Research Article - Der Pharma Chemica ( 2022) Volume 14, Issue 6
Eco-friendly Synthesis of α -Aminonitriles Catalysed by Epzg
Rahul Patil1*, Ankush Mali1, Shivaji Burungale1, Uday Lad1, Sanjay Jadhav1 and Uttam More22Department of Chemistry, S. G. M. College, Karad-415 124, India
Rahul Patil, Department of Chemistry, Yashwantrao Chavan College of Science, Karad-415 124, India, Email: rspatilorg@gmail.com
Received: 06-Jun-2022, Manuscript No. dpc-22-65962; Editor assigned: 08-Jun-2022, Pre QC No. dpc-22-65962; Reviewed: 22-Jun-2022, QC No. dpc-22-65962; Revised: 23-Jun-2022, Manuscript No. dpc-22-65962; Published: 30-Jun-2022, DOI: 10.4172/0975-413X.14.6.31-37
Abstract
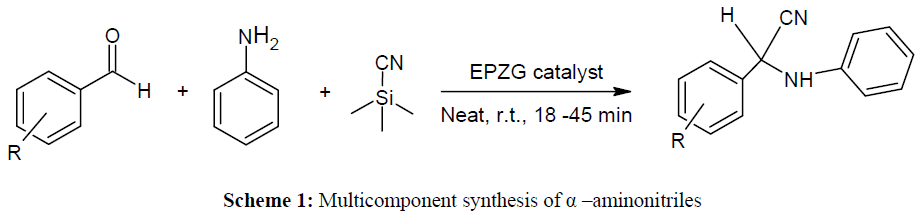
A simple, proficient and ecofriendly procedure has been developed for the three component coupling of aromatic aldehyde, substituted amines and trimethylsilyl cyanide produce α-amino nitriles. The α-amino nitriles are synthesized in high yields (90-91%) in a few minutes (18-45 min) under solvent-free conditions using EPZG catalyst at room temperature.
Keywords
α-amino nitriles; EPZG; Ecofriendly; Room temperature
INTRODUCTION
Nitriles are the building blocks of most biologically active substances and natural products. However, amides and carboxylic acids [1-2] have synthesized from nitriles, due to this α-aminonitriles have occupied great position in synthetic organic chemistry, this growing demand of nitriles, satisfied by Strecker reaction [3]. Strecker in 1850 reported the synthesis of α-aminonitriles by multicomponent condensation of aldehyde, amine and hydrogen cyanide [3] hydrocyanation of emines is thus basic C-C bond formation reaction [4] involves conversion of nitriles to carbonyl group [5-6]. Modified Strecker reaction i.e. synthesis of optically active α-amino acid by the hydration of cyanide [7], α-aminonitriles is acts a precursor fragment for the synthesis of α-amino acid [8], imidazole and several biologically active compounds [9] containing nitrogen atom. Bifunctionality of α-aminonitriles acts as a building blocks of pharmaceutical industries [11], such as serine protease inhibitors [12], (-, +)phtalascidine 650 [13] and also in the synthesis of boron containing retinoids [14]. Synthesis of heterocyclic moiety such as 1,2,3-diazaphospholidines, imidazole, oxazoles, and isothiozoles [15] derived from 2-amino-2-alkyl(aryl) propanenitriles as a starting material. Synthesis of 5-amino-4H-imidazoles was achieved by reacting α-aminonitriles with imidoester which is a key material of many biological compounds. Different ptotocols have been eported for the synthesis of α–aminonitriles such as Formic acid [16], ammonium chloride [17], PPh3/DEAD[18], Bicyclic Guanidine [19], polyethylene glycol (PEG-OSO3H) [20], MgI2 [21], sulphated polyborate [22], PEG -400 [23], Zn(CN) [24], cinchona-based thiourea alkaloid [25], 5mol % to 20mol % L –prolineamide derived N,N’-dioxide [26], Ga(OTF)3[27], Nafion –H and NafionSAC-13 [28], SBA-15 supported sulphonic acid [29], indium (III) iodide [30], mesoporous MCM-41 catalyst [31], ionic liquid [bmim]BF4 or MgBr2.OEt2 [32], Bismuth Nitrate [33], Fe3O4@SiO2@Me&Et-PhSO3H [34], Task- Specific ionic liquid [35], chiral ammonium trifluoroacetate, potassium hexacyanoferrate (II) [36], Silica based Scandium (III) [37], Pd(II) [38], magnetically separable nanoparticles [39,40]. We have reported here environmentally green EPZG as catalyst for the synthesis of α–aminonitriles. EPZG is a FeCl3 supported on clay.
