Research Article - Der Pharma Chemica ( 2018) Volume 10, Issue 3
Effect of Untreated Olive Mill Wastewater on Seed Germination, Seedling Growth and Biochemical of Maize (Zea mays L.)
Manar Abou-Hassan*, Ahmad Malo and Nadim Almhana
Department of Chemistry, Faculty of Science, Damascus University, Damascus, Syria
- *Corresponding Author:
- Manar Abou-Hassan
Department of Chemistry
Faculty of Science
Damascus University
Damascus, Syria
Abstract
Aims and objectives: To determine the effect of OMW on seed germination and seedling growth of maize (Zea mays L). The study had following objectives: (1) To study the effect of different concentrations of OMW on seed germination and seedling growth of maize. (2) To determine the percentage phytotoxicity of OMW on maize. (3) To determine contents biochemical for both the endosperm and the embryo during germination. Olive Mill Wastewater (OMW) is considered as phytotoxic and thus an environmentally hazardous material, it is one of the most severe environmental factors that reduces and limits growth and development of plants. This study was conducted under laboratory conditions in order to evaluate the effect of OMW at three concentrations on seeds germination and seedling growth of maize (Zea mays L.). Seeds were soaked then placed in petri-dishes and irrigated with 1, 5 and 10% v/v concentrations of OMW. A control was moistened with distilled water. The germination percentage, root and shoot length, phytotoxicity percentage of root and shoot, and contents biochemical like total proteins, oil, total soluble sugars and starch for both the endosperm and the embryo of maize were observed. The results obtained showed beneficial effects using low concentrations of OMW whereas the treated plants with 1% of OMW showed a slight improvement in all the above growth parameters and contents biochemical, but the highest levels 5 and 10% of OMW had a negative effect compared with control.
Keywords
Zea mays L., Olive mill wastewater, Germination, Seedling growth, Morphology parameter, Biochemical contents endosperm, Embryo
Introduction
Olive Mill Wastewater (OMW), a by-product of the olive mill industry, is produced by traditional and industrial olive mills in large amount in Mediterranean countries over a limited time period usually from October-December. The annual production of OMW in these countries reached 30 million cubic meters [1]. OMW is a critical problem, it contains an enormous supply of organic matter, Chemical Oxygen Demand (COD) between 40 and 210 gm-3 and Biochemical Oxygen Demand (BOD5) between 10 and 150 gm-3 [2].
Agricultural irrigation with wastewater effluents became a common practice in arid and semiarid regions, where it was used as a readily available and inexpensive option to fresh water. Some authors indicated that OMW spreading does not result in heavy metal accumulation in the soil. Therefore, they considered that its environmental pollution risk was low [3]. However, recent studies found that the addition of unprocessed OMW causes significant shifts in the structure and function of microbial communities which in turn influences the soil fertility [4]. The removal of this waste is a problem for the whole community in general and for the producers and millers in particular. This is due to the great toxicity that OMW exhibited against microorganism and plant during seed germination and plant growth [5]. In fact, several authors attribute OMW toxicity to their phenolic content, high percentage of salt, acidic pH, as well as short and long chain fatty acids and antimicrobial [6,7].
OMW application causes serious environmental problems due to its antibacterial effects and its phytotoxicity [8]. The high COD value and the presence of phytotoxic and antibacterial polyphenols in OMW can be a serious pollution risk for superficial and underground waters [9]. Moreover, the presence of phenolic compounds in OMW makes them highly toxic and ecologically noxious [10,11].
Maize (Zea_mays L.) is one of the most economically important food crops in the world after wheat and rice, it possesses high nutritive value and is important as a coarse grain [12]. Germination is the first stage and one of the important and sensitive stages of the plant life cycle, it is an important process in seedling growth. This stage of growth is strictly influenced by environmental factors [13].
Keeping in view the importance of maize as major food crop and the excessive use of olive mill wastewater in agricultural irrigation with wastewater effluents the present study has been designed to study the effect of different concentrations of these OMW on germination and seedling growth of maize.
The study was conducted in October of 2016, in the Biochemistry Research Laboratory, Department of Chemistry, Faculty of Science, Damascus University, Syria.
Plant material
The Seeds of maize (Zea mays L. Ghouta 82) was caught from the General Institution for plenitude of seeds (Aleppo-Syria).
Chemical material
All chemicals used were of analytical grade and were purchased from Sigma-Aldrich Co.
Soaking and germination
Healthy seeds of uniform size were used for the experiment, after surface sterilization with 0.1% NaOCl the maize seeds washed thoroughly with distilled water [14]. The seeds were soaked for 24 h at room temperature (24°C) in different concentration of OMW (1, 5 and 10 v/v), used the distilled water as control then allowed to germinate on moist paper towels in perti dishes at 37°C in darkness for 6 days (144 h). The experimental seed were kept moist by regularly adding of test solution if required [15,16].
The endosperm and the embryo tissues carefully removed, and ground both of them into fine powder for chemical analysis. The chemical analyses were carried out on both the endosperm and the embryo at the soak and during germination (24 to 144 h).
