solution of cityl tri methyl ammonium bromide (Sigma chemicals) was prepared by dissolving one gram in hot distilled water and diluting to the volume in a 100 ml volumetric flask.
PREPARATIONS OF SAMPLE SOLUTIONS
Zinc effluents [16]
The effluents collected from the zinc smelting industry Hindustan zinc Ltd, Visakhapatnam, were filtered and then treated with NH4OH solution (2 ml) to precipitate zinc as zinc hydroxide. This was filtered through whatman (No 40) filter paper. The filtrate collected was diluted to 50 ml with distilled water. Different aliquots were taken different 10ml volumetric flasks and the pH was adjusted to 6.5 with NH4Cl (2M) solution and then treated with suitable amounts of HNAHBH, 1% CTAB and 1000 μg of tartrate (to mask copper). The resultant solutions were diluted to the volume with distilled water and the absorbance was measured at 450 nm. From the measured absorbance values, the amounts of indium were computed from predetermined calibration plot.
Alloy sample
The synthetic sample of an alloy of gold, silver and indium (15: 4: 1) is used as decorative metal in jewellery. The amount of indium present in this alloy was determined by the proposed method after preparing the alloy sample according to the recommended procedure [17]. A known amount of alloy was dissolved in minimum volume of in dilute HNO3 and diluted to known volume with distilled water. The amount of indium present in known aliquot solution was determined from the measured absorbance values and pre-determined calibration plot.
PROCEDURE
Direct spectrophotometry
In each of a set of 10 ml volumetric flasks, 4 ml of buffer solution (pH 6.5), and 0.3 ml of HNAHBH (1x10-2M) and 0.5 ml of CTAB (1%) were taken and various volumes of 1x10-4M indium (III) solution were added. The resultant solutions were made up to the mark with distilled water. The absorbance was measured at 450 nm against the reagent blank. The calibration plot was prepared by plotting the absorbance against the amount of indium (III).
Derivative spectrophotometry
Second and third order derivative spectra were recorded for the above solutions with a scan speed of fast (nearly 2400 nm min-1) and split width of 1 nm with nine degrees of freedom in the wavelength region 350-600 nm. The derivative amplitudes were measured at 445 nm, 480 nm for the second derivative and at 433.5 nm, 465 nm and 492 nm for the third derivative curves and plotted against amount of indium (III) to obtain the calibration plots.
RESULTS AND DISCUSSION
Direct method
Absorption spectrum
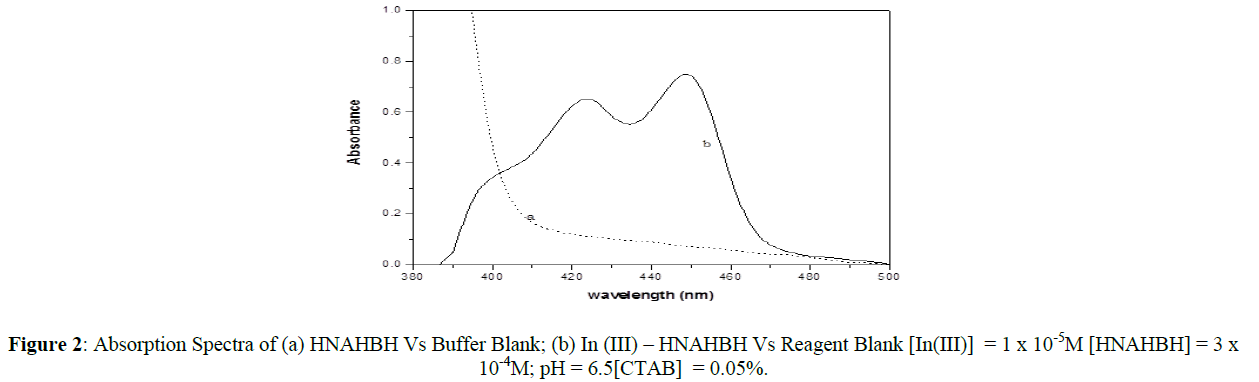
The absorption spectra of greenish yellow colored [In(III)-HNAHBH] solution and almost colorless HNAHBH solution at pH 6.5 were recorded in the wave length region 380 – 500 nm against the reagent blank and buffer blank respectively and presented in figure 2. From the spectrum, it can be seen that there is a small unresolved peak at 400 nm, another clear peak at 425 nm and another peak with maximum absorption at 450 nm. The absorption for the reagent blank is considerably high at 400 nm and less at 425 nm and 450 nm. The analytical studies were carried out at 450 nm as the absorbance for the complex was maximum and the absorbance of the blank was minimum at this wavelength.