Present work
It was clear from the literature review that α–aminonitriles has greater utility in medicinal chemistry as well as in agricultural fields. we have report herein Lewis acid EPZGR [41-51] catalyzed solvent free synthesis of α–aminonitriles at room temperature (Scheme 1) (Table 1-4).
| Sr. No | catalyst | Amount | Time in minute | Yield |
|---|---|---|---|---|
| mg | % | |||
| >1 | EPZGR | 10 | 40 | 60 |
| 2 | EPZGR | 15 | 34 | 88 |
| 3 | EPZGR | 20 | 30 | 91 |
| 4 | EPZGR | 25 | 30 | 91 |
| 5 | EPZGR | 30 | 30 | 91 |
| Reaction condition: Benzaldehyde (1mmol); aniline (1mmol); trimethylsilyl cyanide (1mmol); EPZGR catalyst 20mg; at room temperature. | ||||
| Entry | Solvent | Time (Min.) | Yield% |
|---|---|---|---|
| >1 | Water | 60 | 69 |
| 2 | Alcohol | 52 | 58 |
| 3 | Solvent free | 30 | 91 |
| 4 | Chloroform | 64 | 69 |
| 5 | Dichloromethane | 70 | 54 |
| 6 | Dimethylsulphoxide | 90 | 53 |
| Reaction condition: -Benzaldehyde (1mmol); aniline (1mmol); trimethylsilyl cyanide(1mmol); EPZGR catalyst 20mg; at room temperature. | |||
| Sr. No. | Catalyst | Amount mg | Time in minute | Yield% |
|---|---|---|---|---|
| >1 | EPZGR | 20 | 30 | 91 |
| 2 | EPZGR | 20 | 30 | 91 |
| 3 | EPZGR | 20 | 31 | 90 |
| 4 | EPZGR | 20 | 32 | 89 |
| 5 | EPZGR | 20 | 32 | 88 |
| Reaction condition: Benzaldehyde (1mmol); aniline (1mmol); trimethylsilyl cyanide (1mmol); EPZGR catalyst 20mg; at room temperature. | ||||
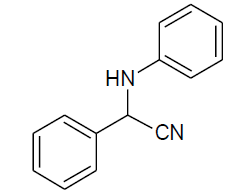
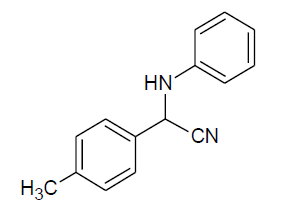
| Entry | Aldehyde | Amine | Product | Time min. | Yield % |
|---|---|---|---|---|---|
| > a |
|
 |
 |
30 | 94 |
| b | 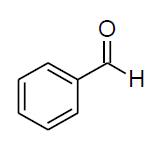 |
 |
 |
30 | 91 |
| c | 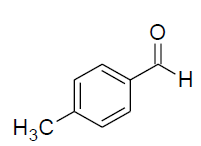 |
 |
 |
18 | 93 |
| d | 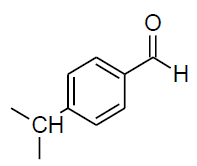 |
 |
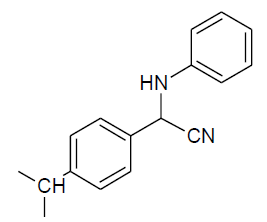 |
29 | 91 |
| e | 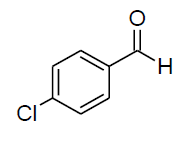 |
 |
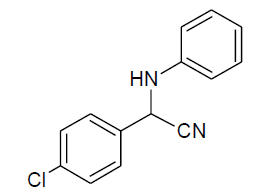 |
21 | 93 |
| f | 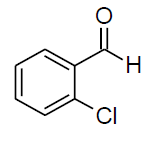 |
 |
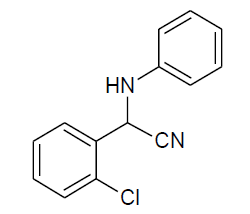 |
45 | 87 |
| g | 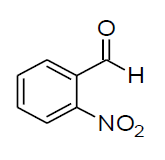 |
 |
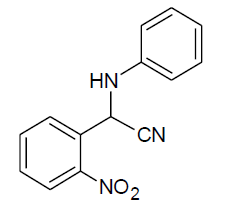 |
22 | 89 |
| h | 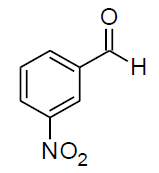 |
 |
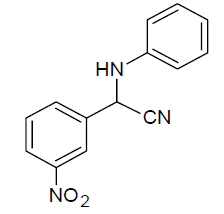 |
27 | 90 |
| i | 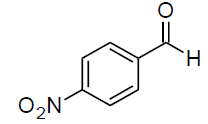 |
 |
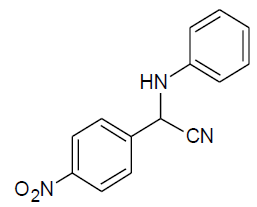 |
20 | 92 |
| j | 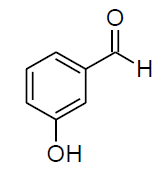 |
 |
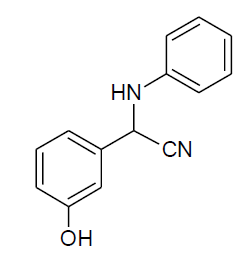 |
42 | 83 |
| k | 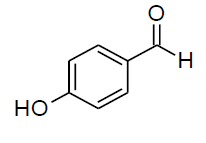 |
 |
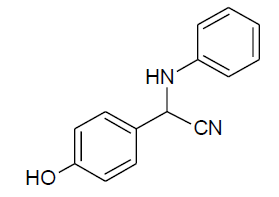 |
25 | 87 |
| l | 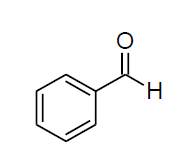 |
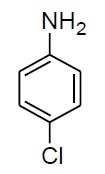 |
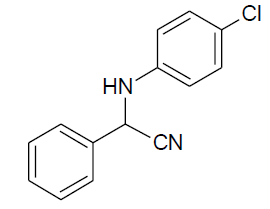 |
27 | 90 |
| m |  |
 |
 |
32 | 91 |
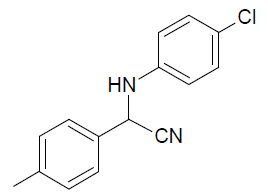
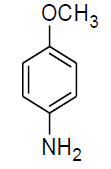
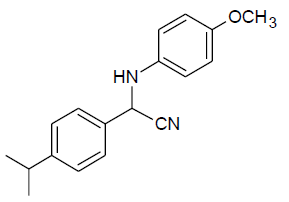
| n | 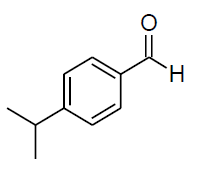 |
 |
 |
31 | 89 |
| o | 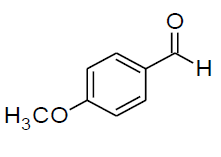 |
 |
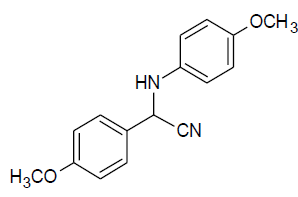 |
30 | 88 |
| Reaction condition: Benzaldehyde (1mmol); aniline (1mmol); trimethylsilyl cyanide (1mmol); EPZGR catalyst 20mg; at room temperature. | |||||
EXPERIMENTAL SECTION
General
Various substituted aldehyde (Sigma-Aldrich), aniline (Himedia), Trimethylsilyl cyanide (Himedia) were used as received without purification. Melting point measures by melting point apparatus made by Equiptronics model No – EQ- 730A. IR spectra were recorded on FT-IR -7600 Lambda Scientific Spectrometer. NMR Spectra were recorded on a Bruker AC400 MHz spectrometer in CDCl3 using tetramethyl silane as an internal standard material.