OMW characterization
The pH was measured using a glass electrode. The phenolic content of OMW was determined by extraction with ethyl acetate after remove oil with hexane [17], Total phenolic content was determined by a colorimetric method using Folin-Ciocalteu reagent [18].
Morphology parameter
Germination percentage (%G)
The germination percentage is the proportion, expressed as percentage of germinated seeds to the total number of viable seeds that were tested by following formula according to International Rules for Seed Testing Association (ISTA) [19].
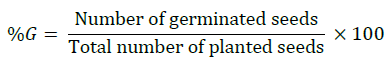
Seedling growth
The seedling growth was harvested after six days. The root length and shoot length were measured by using a centimeter scale, root length was measured from the main apex to the crown whereas shoot length was measured from the crown to the main apex [20].
Percentage phytotoxicity
Percentage phytotoxicity of OMW on root and shoot growth was calculated after six days of seedling growth. The following formula was used for calculating the percentage phytotoxicity [21].
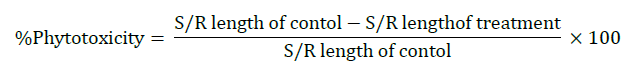
Where, S/R=Shoot/ Root.
Biochemical contents
Determination of protein
Total proteins were extracted from samples many times using 50 mM phosphate buffer (pH 7.8) until the negative detection of proteins in the final extraction and after each extraction was centrifuged in micro-centrifuge machine (BOECO 1610-13, Germany) for 10 min at 5,000 rpm [22]. The supernatant was separated in each extraction, and total proteins concentration in supernatants was measured by dye binding assay as described by Bradford using BSA as standard [23].
Determination of oil
The ground endosperms and embryos were extracted many times with n-hexane to remove most of the oil until the negative detection of oil in the final extraction, 12 h for each extraction with continuous stirring. In each extraction the solution was subjected to centrifugation in micro-centrifuge machine (BOECO 1610-13, Germany) for 10 min at 5,000 rpm. The supernatants were combined and evaporated by rotary evaporator. The weight of the oil was determined and the results were expressed in terms of weight in mg of total oil per gram of dry seeds [24].
Determination of carbohydrate
For total soluble sugars, the ground endosperms and embryos were homogenized in 10.0 ml of 80% (v/v) ethanol in a mortar and the mixture boiled for 10 min. The samples were centrifuged in micro-centrifuge machine (BOECO 1610-13, Germany) at 5000 rpm for 10 min and the supernatant removed. Two additional extraction using 10.0 ml of hot 80% ethanol were carried out. The supernatant fractions were combined and soluble sugars were then determined by anthrone method at 620 nm. The quantity of total soluble sugars was expressed as mg per gram of dry seeds. The precipitate is retained for the analysis of starch [25]. For starch estimation the precipitates were dried in hot water bath and resuspended in 5 ml water. Subsequently 6.5 ml of 52% (v/v) perchloric acid was added to the residue and the contents were centrifuged in micro-centrifuge machine (BOECO 1610-13, Germany). The supernatant was decanted and collected and the whole procedure for starch estimation was repeated thrice. The supernatants were combined and glucose was determined by anthrone method at 620 nm. In acidic medium starch is hydrolyzed to glucose, so the quantity of starch was calculated in terms of glucose equivalent and factor 0.9 was used to convert the values of glucose to starch, the quantity of starch was expressed as mg per gram of dry seeds [26].
Analytical methods
The data was subjected to one way Analysis of Variance (ANOVA) IBM SPSS software package for Windows (Version 20, SPSS Inc., Chicago, IL), the statistical significance was evaluated at P ≤ 0.05. The results were presented as mean ± standard deviation based on three replications.
Results and Discussion
Effect of OMW waters on morphology parameter
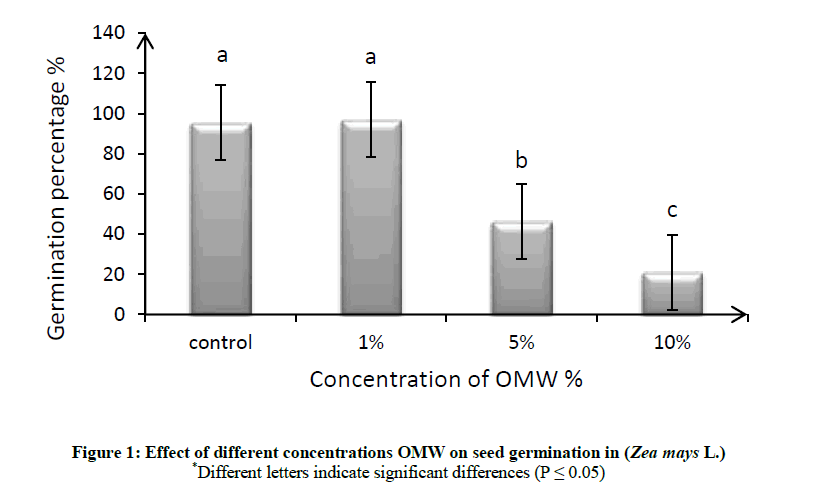
Effect of different concentrations (1, 5 and 10% v/v) of OMW were studied on germination percentage of maize seeds (Zea mays L.), distilled water without any OMW was used as control. Figure 1 and Tables 1, 2 showed although the differences in germination percentage were insignificant compared with the control, it could be considered that the lower concentration (1% v/v) of OMW was beneficial effect on germination percentage as compare to the control, but the higher concentration (5% v/v) was inhibitory and the most suppressive concentration was the (10% v/v) that caused about 21.90% inhibition in germinability of maize.
| Con. OMW (% v/v) | 1% | 5% | 10% | 100% |
|---|---|---|---|---|
| pH | 5.37a ± 0.005 | 5.25b ± 0.01 | 5.23b ± 0.03 | 5.16c ± 0.01 |
| Con. Phenol (ppm) | 40.07a ± 4.59 | 76.92b ± 2.91 | 138.46c ± 3.43 | 1261.50d ± 14.21 |
*Values are mean ± standard deviation; *Different letters within row indicate significant differences (P ≤ 0.05)
Table 1: Shows the concentration of phenols in untreated OMW at three different concentrations
| Conc. (% v/v) | RL (cm) | SL (cm) |
|---|---|---|
| Control | 0.50a ± 9.5 | ± 0.28a ± 8.3 |
| 1% | 10.3b ± 0.30 | 8.8a ± 0.20 |
| 5% | 1.28c ± 0.07 | 1.63b ± 0.15 |
| 10% | 0.4d ± 0.10 | 0.7c ± 0.10 |
*Values are mean ± standard deviation; *Different letters within columns indicate significant differences (P ≤ 0.05)
Table 2: Effects of different concentration of OMW on root and shoot length and germination percentage of (Zea mays L.)
The germination percentage decreased with increasing in OMW concentration as shown in Figure 1. So we can say that the higher level of OMW produced toxic effect in seed germination, thus increasing concentration of OMW significantly reduce the strength of germination as compare to the lowest concentration of OMW and the control. Therefore, it can be suggested that the germination inhibition is principally owing to the phenolic compounds highly present in untreated olive mill wastewater [27,28]. Muscolo et al. tested effect of OMW on seed germination of fava, sulla, chicory and wheat, they found the seed germination percentage for each species examined decreased with increasing OMW concentrations [29]. OMW phytotoxicity is a complex property, since more than one compound can be responsible for it. Polyphenols are not necessarily the sole compounds responsible for phytotoxicity properties of OMW, however, they have been claimed as the major ones responsible for phytotoxicity. Numerous works have suggested that phenolic compounds are implicated in the OMW germinability suppression or reduction and that they are able to affect the germinability [30,31].
The results indicated that the higher concentrations of OMW (5 and 10% v/v) lead to a significant decrease in length of root and shoot. While, the lower concentration (1% v/v) of OMW was better than control. Indeed, as according to Table 1, the lower amount of phenolic compounds in (1% v/v) olive mill wastewater (15.38 ppm) had no harmful effect on plant, but the higher amount of phenolic compounds in (5 and 10% v/v) olive mill wastewater (76.92 and 138.46 ppm) respectively had a harmful and great effect on germination percentage and length of root and shoot.
The present results revealed that the lower concentrations (1% v/v) of OMW have no negative effect on germination of maize. In accordance with these results, Hanifi et al. reported a relative tolerance of maize germination to OMW [32]. While, Asfi et al. proved that OMW caused an evident concentration dependent inhibition in germinability of spinach [33]. Root and shoot growth of maize decreased with increasing OMW concentration (Table 2).
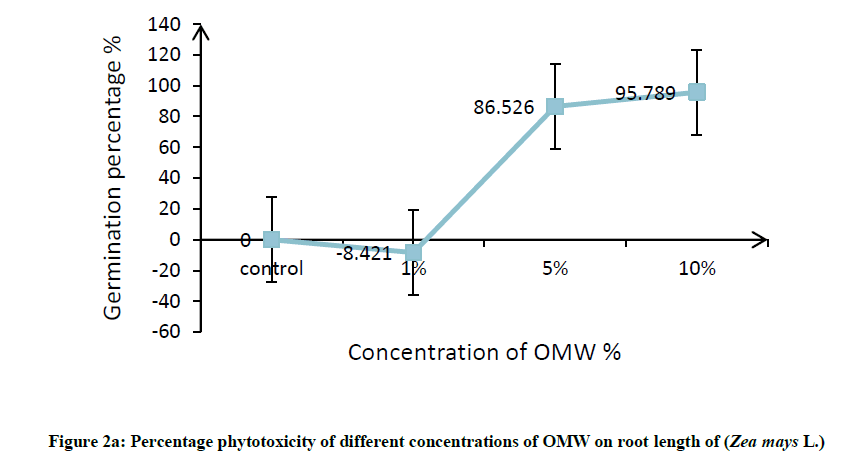
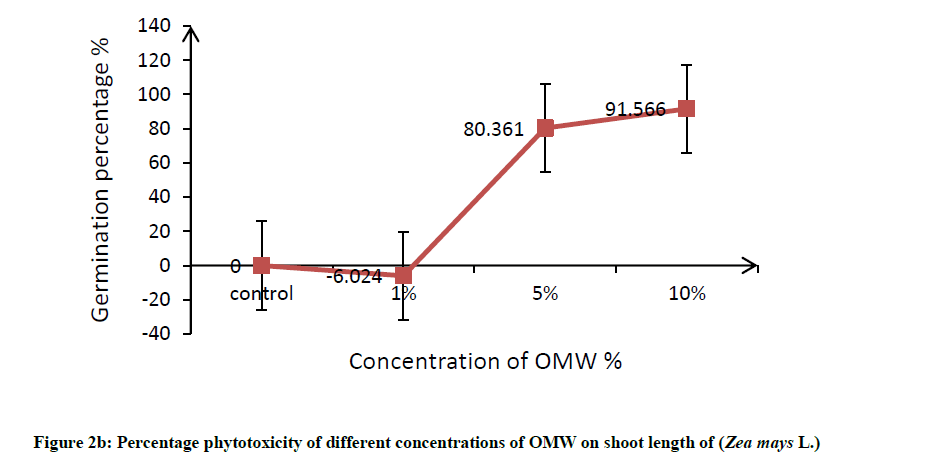
Root was found to be more affected than shoot, it can be attributed to roots are the organs in direct contact with the toxic factor, this effects on root elongation where it causes discomposure of cellular division and elongation of cells. In this connection, Hanifi et al. reported that OMW at concentration of 12.5% (v/v) inhibited the shoot and radicle elongation as well as the biomass of maize seedlings [32]. In addition, Asfi et al. reported that spinach shoot and root length were reduced in response to OMW, where root was the most affected organ [33]. The percentage phytotoxicity of different concentration of OMW on root length and shoot length of maize was calculated and results are shown in Figure 2a and 2b, percentage phytotoxicity of root and shoot increased with increasing OMW concentration.
The phytotoxic effect on higher plants is especially severe during germination and seedling development [34], OMW phytotoxicity has been mainly attributed to the phenolic and organic acid content [10,35]. Piotrowska et al. observed that soil became highly phytotoxic after addition of olive mill wastewater [28], Rinaldi et al. showed that crude olive mill wastewater has phytotoxic effects on barley, durum and soft wheat [3].
Effect of OMW waters on biochemical contents
When studying the effect of OMW on the total protein content of maize (Zea mays L.) during germination, we found that the total protein content in the endosperm increased and in the embryo decreased linearly with increasing the concentration of OMW in the growth medium (Tables 3 and 4).
| Germination time (h) | control | OMW % (v/v) | ||
|---|---|---|---|---|
| 1% | 5% | 10% | ||
| Soak | 4.410a ± 1.18 | 4.114a ± 0.54 | 4.809a ± 0.25 | 5.064a ± 0.17 |
| 24 | 4.035a ± 0.27 | 3.827a,b ± 0.20 | 4.378a ± 0.36 | 4.697a,c ± 0.47 |
| 48 | 2.702a ± 0.30 | 2.438a ± 0.11 | 3.284b ± 0.19 | 4.043c ± 0.16 |
| 72 | 2.518a ± 0.35 | 2.327a ± 0.27 | 3.021a ± 0.24 | 3.851b ± 0.26 |
| 96 | 2.454a ± 0.38 | 2.215a ± 0.22 | 2.806a ± 0.31 | 3.779b ± 0.32 |
| 120 | 2.287a ± 0.29 | 2.936a ± 0.16 | 2.566a ± 0.23 | 3.731b ± 0.39 |
| 144 | 2.127a ± 0.29 | 1.561a,c ± 0.22 | 2.367a,d ± 0.26 | 3.715b ± 0.29 |
*Values are mean ± standard deviation; *Different letters within row indicate significant differences (P ≤ 0.05)
Table 3: Change of protein content (mg protein/g dry seeds) in the endosperm of Zea mays L. during germination
| Germination time (h) | control | OMW % (v/v) | ||
|---|---|---|---|---|
| 1% | 5% | 10% | ||
| Soak | 2.710a ± 0.21 | 3.101a,c ± 0.18 | 2.407a,d ± 0.27 | 0.994b ± 0.15 |
| 24 | 3.803a ± 0.94 | 3.931a ± 0.16 | 3.125a,b ± 0.09 | 2.143b ± 0.15 |
| 48 | 5.974a ± 0.61 | 6.181a ± 0.16 | 4.920b ± 0.19 | 3.604c ± 0.17 |
| 72 | 6.804a ± 0.68 | 6.948a ± 0.20 | 5.104b ± 0.23 | 3.787c ± 0.15 |
| 96 | 6.916a ± 0.69 | 7.067a ± 0.10 | 5.415b ± 0.26 | 3.851c ± 0.15 |
| 120 | 7.115a ± 0.47 | 7.490a ± 0.23 | 5.607b ± 0.25 | 3.89 c ± 0.19 |
| 144 | 7.498a ± 0.38 | 7.650a ± 0.17 | 5.886b ± 0.35 | 3.931c ± 0.18 |
*Values are mean ± standard deviation; *Different letters within row indicate significant differences (P ≤ 0.05)
Table 4: Change of protein content (mg protein/g dry seeds) in the embryo of Zea mays L. during germination
Results showed that the total protein content in the endosperm at 1% of OMW was insignificant less than the control, but when the concentration of OMW increased, the total protein content significantly increased. While the total protein content in the embryo at 1% of OMW was insignificant higher than control then significantly decreased with increasing concentration of OMW.
Zhou et al., demonstrated the influence of allelochemicals on protein metabolism [36]. The increase in the total protein content in the endosperm at increase concentration of OMW may be due to the activity of protease enzyme, as OMW have contributed to inhibition of protease enzyme and reduced its activity in the hydrolysis of proteins, thus increasing total protein content in the endosperm with increased concentration of toxic agent. Total protein content in the embryo of maize plant decreased with the increased concentration of OMW, which may be due to decrease in the content of nitrogen, and as nitrogen is the precursor for the synthesis of amino acids which are the building blocks of protein [37].
Saleh et al. reported that treatment of maize seedlings with lower concentrations of olive processing waste extract significantly increased the protein content of radicle tissues, while the higher concentrations were inhibitory. Regarding the coleoptile tissues, all the tested concentrations had no obvious impact on the level of soluble proteins except for 9.0% that caused a significant reduction in the level of proteins [38].
The changes in the oil content of maize (Zea mays L.) seeds as affected by OMW are shown in Tables 5 and 6. Results obtained show the oil content in the endosperm at 1% of OMW was less than the control, but when the concentration of OMW increased, the total oil content significantly increased. In the embryo the oil content at 1% of OMW was significant higher than control and at 5% of OMW the oil content significantly decreased then significantly increased again at 10% of OMW.
| Germination time (h) | Control | OMW % (v/v) | ||
|---|---|---|---|---|
| 1% | 5% | 10% | ||
| Soak | 255.5a ± 3.98 | 243.1b ± 2.70 | 271.6c ± 2.68 | 364.2d ± 3.57 |
| 24 | 244.8a ± 4.45 | 237.3a ± 4.25 | 270.3b ± 4.61 | 323.8c ± 3.73 |
| 48 | 236.1a ± 3.57 | 214.4b ± 5.46 | 252.1c ± 4.63 | 314.6d ± 3.26 |
| 72 | 219.5a ± 2.82 | 200.3b ± 4.71 | 231.8c ± 3.47 | 295.1d ± 4.40 |
| 96 | 204.2a ± 5.17 | 198.6a ± 2.19 | 224.6b ± 3.60 | 268.8c ± 2.95 |
| 120 | 157.7a ± 3.60 | 150.5a ± 1.57 | 181.1b ± 3.74 | 247.4c ± 2.52 |
| 144 | 113.1a ± 2.62 | 102.7b ± 4.24 | 142.9c ± 3.83 | 228.1d ± 2.80 |
*Values are mean ± standard deviation; *Different letters within row indicate significant differences (P ≤ 0.05)
Table 5: Change of oil content (mg oil/g dry seeds) in the endosperm of Zea mays L. during germination
| Germination time (h) | control | OMW % (v/v) | ||
|---|---|---|---|---|
| 1% | 5% | 10% | ||
| Soak | 126.5a ± 3.10 | 157.4b ± 2.52 | 101.8c ± 4.63 | 127.1a ± 4.09 |
| 24 | 162.2a ± 4.55 | 184.9b ± 4.07 | 126.5c ± 2.46 | 157.2a ± 6.07 |
| 48 | 225.8a ± 8.83 | 245.2a ± 5.42 | 185.6b ± 4.32 | 200.9b ± 5.59 |
| 72 | 357.6a ± 5.95 | 384.6b ± 3.90 | 266.4c ± 5.10 | 371.8d ± 4.19 |
| 96 | 435.1a ± 5.17 | 469.1b ± 6.25 | 372.0c ± 7.41 | 473.6b ± 3.05 |
| 120 | 529.3a ± 2.68 | 567.7b ± 5.66 | 435.9c ± 7.30 | 541.8a ± 3.50 |
| 144 | 615.1a ± 12.42 | 674.3b ± 3.70 | 599.3a ± 4.35 | 656.9b ± 5.28 |
*Values are mean ± standard deviation; *Different letters within row indicate significant differences (P ≤ 0.05)
Table 6: Change of oil content (mg oil/g dry seeds) in the embryo of Zea mays L. during germination
The increase in the oil content in the endosperm at increase concentration of OMW may be due to the inhibited or reduced the activity of the lipase enzyme, whereas OMW have contributed to inhibition of lipase enzyme and reduced its activity in the hydrolysis of triacylglycerols to release glycerols and free fatty acids. In the embryo there was decrease in the oil content with increasing OMW concentration up to 5%, this may be attributed to the increasing OMW concentration leads to reduce the division and elongation of cells. In addition to the decrease in the release of free fatty acids in the endosperm as a result of decreased activity of lipase enzyme has led to decrease in the construction of lipid membranes in the embryo cells with increased concentration of OMW, Satyanarayana et al. reported that the liberated fatty acids might be used for the formation of membrane lipids in the growing embryos [39]. In the embryo, with the increase concentration of OMW to 10%, the oil content increased as a result of the transformation of part of the soluble sugars in embryo cells into oil during metabolism.
The changes in Total Soluble Sugar (TSS) content in the endosperm and the embryo during germination under effect different concentration of OMW as shown in Tables 7 and 8. In endosperm total soluble sugar declined at 1% of OMW and was significant less than control, but with increasing concentration of OMW the total soluble sugar significantly increased, this may be due to slower mobilization of starch as well as further utilization of sugars for growth of axis, Bhushan et al. reported that the total soluble sugar content in endosperm of seeds in lead solutions was higher than control [40].
| Germination time (h) | control | OMW % (v/v) | ||
|---|---|---|---|---|
| 1% | 5% | 10% | ||
| Soak | 11.438a ± 0.32 | 8.887b ± 0.26 | 12.295c ± 0.33 | 15.234d ± 0.31 |
| 24 | 12.165a ± 0.22 | 9.625b ± 0.39 | 14.081c ± 0.29 | 15.336d ± 0.43 |
| 48 | 13.010a ± 0.12 | 10.193b ± 0.32 | 14.887c ± 0.31 | 15.867d ± 0.25 |
| 72 | 13.81a ± 0.23 | 11.301b ± 0.22 | 15.355c ± 0.21 | 16.060c ± 0.44 |
| 96 | 14.689a ± 0.30 | 12.490b ± 0.29 | 15.928c ± 0.21 | 17.419d ± 0.21 |
| 120 | 15.754a ± 0.13 | 13.766b ± 0.34 | 16.374a ± 0.23 | 18.711c ± 0.34 |
| 144 | 16.112a ± 0.18 | 14.257b ± 0.25 | 16.683a ± 0.49 | 19.504c ± 0.40 |
*Values are mean ± standard deviation; *Different letters within row indicate significant differences (P ≤ 0.05)
Table 7: Change of TSS content (mg TSS/g dry seeds) in the endosperm of Zea mays L. during germination
| Germination time (h) | control | OMW % (v/v) | ||
|---|---|---|---|---|
| 1% | 5% | 10% | ||
| Soak | 20.397a ± 0.38 | 19.989a ± 0.24 | 22.479b ± 0.28 | 15.132c ± 0.22 |
| 24 | 19.527a ± 0.31 | 19.003a ± 0.43 | 21.058b ± 0.19 | 13.836c ± 0.27 |
| 48 | 18.989a ± 0.40 | 18.887a ± 0.19 | 19.826b ± 0.10 | 12.336c ± 0.24 |
| 72 | 18.513a ± 0.26 | 18.245a,c ± 0.27 | 19.131a,d ± 0.16 | 12.179b ± 0.31 |
| 96 | 18.034a ± 0.16 | 17.983a,c ± 0.39 | 18.806a,d ± 0.14 | 12.052b ± 0.40 |
| 120 | 17.817a ± 0.16 | 17.651a ± 0.40 | 18.254a, ± 0.33 | 11.993b ± 0.14 |
| 144 | 17.581a ± 0.34 | 17.377a ± 0.24 | 17.948a ± 0.36 | 11.846b ± 0.18 |
*Values are mean ± standard deviation; *Different letters within row indicate significant differences (P ≤ 0.05)
Table 8: Change of TSS content (mg TSS/g dry seeds) in the embryo of Zea mays L. during germination
Gopal et al. reported that accumulation of soluble saccharides and polysaccharides content in seeds due to their negative effect on amylase activity [41]. El-Darier (2002) reported that treatment of maize seedlings with water extract of Eucalyptus rostrata leaf-litter leads to accumulation of sugars [42].
In the embryo, total soluble sugar at 1% of OMW was insignificant less than control, and significantly increased with increasing concentration of OMW up to 5% concentration and then significantly decreased again at 10% concentration. This decrease in high concentrations of OMW may be due to either the conversion the total soluble sugar to oil, where this concentration was observed increase the oil content in the embryo, or as some researchers suggested that the soluble sugars and some amino acids can have a protective role, regulating osmotic potential and also with a more direct detoxification role of Reactive Oxygen Species (ROS) [43,44], Ali et al. reported in his study on Brassica napus L. that using high concentrations of cadmium ions, there was a decrease in the total soluble sugars, which probably due to general damage in the plant metabolism [45]. Deef (2007) also reported that low concentration of toxic factor treatment exhibit an increase in the total carbohydrates content in growing axis and reverse is true for the high concentrations [46]. Mechri et al. reported that application of OMW increased the soluble sugars in leaves of olive trees [47].
Starch content significantly increased with increasing OMW concentration in the endosperm, starch content at 1% of OMW was significant less than control, and reverse is true for the embryo (Tables 9 and 10).
| Germination time (h) | Control | OMW % (v/v) | ||
|---|---|---|---|---|
| 1% | 5% | 10% | ||
| Soak | 614.795a ± 12.49 | 604.591a,c ± 5.47 | 632.144a,d ± 2.89 | 656.632b ± 4.17 |
| 24 | 592.602a ± 10.86 | 538.780b ± 7.12 | 620.456c ± 6.57 | 652.865d ± 5.61 |
| 48 | 576.780a ± 12.82 | 460.969b ± 4.51 | 616.581c ± 12.95 | 649.234d ± 5.16 |
| 72 | 425.010a ± 35.45 | 401.137a ± 36.12 | 539.147b ± 2.53 | 613.374c ± 5.35 |
| 96 | 305.130a ± 16.20 | 295.641a ± 4.66 | 374.592b ± 7.04 | 522.529c ± 6.68 |
| 120 | 243.851a ± 15.28 | 210.583b ± 3.37 | 308.936c ± 7.70 | 468.861d ± 5.99 |
| 144 | 151.762a ± 8.61 | 133.017b ± 7.38 | 273.214c ± 3.02 | 406.887d ± 5.94 |
*Values are mean ± standard deviation; *Different letters within row indicate significant differences (P ≤ 0.05)
Table 9: Change of starch content (mg starch/g dry seeds) in the endosperm of Zea mays L. during germination
| Germination time (h) | Control | OMW % (v/v) | ||
|---|---|---|---|---|
| 1% | 5% | 10% | ||
| Soak | 3.341a ± 0.24 | 3.698a ± 0.33 | 1.607b ± 0.31 | 1.147b ± 0.19 |
| 24 | 4.192a ± 0.30 | 4.526a ± 0.19 | 2.817b ± 0.22 | 1.930c ± 0.10 |
| 48 | 6.198a ± 0.35 | 6.862b ± 0.18 | 4.515c ± 0.14 | 3.647d ± 0.20 |
| 72 | 6.781a ± 0.47 | 6.946a ± 0.20 | 4.613b ± 0.19 | 3.720c ± 0.39 |
| 96 | 6.956a ± 0.27 | 7.155a ± 0.19 | 4.696b ± 0.21 | 3.852c ± 0.15 |
| 120 | 7.082a ± 0.06 | 7.378a ± 0.26 | 4.771b ± 0.16 | 3.924c ± 0.17 |
| 144 | 7.117a ± 0.10 | 7.423a ± 0.27 | 4.821b ± 0.20 | 4.005c ± 0.15 |
*Values are mean ± standard deviation; *Different letters within row indicate significant differences (P ≤ 0.05)
Table 10: Change of starch content (mg starch/g dry seeds) in the embryo of Zea mays L. during germination
The increase in the starch content in the endosperm with increasing concentration of OMW may be due to the activity of amylase enzyme, as OMW contributed to reduce activity of amylase in the hydrolysis of starch [41]. Curtailed degradation of starch in response to stress was proposed by Mishra et al. they suggested that starch in the endosperm is not much solubilized by amylase and could not be consumed by the embryo [48].
Starch content in the embryo of maize (Z. mays L.) plant significantly decreased with increasing concentration of OMW, which may be due either to decrease the activity of synthesis enzymes in the embryo cells or as a result of decreased starch hydrolysis in the endosperm due to increased concentration of OMW, led that to decrease in the proportion of transmittal to the embryo.
Conclusion
OMW constitute a serious environmental problem, it can effect on germination and seedling growth of maize (Z. mays L.) where the effect is concentration dependent, since the low concentrations (1%) of OMW showed an improvement in morphological growth and chemical composition, while the higher concentrations showed clear toxicity on maize plant. The main reason for this is high phenolic content in untreated OMW.
Recommendation
Based on what was reached in this study, it is recommended to treat the OMW before use in the process of agricultural irrigation or dilution with water to the extent that it is non-toxic to germination seeds and growth of plants.
References
- A. D’Annibale, R. Casa, F. Pieruccetti, M. Ricci, R. Marabottini, Chemosphere., 2004, 54, 887-894.
- I. Saadi, Y. Laor, M. Raviv, S. Medina, Chemosphere., 2007, 66, 75-88.
- M. Rinaldi, G. Rana, M. Introna, Field Crops Res., 2003, 84, 319-326.
- A. Mekki, A. Dhouib, F. Aloui, S. Sayadi, Agronomy for Sustainable Development., 2006, 26(1), 61-67.
- A. Mekki, A. Dhouib, F. Fekia, S. Sayadi, Ecotoxicology and Environmental Safety, 2008, 69, 488-495.
- G. Celano, D. Smejkalov, R. Spaccini, A. Piccolo, Environ. Sci. Technol., 2008, 42, 4896-4901.
- C.J. McNamara, C.C. Anastasiou, V. O'Flahertyd, R. Mitchell, Int. Biodeterior. Biodegradation., 2006, 61, 127-134.
- E. Moreno, J. Perez, A. Ramos-Cormenzana, J. Martinez, Microbios., 1987, 51, 169-174.
- R. Levi-Menzi, A. Saviozzi, R. Riffaldi, L. Falzo, Olivae., 1992, 40, 20-25.
- A. Mekki, A. Dhouib, F. Fekia, S. Sayadi, Inter. J. Recycling of Organic Waste in Agriculture, 2013, 2(15), 1-7.
- G. Aggelis, D. Iconomou, M. Christou, D. Bokas, S. Kotzailias, G. Christou, V. Tsagou, S. Papanikolaou, Water Res., 2003, 37, 3897-3904.
- K.T.G.K. Perera, T.K. Weerasinghe, Inter. J. Scientific and Res. Publications, 2014, 7(4), 1-6.
- R. Sozharajan, S. Natarajan, Int. Lett. Natural Sci., 2014, 12, 5-15.
- S. Aliu, I. Rusinovci, S. Fetahu, B. Gashi, E. Simeonovska, L. Rozman, Acta Agriculturae Slovenica., 2015, 105(1), 85-94.
- M.G. Dawood, M.S. Dawood, B.Y. Reyad, A.S.M. El-Sayed, S.H. El-Gayar, Sci. Agric., 2013, 2(3), 77-82.
- P.R. Malekzadeh, M. Sheikhakbari, A.A. Hatamnia, Iran. J. Plant Physiol., 2015, 5(2), 1289-1296.
- A. Yangui, M.H. Abessi, M. Abderrabba, J. Chem. Pharm. Res., 2015, 7(3), 171-177.
- K.W. Lee, Y.J. Kim, H.J. Lee, C.Y. Lee, J. Agric. Food Chem., 2003, 51(25), 7292-7295.
- International Rules for Seed Testing (ISTA), Association, Chapter 5: Germination test, 2008, 1-57.
- A. Houshmandfar, F. Moraghebi, Afric. J. Agric. Res., 2011, 6(5), 1182-1187.
- A. Gang, A. Vyas, H. Vyas, J. Environ. Res. Development., 2013, 8(2), 206-213.
- A. Hameed, T.M. Shah, B.M. Atta, N. Iqbal, M.A. Haq, H. Ali, Pak. J. Bot., 2009, 41(2), 703-710.
- A. Jaleel, K. Jayakumar, Z. Chang-Xing, M. Iqbal, J. Sci. Res., 2009, 1(1), 128-137.
- D.K. Saxena, S.K. Sharma, S.S. Sambi, ARPN J Engineering and Applied Sci., 2009, 6(1), 84-89.
- M. Tonguç, R. Elkoyunu, S. Erbaş, Y. Karakurt, Turk. J. Biol., 2012, 36, 107-112.
- S. Basu, A. Roychoudhury, S. Sanyal, D.N. Sengupta, Indian J. Biochem. Biophys., 2012, 49, 115-123.
- D.P. Komilis, E. Karatzas, C.P. Halvadakis, J. Environ. Manage., 2005, 74, 339-348.
- A. Piotrowska, G. Iamarino, M.A Rao, L. Gianfreda, Soil Biol. Biochem., 2006, 38, 600-610.
- A. Muscolo, M. Sidari, C. Mallamaci, E. Attina, Terresr. Aqua. Environ. Toxicol., 2010, 4, 75-83.
- A. Muscolo, M.R. Panuccio, M. Sidari, Plant Growth Regulation., 2001, 35, 31-35.
- A. Muscolo, M.R. Panuccio, M. Sidari, Plant Growth Regulation., 2002, 37, 1-5.
- S. Hanifi, I.E. Hadrami, Agron. Sust. Develop., 2008, 28(2), 313-319.
- M. Asfi, G. Ouzounidou, M. Moustakas, Environ. Sci. Pollut. Res., 2012, 19(6), 2363-2371.
- A. Stokłosa, T. Hura, E.S. Rodzynkiewicz, T. Dąbkowska, A. Lepiarczyk, Acta Agrobotanica., 2008, 61(2), 205-219.
- M. Isidori, M. Lavorgna, A. Nardelli, A. Parrella, J. Agric. Food Chem., 2005, 53, 8414-8417.
- Y.H. Zhou, J.Q. Yu, Manuel J. Reigosa., 2006, 127-139.
- J.K. Datta, A. Bandhyopadhyay, A. Banerjee, N.K. Mondal, J. Agric. Technol., 2011, 7(2), 395-402.
- A.M. Saleh, Egypt. J. Exp. Biol., 2013, 9(1), 35-39.
- B. Satyanarayana, P. Subhashinidevi, A. Arundhati, Not. Sci. Biol., 2011, 3(3), 105-108.
- B. Bhushan, K. Gupta, J. Appl. Sci. Environ. Manage., 2008, 12(2), 29-33.
- R. Gopal, A.H. Rizvi, Chemosphere., 2008, 70(9), 1539-1544.
- S. El-Darier, Pak. J. Biol. Sci., 2002, 5(1), 6-11.
- E. Keunen, D. Peshev, J. Vangronsveld, E. van den Wim, A. Cuypers, Plant Cell Environ., 2013, 36, 1242-1255.
- S.S. Sharma, K.J. Dietz, J. Exp. Bot., 2006, 57, 711-726.
- B. Ali, R.A. Gill, S. Yang, M.B. Gill, M.A. Farooq, D. Liu, M.K. Daud, S. Ali, W. Zhou, PLoS One., 2015, 10, e0123328.
- H.E. Deef, World J. Agric. Sci., 2007, 3, 322-328.
- B. Mechri, H. Cheheb, O. Boussadia, F. Attia, F. Ben Mariem, M. Braham, M. Hammami, Environ. Exp. Bot., 2011, 71(2), 184-191.
- A. Mishra, M.A. Choudhuri, Indian J. Plant Physiol., 1997, 2(1), 41-44.