Effect of pH
Studies on the effect of variation in pH on the absorbance of the greenish yellow colored [In(III)-HNAHBH] solution showed that the color intensity exhibits maximum and constant absorbance in the pH range 6.0-7.0. Therefore the studies were carried out at pH 6.5.
Effect of reagent concentration
Colour intensity of the experimental solution was found to increase with the increase in the reagent concentration attaining maximum color intensity in the presence of 30 fold excess of the regent. Hence the required reagent concentration (30 fold excess) was maintained throughout the studies.
Effect of surfactants
In the presence of some suitable surfactants, the absorbance of [In(III)-HNAHBH] solution was found to increase. Therefore, the effect of triton X-100, cetyl pyridinium chloride (CPC), sodium dodecyl sulphate (SDS), poly vinyl alcohol (PVA) and cityl tri methyl ammonium bromide (CTAB) on the absorbance of experimental solution was studied and found that of all the surfactants tested, 0.05% of CTAB gives maximum absorbance. Hence the analysis of indium was carried out by measuring the absorbance of the experimental solutions in the presence of 0.05% CTAB.
Validity of Beer’s law
The absorbance data measured for experimental solutions containing different known amounts of indium (III) fitted into a straight line equation A450 = 0.7584C -0.005. Beer’s law was obeyed in the concentration range 0.057-2.869 μg mL-1 of indium. The molar absorptivity, Sandell’s sensitivity, detection limit, determination limit, relative standard deviation, correlation coefficient and other statistical data of the direct method are evaluated and presented in table 1.
| Parameter | Direct method | Second derivative | Third derivative | |||
|---|---|---|---|---|---|---|
| 450 nm | 445 nm | 480 nm | 433.5 nm | 465 nm | 492 nm | |
| Beer’s law range (μg mL-1) | 0.057- | 0.011- | 0.008- | 0.005- | 0.006- | 0.018- |
| 2.869 | 2.181 | 2.181 | 2.181 | 2.181 | 2.181 | |
| Angular coefficient (m) | 0.7584 | 0.9056 | 1.0442 | 0.4321 | 1.6789 | 1.3157 |
| Y-intercept (b) | -0.005 | 0.0015 | 0.0054 | 0.0028 | 0.0104 | 0.0074 |
| Correlation coefficient(r) | 0.9998 | 0.9999 | 0.9999 | 0.9998 | 0.9999 | 0.9999 |
| Relative standard deviation (%) | 1.66 | 0.2 | 0.14 | 0.13 | 0.14 | 0.21 |
| Detection limit (μg mL-1) | 0.004 | 0.0036 | 0.0025 | 0.0027 | 0.0023 | 0.0034 |
| Determination limit (μg mL-) | 0.012 | 0.0108 | 0.0075 | 0.0081 | 0.0069 | 0.0102 |
Effect of foreign ions
The effect of various anions and cations on the absorbance of the experimental solution was studied and their tolerance limits were determined. The amount of foreign ion that brings about a change in absorbance by ± 2% was taken as its tolerance limit.
Stoichiometry and stability constant
The stoichiometry of [In(III)-HNAHBH] complex in solution was determined by Job’s variation method, molar and slope ratio methods and obtained as 1:2 (Metal: Ligand). The stability constant of the complex was calculated from the experimental data of Job’s method as 1.11 x 1020.
APPLICATIONS
To substantiate the validity of the proposed direct method, it was applied for the determination of indium in zinc effluents and in some synthetic alloy samples. The results obtained were compared with those obtained by a reference method or certified values and presented in table 2.