General Experimental Procedure
In a 25ml round bottom flask mixture of aldehyde (1mmol), aniline (1mmol) and Trimethylsilyl cyanide (1mmol) was stirred with EPZGR catalyst 20 mg at room temperature for desired time mentioned in Table 2; Entry b completion of reaction was monitored by TLC. Upon completion of reaction crystallization of the product was carried out in ethanol. All the products were purified by same technique and found to be correct. Further structures of the product were confirmed by 1H NMR, 13C NMR, HRMS and IR.
CONCLUSION
We have developed simple process of synthesis of α-aminonitriles by reacting aldehyde, amine and Trimethylsilyl cyanide. New process which was easily applicable, easy separation of product and catalyst, short reaction time at room temperature. Recyclability of the catalyst was found to be efficient for successive five times without loss of its activity.
Spectral data of synthesized α-aminonitriles.
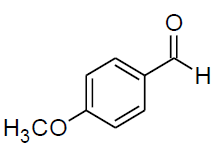
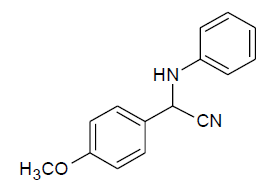
Table 3, Entry a : 2-(4-methoxyphenyl)-2-(phenylamino)acetonitrile, Color: White; M. P.: 94-96ºC; IR (KBr): 3386, 3029, 2249, 1892, 1594, 1498, 1428, 1262, 1192, 1105, 834, 746, 675cm-1;1H NMR (400MHz, CDCl3):δ (ppm):3.86(s, 3H, -OCH3), 4.00-4.02(d, 1H, -NH,J = 8Hz), 5.38- 5.40(d, 1H, -CH, J = 8Hz), 6.78-7.54(m, Ar-H);13C NMR (100 MHz, CDCl3 ): δ (ppm):49.68, 55.45,114.10, 114.65, 118.44, 120.20,125.95, 128.65, 129.58,114.75, 160.45; HRMS:238.16;Calculated mass: 238.28.
Table 3, Entry b : 2-(phenyl)-2-(phenylamino)acetonitrile, Color: White; M. P.: 80-82ºC; IR (KBr):3317, 3036, 2242, 1918, 1994, 1489, 1393, 1271, 1096, 999, 746cm-1;1H NMR(400MHz, CDCl3):δ (ppm): 4.04-4.06 (d, 1H, -NH, J = 8Hz),5.44-5.46 (d, 1H, -CH, J = 8Hz),6.78-7.63 (m, Ar- H); 13C NMR (100 MHz, CDCl3):δ (ppm): 50.19,114.12,118.21, 120.29, 127.29, 129.37, 129.58, 129.60, 133.89, 144.65;
Table 3, Entry d : 2-(4-isopropylphenyl)-2-(phenylamino)acetonitrile, Color:White, M. P.:78-80 ºC.IR (KBr): 3395, 2949, 2897, 2242, 1918, 1603, 1515, 1441, 1296, 1105, 912, 825, 746, 684cm-1;1H NMR(400MHz, CDCl3):δ (ppm): 1.27-1.29 (d, 6H, -CH3, J = 8Hz), 2.93-3.00 (m, 1H, - CH, J = 8Hz),4.00-4.02 (d, 1H, -NH, J = 8Hz), 5.39-5.41 (d, 1H, -CH, J = 8Hz),6.78-7.54 (m, Ar-H); 13C NMR (100 MHz, CDCl3 ): δ (ppm): 23.91, 33.92, 49.94, 114.01, 118.37, 120.16, 127.34,127.43,129.58,131.25,144.75,150.57;
Table 3, Entry f : 2-(2-chlorophenyl)-2-(phenylamino)acetonitrile, Color: White; M. P.: 62-64ºC;IR (KBr):3335, 3020, 2249, 1927, 1594, 1498, 1296, 1236, 1061, 947, 755, 675cm-1; 1H NMR(400MHz, CDCl3): δ (ppm): 4.03-4.0 (d, 1H, -NH, J = 8Hz), 5.73-5.75 (d, 1H, -CH, J = 8Hz), 6.79-7.77 (m, Ar-H);13C NMR (100 MHz, CDCl3):δ (ppm):48.03, 114.24,117.76,120.48,127.82,129.07, 29.60,130.49,131.07,131.7, 133.55,142.52;
Table 3, Entry h : 2-(3-nitrophenyl)-2-(phenylamino)acetonitrile, Color: Yellow; M. P.: 86-88ºC; IR (KBr):3323, 3107, 3062, 2243, 1920, 1613, 1541, 1543, 1226, 1082, 893, 758, 975cm-1; 1H NMR (400MHz, CDCl3):δ (ppm): 4.20-4.22 (d, 1H, -NH, J = 8Hz), 5.58-5.60 (d, 1H, -CH, J = 8Hz), 6.78-8.51 (m, Ar-H);13C NMR (100 MHz, CDCl3 ): δ(ppm): 49.62, 114.58, 117.19,121.09,122.38, 124.53, 129.72,130.50,133.05, 136.09, 143.89, 148.75;
Table 3,Entry j : 2-(3-hydroxyphenyl)-2-(phenylamino)acetonitrile, Color : White; M.P.:86-88ºC IR (KBr): 3402, 3072, 2249, 2010, 1615, 1504, 1348, 1275, 1063, 854, 794, 676cm-1; 1H NMR (400MHz, CDCl3): δ (ppm): 3.98-4.00 (d, 1H, -NH, J = 8Hz), 5.43-5.45 (d, 1H, -CH, J = 8Hz), 6.66-7.74 (m, Ar-H), 9.38 (bs, HO-Ar); 13C NMR (100 MHz, CDCl3): δ(ppm): 49.20,113.96,114.31, 116.30, 117.95, 118.88, 129.08, 129.22, 130.10, 131.08, 132.04, 132.06, 132.09, 133.11, 133.17, 145.77, 158.03; 130.23, 133.34, 133.50, 133.86, 133.94, 133.99, 135.52, 136.99, 150.55, 152.38, 162.95, 165.38, 168.45;
Table 3, Entry l : 2-(phenyl)-2-(4-chlorophenylamino)acetonitrile, Color: White; M. P.:80-82 ºC;IR (KBr): 3317, 3045, 2242, 1603, 1489, 1262, 1069, 834, 693 cm-1; 1H NMR( 400MHz, CDCl3): δ (ppm): 4.09-4.11(d, 1H, -NH, J = Hz), 5.38-5.40(d, 1H, -CH, J = 8Hz), 6.69-7.60 (m, Ar-H); 13C NMR (100 MHz, CDCl3 ): δ(ppm):50.28, 115.38, 117.89, 122.22, 125.14, 127.23, 128.85,128.89,129.25,129.43, 129.48, 129.71,133.48,143.20,160.80;
Table 3, Entry n : 2-(4-isopropylphenyl)-2-(4-methoxyphenylamino)acetonitrile, Color: White;M. P.:78-80 ºC;IR (KBr): 3395, 2958, 2224, 1603, 1498, 1236, 1025, 834, 755, 650cm-1;1H NMR( 400MHz, CDCl3):δ (ppm): 1.27-1.29 (d, 6H, 2CH3 ofisopropyl gr.J = 8Hz), 2.93-3.00(m, 1H, -CH of isopropyl gr. J = 8Hz), 3.78 (s, OCH3),5.31-5.33(d, 1H, -CH, J8Hz),6.76-7.54(m, Ar-H); 13C NMR (100 MHz, CDCl3): δ (ppm): 24.02, 34.15,51.28,55.66,114.99,116.14,118.64,127.30,127.34, δ = 131.51,138.43, 154.02;
Table 3, Entry o : 2-(4-methoxyphenyl)-2-(4-methoxyphenylamino)acetonitrile, Color: White; M. P.:134-134 ºC; IR (KBr): 3530, 2942, 2248, 1630, 1460, 1251, 989, 874, 649cm-1; 1H NMR( 400MHz, CDCl3):δ (ppm): 3.49 (s, -OCH3),4.04 (s, -OCH3),5.30-5.32 (d, 1H, -CH, J = 8Hz),6.29-8.14 (m, Ar-H);13C NMR (100 MHz, CDCl3): δ (ppm): 54.17,55.12, 115.22, 117.33, 119.43, 126.19, 127.56,130.49,139.12,156.62;
Table 3, Entry p : 2-(phenyl)-2-(4-nitrophenylamino)acetonitrile, Color: White; M. P.:94-96 ºC ;IR (KBr): 3381,2949, 2246, 1612, 1479, 1269, 1015,843, 664cm-1; 1H NMR( 400MHz, CDCl3) :δ (ppm): 4.96-4.98 (d, 1H, -CH, J = 8Hz),5.56-5.58(d, 1H, -CH, J = 8Hz),6.60-8.43 (m, Ar-H);13C NMR (100 MHz, CDCl3): δ (ppm): 49.14, 114.52, 118.11, 123.02, 124.11, 128.05,128.95,130.14,131.44,132.64, 145.59, 161.12;
Table 3, Entry q : 2-(2-chlorophenyl)-2-(4-nitrophenylamino)acetonitrile, Color: White; M. P.:104-106 ºC ; IR (KBr): 3340, 3012, 2246, 1970, 1592, 1498, 1293, 1242, 1063, 945, 789, 674 cm-1; 1H NMR( 400MHz, CDCl3): δ (ppm): 4.40-4.42 (d, 1H, -CH, J = 8Hz),5.80-5.82 (d, 1H, -CH, J = 8Hz),6.60-8.30 (m, Ar-H); 13C NMR (100 MHz, CDCl3): δ (ppm): 48.72, 115.88, 117.72, 124.19, 129.55, 129.91, 129.98,130.12,130.33,131.27, 132.46, 158.17;
REFERENCES
- Rappoport Z, John Wiley & Sons Ltd, New York, NY, USA, 1970, 52: p. 1737-1746.
- Zhang G, Zheng D, NieJ Wang T, et al., Org Biomol Chem. 2010, 8: p. 1399-1405.
- Strecker A. Liebigs Ann Chem. 1850, 75: p. 27-45.
- Yet L. Angew Chem Int Ed. 2001, 40: p. 875-877.
- Enders D, Kirchhoff J, Gerdes P, et al., Eur J Org Chem. 1998, 1: p. 63-72.
- Danielson M, Falke J. Annu Rev Biophys Biomol Struct. 1996, 25: p. 163-195, 1996.
- Shafran Y, Bakulev V, Mokrushin V. Russ Chem Rev. 1989, 58: p. 148-162..
- Matier W, Owens D, Comer W, et al., J Med Chem. 1973, 16: p. 901-908.
- Martinez, Corey E. Org Lett. 1999, 1: p. 75-78.
- Enders D, Shilvock J. Chem Soc Rev. 2000, 29: p. 359-373.
- ArasappanA, VenkatramanS, Padilla A, et al., Tetrahedron Lett. 2007, 48: p. 6343.
- Razafindrabe C, Aubry S, Bourdon B, et al., Tetrahedron. 2010, 66: p. 9061-9066.
- Das B, Anguiano J, Mahalingam S. Tetrahedron Lett. 2009, 50: p. 5670-5672.
- Drabina P, Sedlak M. Arkivoc. 2012, p.152.
- Avendano C, Ramos T, Gomez-Molinero E. J Heterocycl Chem. 1985, 22: p. 537.
- Roshani M., Ghafuri H. RSC Adv. 2013, p. 1-3.
- Nammalwar B, Fortenberry C, Bunce R. Tetrahedron Lett. 2014, 55: p. 379-381.
- Chaturvedi D, Chaturvedi A, Mishra N, et al., Tetrahedron Lett. 2012, 55: p. 5398-5401.
- Li J, Jing W, Han K, et al., J Org Chem. 2003, 68: p. 8786-8789.
- Shekouhy M. Catal Sci Technol. 2012, 2: p. 1010-1020.
- Li P, Zhang Y, Chen Z, et al., Tetrahedron Lett. 2017, 58: p.1854-1858.
- Indalkar K, Khatri C, Chaturbhuj G. Tetrahedron Lett. 2017, 58: p. 2144-2148.
- Hu X, Ma Y, Li Z. J Organomet Chem. 2012, 705: p. 70-74.
- Shah S, Singh B. Tetrahedron Lett. 2012, 53: p. 151-156.
- Dong Y, Tian. Chem Commun. 2012, 48: p. 4899-4901.
- Huang J, Liu X, Wen Y, et al., J Org Chem. 2007, 72: p. 204-208.
- Prakash G, Mathew T, Panja C, et al., Proc Natl Acad Sci. 2007, 104: p. 3703-3706.
- Prakash G, Elizabeth T, Bychinskaya T, et al., Green chem. 2008, 10: p. 1105-1110.
- Karimi B, Zareyee D. J Mater Chem. 2009, 19: p. 8665-8670.
- Shen Z, Jun S, Loh T. Tetrahedron. 2008, 64: p. 8159-8163.
- Maleki. Green Chem Lett Rev. 2018, 11: p. 36-46.
- Safa K, Zeinolabedini A, Abbasi H, et al., J Iran Chem Soc. 2013, 10: p. 447-452.
- Mansoor S, AswinK, Logaiya K. et al., J Saudi Chem Soc. 2016, 20: p. 138-150.
- Mobaraki A, Movassagh B, Karimi B. ACS Comb Sci. 2014, 16: p. 352-358.
- Huang J, Corey E. Org Lett. 2004, 6: p. 5027-5029.
- Zheng Li, Yuanhong Ma, Jun Xu, et al., Tetrahedron Lett. 2010, 51: p. 2022-2026.
- Karimi B, Safari A. J Organmet Chem. 2008, 693: p. 2967-2970.
- Jarusiewicz J, Choe Y, Yoo K. et al., J Org Chem. 2009, 7: p. 74.
- Baghery S, Zolfigol M, Schirhagel R, et al., Nag N Appl Organometal Chem. 2017.
- Rakhtshah J. Sadegh Salehzadeh Res. chem. Intermed. 2017.
- Envirocats-Supported Reagents: Product information, Contract Chemicals, UK, 1994.
- Ponde D, Borate H, SudlaiA, et al., Tetrahedron Lett.1996, 37: p. 4605.
- Bandgar BP, Kasture SP, Tidake K, et al., Green Chem. 2000, 2, 152.
- Upadhya T, Daniel T, Sudlai A, et al., Synth Commun. 1996, 26: p. 4539.
- Sonali Ghandande and Supriya Mahajan. Indian J Chem. 2005, p. 188-192.
- Bandgar BP, Uppalla LS and Sadavarte VS. Green Chemistry. 2001, 3: p. 39-41.
- Bandgar BP, Zirange MB, Wadgaonkar PP. Synlett. 1996, 2: p. 149.
- Bandgar BP, Wadgaonkar PP. Synthetic Communication. 1997, 27: p. 2069.
- Bandgar BP, Hazare CT, Wadgaonkar PP. J Chem Res(S). 1995, 1: p. 90.
- Bandgar BP, Jagtap SR, Ghodeshwar SB, et al., Synthetic Communication. 1995, 25: p. 2993.
- Rahul Patil, Uday Lad, Suresh Shendage, et al., Rasayan J Chem. 2020, 13: p. 1735-1743.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref