Research Article - Der Pharma Chemica ( 2021) Volume 13, Issue 3
Dyeing of cotton fabric with Eco-friendly Natural Dye obtained from Tecoma capensis using single mordants
Kumaresan M*Kumaresan M, Department of Chemistry, Arasu Engineering College, Kumbakonam, Tanjore, India, Email: mkumsrenu@gmail.com
Received: 29-Jan-2021 Accepted Date: Mar 22, 2021 ; Published: 30-Mar-2021
Abstract
Cotton fabric was dyed with natural dyes extracted from the flower of Tecomacapensis. The fastness properties of the dry dye finishing material and colour strength of dyed fabric were determined and the comparison was studied. Based on the comparative study, Tecomacapensis dye in simultaneous mordanting method with 3% mordant combination gives better results.
Keywords
Fastness, Mordant, Natural dye, Tecomacapensis, Cotton
Introduction
Before the 19th century natural dyes imparts colour for the textiles, pots and decorative purposes. Recently, there is an interest in the use of natural dyes; due to the result of stringent environmental standards was given by many countries. The reason is the toxic and allergic reactions associated with synthetic dyes. Environmental pollution is due to the discharge of dyeing industry effluents which results more pollution problems. About 150 years before all dyes were natural substances, derived from minerals, plants and animals. The natural dyes obtained from the parts of various plants and animals are colour giving molecules[1] which impart colour to the textile materials (Figure 1).
The present investigation deals with the extraction of natural dyes from the flower of Tecomacapensis grows to 2–3 m (7–10 ft) in height and a similar width. Normally evergreen, it may lose its leaves in colder climates. In certain habitats it may scramble, meaning that it shoots out long growth tips which lean on the stems and branches of other plants grow in all warm and damp parts of India. The flowers are tubular, narrow, about 7.5 cm (3 in) long, and are produced at different times throughout the year. They are grouped in 10–15 cm (4–6 in) long terminal clusters. The flower colour ranges from orange to orange-red to apricot.
Tecomacapensis has been in cultivation for many years and is often used for hedging, as it is a scrambling shrub. It can be propagated from cuttings or by removing rooted suckers during the active growth phase. It can be planted in semi-shade to full sun. Tolerating temperatures down to 5°C (41 °F), it can be grown in mild temperate areas with the protection of a warm wall. Otherwise it can be grown in a container and taken indoors through the winter months. To keep this shrub clean and tidy, it must be pruned back in late winter to promote new growth and flowers. The application of a balanced fertilizer after pruning will enhance the growth and flowering.
Materials and Methods
Materials
Conventionally desized, scoured and H2O2 (1%) a plain cotton fabric (220 ends/ dm, 180 picks/dm,120 g/m2) fabric obtained from Gandhi Trust, Dindugal, was used for this study. Analytical reagents (AR) grade stannous chloride, potassium dichromate aluminium sulphate, nickel sulphate, ferrous sulphate, commercial grade acetic acid, common salt, sodium carbonate were used. An excellent natural mordant myrobolan (Terminaliachebula) powder was used for the study. Depending upon the mordant used, the flower of Tecomacapensis extract gave varieties of shades.
Methods
Extraction of colour component
For the optimizing [2-4] process, the ethanol extraction of dye liquor was carried out under varying conditions, such as temperature of extraction, time of extraction and material-to-liquor ratio. In each and every cases, the optical density or absorbance value at a particular maximum absorbance wavelength (λ420nm) for the ethanol extract of plant parts were estimated by using Hitachi-U-2000 UV-VIS absorbance spectrometer.
Dyeing of cotton with the extract of flower of Opuntiaficus-indica
The wetted out samples of cotton were entered into dye baths containing required amount of dye extract and water. After 10 minutes, required amount of sodium carbonate and sodium chloride were added. The dyeing was carried out for one hour at 60°C. The samples were dried in air without washing to make them ready for pre, simultaneous and post-mordanting using myrobolan and metallic salts.
Pre-Mordanting of fabric with myrobolan and metallic salts
The cotton samples with or without pre-mordanting were further mordanted prior to dyeing using 1-3% of any one of the chemical mordants, such as, stannous chloride, nickel sulphate, potassium dichromate, aluminium sulphate copper sulphate and the myrobolan, at 60°C for 30 min with material-to-liquor ratio of 1:20. Then the cotton samples were treated with metal salts followed by the dye extract.
Simultaneous mordanting of cotton samples with myrobolan and metallic salts
The cotton samples were treated with both dye extract and metal salts simultaneously, using 1-3% of any one of the chemical mordants, such as potassium dichromate, stannous chloride, copper sulphate, aluminium sulphate, nickel sulphate and the myrobolan, at 60°C for 30 min with material-to-liquor ratio of 1:20.
Post-Mordanting of cotton samples with myrobolan and metallic salts
The samples were dyed with dye extract. The cotton samples were entered into different dye baths containing required amount of dye extract and water. After 10 minutes, required amount of sodium sulphate was added. After 20 minutes, required amount of sodium chloride was added. The dyeing was carried out for one hour at 50°C. The dyed cotton samples were taken out, squeezed and used for treatment with metal salts process without washing. The dyed samples were treated with different metal salts using 1-3% of any one of the chemical mordants, such as potassium dichromate, stannous chloride, aluminium sulphate, nickel sulphate, copper sulphate and the myrobolan, at 60°C for 30 min with material-to-liquor ratio of 1:20.
In all the three dyeing methods, after the dyeing is over, the dyed samples were repeatedly washed with water and then dried in air. Finally, the dyed cotton samples were subjected to soaping with 2gpl soap solution at 50°C for 10 min, followed by repeated water wash and drying under sun light.
Determination of surface colour strength (K/S value)
The K/S value of the undyed and dyed sample was determined by measuring surface reflectance of the samples using a computer-aided Macbeth 2020 plus reflectance spectrophotometer, [5-7] using the following Kubelka Munk equation with the help of relevant software:
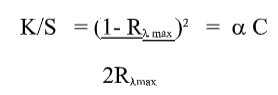
Where, K is the coefficient of absorption; S the coefficient of scattering; Cd, the concentration of the due and Rλmaxthe surface reflectance value of the sample at a particular wavelength, where maximum absorption occurs for a particular dye/colour component.
Evaluation of Colour Fastness
The colour fastness [8] to washing of the dyed samples was determined as per IS: 764-1984 method using a Sasmira launder-O-meter following Is-3 wash fastness method. The wash fastness rating was assessed using grey scale as per ISO-05-A02 (loss of shade depth) and ISO-105-AO3 (extent of staining) and the same was cross-checked by measuring the loss of depth of colour and staining using Macbeth 2020 plus computer-aided colour measurement system attached with relevant software. Colour fastness to rubbing (dry and wet) was assessed as per IS: 766-1984 method using a manually operated crock meter and grey scale as per ISO-105-AO3 (extent of staining).
The colour fastness to exposure to light (Sun) was determined as per IS: 2454-1984 method. The sample was exposed to UV light in a Shirley MBTF Microsal fade-O-meter (having 500 watt Philips mercury bulb tungsten filament lamp simulating day light) along with the eight blue wool standards (BS 1006: BOI: 1978). The fading of each sample was observed against the fading of blue wool standards (1-8).
The colour fastness to perspiration assessed according to IS 971-1983 composite specimen was prepared by placing the test specimen between two adjacent pieces of fabrics of cotton and stitched all among four sides. The sample was soaked in the test solution (acidic/alkaline) separately with MLR 1:50 for 30 minutes at room temperature. The sample was then placed between two glass plates of perspirometer under load of 4.5kgs (10 lbs). The apparatus was kept in the oven for four hours at 37 ± 2°C. At the end of this period the specimen was removed and dried in air at a temperature not exceeding 60°C. The test cotton samples were graded for change in colour and staining using grey scales.
Results and Discussions
The colour strength values of cotton samples with the flower of Tecomacapensis obtained in this study by using single mordanting method are presented and compared in Tables 1, 2 and 3.
| Mordant concentration:1% | K/S(λ=420 nm) | ||
|---|---|---|---|
| Pre mordanting | Simultaneous mordanting |
Post mordanting | |
| Nickel sulphate | 1.50 | 2.48 | 2.11 |
| Aluminiumsulphate | 1.57 | 2.83 | 2.68 |
| Potassium dichromate | 1.30 | 1.36 | 1.40 |
| Ferrous sulphate | 1.82 | 2.93 | 2.79 |
| Stannous chloride | 1.73 | 2.60 | 2.44 |
| Myrobolan | 1.23 | 1.28 | 1.36 |
Table 1: Surface colour strength of Tecomacapensis dyedcotton fabric after pre, simultaneous and post mordanting methods by using 1% mordant concentration. K/S value without mordant: cotton-1.52
| Mordant concentration:2% | K/S(λ=420 nm) | ||
|---|---|---|---|
| Pre mordanting | Simultaneous mordanting |
Post mordanting | |
| Nickel sulphate | 1.50 | 2.52 | 2.22 |
| Aluminiumsulphate | 1.81 | 2.87 | 2.67 |
| Potassium dichromate | 1.30 | 1.31 | 1.42 |
| Ferrous sulphate | 1.85 | 3.03 | 2.89 |
| Stannous chloride | 1.71 | 2.74 | 2.44 |
| Myrobolan | 1.23 | 1.28 | 1.32 |
Table 2: Surface colour strength of Tecomacapensis dyed cotton fabric after pre, simultaneous and post mordanting methods by using 2% mordant concentration. K/S value without mordant: cotton-1.52
| Mordant concentration:3% | K/S(λ=420 nm) | ||
|---|---|---|---|
| Pre mordanting | Simultaneous mordanting |
Post mordanting | |
| Nickel sulphate | 1.47 | 2.51 | 2.21 |
| Aluminiumsulphate | 1.82 | 2.90 | 2.73 |
| Potassium dichromate | 1.33 | 1.30 | 1.48 |
| Ferrous sulphate | 1.92 | 3.13 | 2.84 |
| Stannous chloride | 1.80 | 2.87 | 2.41 |
| Myrobolan | 1.27 | 1.38 | 1.43 |
Table 3: Surface colour strength of Tecomacapensis dyed cotton fabric after pre, simultaneous and post mordanting methods by using 3% mordant concentration. K/S value without mordant: cotton-1.52
From the above results, it was observed that Tecomacapensis showed better colour strength values. In all the three dyeing methods, simultaneous method gave excellent results. Comparing all the three dyeing methods, the mordants ferrous sulphate and aluminiumsulphate show excellent colour strength values. For dyeing of cotton, 1%, 2% and 3% mordant concentrations were used for the present study. Among the three concentrations, 3% mordant concentration gave better results.
Conclusion
Comparing all the three dyeing methods, simultaneous method gave excellent results. Similarly, comparing the three dyeing methods, the mordants ferrous sulphate and aluminiumsulphate show excellent results. From the study of fastness properties and colour strength of the dyed samples, Tecomacapensis in simultaneous mordanting method with 3% mordant combination gives better results
Acknowledgment
The authors are thankful to Dr .T. Balamurugan, Principal, Arasu Engineering College for his support in the analysis of fastness properties of the dyed samples of this work.
References
- B Anderson, Angus and Robinson, Singapore, 1971, p. 24-28.
- S Boonroeng, P Boonkerdrum, Chadee and R Sangkumpra. University of Technology Lanna. 2009, p. 1-7.
- M Kumaresan and PN Palanisamy. Int J App Eng Res. 2010, 5(12): p.2031-2037.
- M Kumaresan, PN Palanisamy and PE Kumar. Nature Environment and Pollution Technology. 2010, 5(12): p.547-542.
- M Kumaresan, PN Palanisamy and PE Kumar. Eur J Sci Res. 2011, 52(3): p. 306-312
- M Kumaresan, PN Palanisamy and PE Kumar. Ind J Fib Tex Res. 2012, 37(2): p.194-198.
- M Kumaresan. Int J Der Pharma Chemica. 2015, 7(4): 257-260.
- M Kumaresan. Management of Environmental Quality. 2016, 27(1): p. 15-21