| Sample | Indium found (μg mL-1) | Relative error (%) | |
|---|---|---|---|
| Proposed method* | AAS method* | ||
| Zinc effluents | 0.246 ± 0.006 | 0.242 ± 0.002 | 1.65 |
| 1 | |||
| 2 | 0.208 ± 0.009 | 0.210 ± 0.003 | 0.95 |
| 3 | 0.232 ± 0.004 | 0.235 ± 0.001 | 1.27 |
| Alloy | Taken | Found * | |
| (Green gold) | 1.42 | ||
| 75Au;20Ag;5 In | 0.8 | ||
| Composition(%) | 0.5 | 0.504 ± 0.004 | 1.04 |
| 1 | 1.012 ± 0.010 | ||
| 2 | 1.980 ± 0.008 | ||
| *Average of five determinations ± SD | |||
Derivative methods
In order to improve the sensitivity and selectivity of the proposed method, the absorbance data was derivatized twice and thrice and plotted against the wavelength which gave the resultant second and third order derivative curves respectively. The derivative amplitudes at certain wavelengths were found to be proportional to the amount of indium thus obeying Beer’s law. Therefore, the method was exploited to develop second and third order derivative spectrophotometric methods for the determination of indium.
Derivative curves
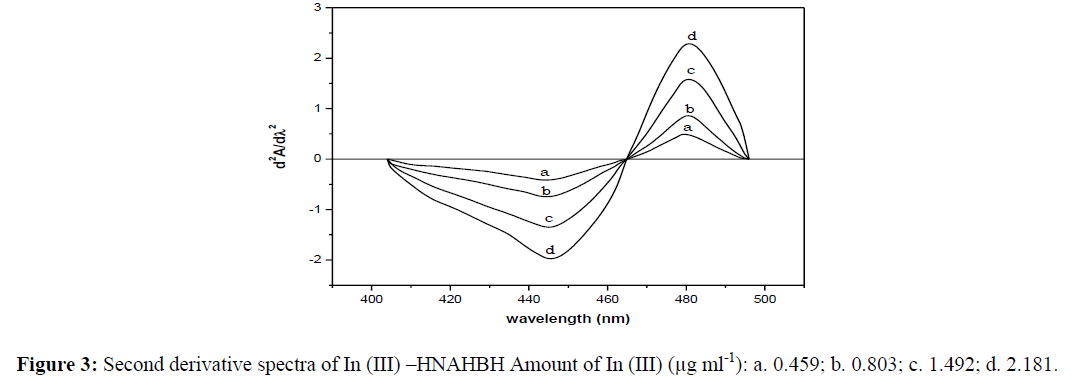
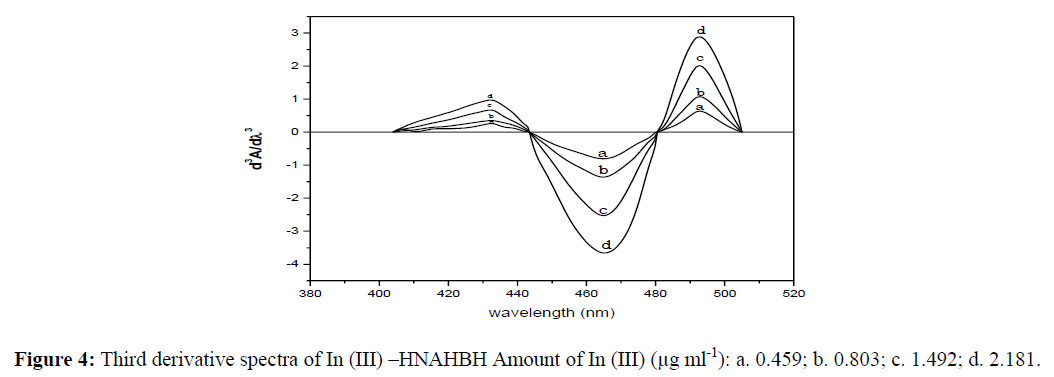
0.018-2.181 μg of indium were treated with buffer solution (pH 6.5), HNAHBH (0.75 ml, 1 x 10-2), and CTAB (0.5 ml, 1%) and diluted to the mark with distilled water in 10 ml volumetric flasks. The second and third order derivative curves were recorded for the above solutions in the wavelength region 350-600 nm and shown in figures 3 and 4 respectively. The derivative amplitudes were measured by peak zero method. The second order curves showed maximum amplitudes at 445 nm and 480 nm with zero cross 465 nm. The third order curves exhibited maximum amplitudes at 433.5 nm, 465 nm and 492 nm with zero cross at 443.5 nm and 480.5 nm.
Determination of indium (III)
The derivative amplitudes of the second order curves measured at 445 nm and 480 nm and the third order curves at 433.5 nm, 465 nm and 492 nm were plotted against the amount of indium. The curves were found to be linear obeying Beer’s law in the range 0.011-2.181 μg mL-1 (445 nm) and 0.008-2.181 μg mL-1 (480 nm) in the second order derivative method. In third derivative method, the Beer’s law ranges were calculated as 0.005 - 2.181 μg mL-1 (433.5 nm), 0.006-2.181 μg mL-1 (465 nm) and 0.018-2.181 μg mL-1 (492 nm). The other analytical and statistical results of the derivative methods are presented in table 1.
Effect of diverse ions
The tolerance limits of some metal ions which showed serious interference in direct method were evaluated in derivative methods and shown in table 3. Except Y (III), all tested ions possess much higher tolerance levels in derivative methods than in direct method. This indicates the greater selectivity of derivative methods compared to direct method (Table 3).
| Foreign ion | Tolerance limit(in folds) | |||||
|---|---|---|---|---|---|---|
| Zero order | Second derivative | Third derivative | ||||
| 445 nm | 480 nm | 433.5 nm | 465 nm | 492 nm | ||
| Ga(III) | <1 | equal | 4 | 4 | 2 | 80 |
| Al (III) | <1 | 70 | 3 | interferes | 6 | 3 |
| La(III) | <1 | 6 | >100 | >100 | >100 | >100 |
| Y(III) | <1 | interferes | equal | interferes | interferes | equal |
| V(V) | <1 | 2 | 4 | >100 | 2 | 5 |
| Pd(II) | <1 | >100 | 10 | Equal | >100 | 60 |
Applications
The third order derivative spectrophotometric method at 465 nm was employed for the determination of indium in some synthetic mixtures of useful compositions and in reference materials. The results obtained are given in table 4. The recovery percentages and the relative standard deviations of the results obtained indicate that the proposed derivative method is quite suitable for the analysis of indium containing natural materials.
| Sample & Composition | Amount of indium (μg mL-1) | RSD | |
|---|---|---|---|
| (%) | |||
| Taken | Found*(recovery percentage) | ||
| S1: 4.0, Ga; 2.6,In; 3.4,Tl; 90.0,SiO2 (in %) | 0.168 | 0.170 (101.2) | 1.12 |
| 0.42 | 0.426 (101.4) | 0.64 | |
| 1.46 | 1.450 (99.3 ) | 0.78 | |
| S2: 40,In; 200,Ca; 200,Ba; 200,Sr | 1.85 | 1.88 (101.6) | 0.82 |
| (in µg) | |||
| Carbon steel (JSS, 061-2) | 1.58a | 1.57 (99.4) | 0.45 |
| Si,0.26; C,0.64;Cu,0.061; Cr,0.019; i,0.019;P,0.01; Mn,0.49; S,0.012; Al,0.034; N,0.038(in %) | |||
| 2.06a | 2.08 (100.9) | 0.42 | |
| *Average of six determinations a: amount of indium added. | |||
CONCLUSION
The results of the present direct and derivative methods were compared with those of some of the already reported methods and presented [18-25] in table 5. The comparison shows that both direct and derivative methods which proposed are more sensitive than the majority of the reported methods. Further, the derivative methods are found to be more selective than the large number of the reported methods. Thus, enhancing the applicability of methods in the analysis of natural complex materials (Table 5).
| Reagent | Beer’s law range (μg mL-1) |
ε x 104 (L mol-1cm-1) |
Interference | |
|---|---|---|---|---|
| 2-(5-Bromo-2-pyridylazo)- 5-diethylaminophenol and cetylpyridinium chloride |
0.07-1.52 | 5.85 | Ga(III),Tl(III),Cu(II),Co(II), Ni(II),Mn(II),Zn(II),EDTA |
13 |
| Alizarin red S | 0.3-2.8 | 0.265 | EDTA, citrate and many metal ions interferred | 18 |
| 4-Hydroxy-3-salicylidene amino-benzene sulphonic acid | 0.04-2.0 | 3.16 | Al(III),Ga(III),Cu(II),Fe(III) | 8 |
| 4(2-Pyridylazo)resorcinol | 0.18-3.7 | 3.0 | Al(III),Ga(III),Tl(III) | 10 |
| 1-(2-Pyridylazo)-2-naphthol and cetyltrimethylammonium bromide | - | 1.91 | Cu(II),Zn(II),Ni(II),Co(II), Fe(II),Fe(III),V(V) |
19 |
| 1-(2-yridylmethyli deneami no)-3-(salicylideneamine) thiourea | 0-1.5 | 6.2 | Numerous cations and anions | 20 |
| 2-Oxoguanidine benzoic acid | 0.57-2.3 | - | Ni(II),Hg(II),Cu(II),Sb(II), Bi(III),EDTA |
21 |
| 1-(2-Thiazolylazo)-2-naphthol | 1-3.2 | 2.15 | Ni(II),Cu(II),Ce(III),Sb(II) EDTA |
22 |
| Bromopyrogallolred and cetyltri-methylammonium bromide | 2.9-23.2 | 4.0 | - | 23 |
| Polymer blend 7-(4-formyl- -phenylazo)-8-quinolinol |
0-1.2 | 3.16 | - | 24 |
| 2,4,6-Tris(1-hydroxy-4-sulpho-naphthyl-2-azo)pyrimidine | 0.4-2.4 | 3.65 | Cu(II),Hg(II),Sn(II),EDTA, PO43- |
25 |
| 2-Hydroxy-1-naphthaldehyde-p-hydroxybenzoichydrazone | 0.057-2.869 µg/ml | 8.8 | Interference from Mn(II), Tl(III),U(VI),Mo(VI),Hg(II), Fe(II),Sn(II), Cu(II), Zn(II), Co(II), Ni(II) is eliminated by masking agents. |
Present (Direct) method |
| 2-Hydroxy-1-naphthaldehyde-p-hydroxybenzoichydrazone | 0.008-2.181 µg/ml | - | Tolerance levels of Ga(III),Al(III), La(III), V(V) and Pd(II) are higher than those in zero order method | Present (Third derivative) method |
ACKNOWLEDGEMENTS
The authors are thankful to the authorities of Sri Krishnadevaraya University, Anantapur for providing necessity facilities for carry out this research work.
REFERENCES
- Miyaki K, Hosoda K, Hirata M, et al., J Occup Health. 2003, 45: p. 228.
- Cheng KL, Goydish BL. Anal Chim Acta. 1996, 34: p. 154.
- Huang L, Sang W, Lu S, et al., Fenxi Huaxue, 1984, 12: 556.
- Shijo Y, Shimizu T, Sakai K. Bull Chem Soc Jpn. 1983, 56: p. 105.
- Beschetnova ET, Malinovskaya LN, Golovina AP, et ., Chem Abstr. 1972, 27: p. 2152.
- Marczenko Z, Kalowska H. Chem Anal (Warsaw). 1980, 25: p. 555.
- Solanke KR, Khopkar SM. Anal Chim Acta. 1973, 66: p. 307.
- Deguchi M, Ebisuya N, Morishige K. Bunseki Kagaku. 1984, 33: p. 678.
- Chen X, Han C, Yuan Z. FenxiHuaxue. 1984, 12: p. 135.
- Agarwal YK, Bhatt VJ. Analyst. 1986, 111: p. 57.
- Balog IS, Kish PP, Bagreev VV, et al., Analit Khim. 1990, 45: p. 289.
- Singh VK, Agnihotri NK, Singh HB, et al., Talanta. 2001, 55: p. 799.
- Agnihotri NK, Ratnani S, Singh VK, et al., Anal Sci. 2003, 19: p. 1297.
- Brown EV, Caglioti L, Paolucci G, et al., Academic Press, New York. 1975, p. 6.
- Marczenko Z. Van Halsted press, New York. 1976, p. 291.
- Sharma M, Chandrawanshi SK, Patil KS. Environ Monit Assess. 1996, 41: p. 247.
- Ishwarsingh, Rakeshsaini. Indian J Chem. 1994, 33a: p. 440.
- Otomo M, Tonosaki K. Talanta. 1971, 18: p. 438.
- Sharma RL, Singh HB. Analyst. 1986, 111: p. 551.
- Rosales D, Millan I, Ariza JLG. Talanta. 1986, 33: p. 607.
- Piwowarska B, Buhl F, Ciba T. Chem Anal (Warsaw). 1986, 31: p. 494.
- Charyulu JK, EshwarMC. J IndianChem Soc. 1987, 64: p. 70.
- Jadhav SG, Venkateswarlu Ch. Indian J Chem. 1989, 28A: p. 820.
- Liang S, Zang E. Fresenius Z Anal Chem. 1989, 334: p. 511.
- Singh I, Saini R. Indian J Chem. 1994, 33A: p. 440.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref