Research Article - Der Pharma Chemica ( 2017) Volume 9, Issue 1
Electrochemical Behaviour of Brass in Nacl 3% Polluted by Yeast: Effect of Traizole Derivative
Elkhotfi Y*, Forsal I and Rakib EMElkhotfi Y, Organic and Analytical Chemistry Laboratory, University Sultan Moulay Slimane, Beni Mellal, Morocco, Email: i.taouaf@usms.ma
Abstract
The effect of new corrosion inhibitor namely 1H-1,2,4-Triazol-4-amine,3,5-diphenyl-N-phenyl methylene) (HTADP) on the corrosion of brass in NaCl 3% medium pure and polluted by the yeast (eukaryotic microorganisms) as corrosion inhibitor has been studied by using electrochemical techniques such as potentiodynamic polarization and electrochemical impedance spectroscopy. Results obtained showed that the yeast accelerate the alloy corrosion. And a significant reduction of corrosion rates was observed in both media in the presence of the (HTADP). The inhibition efficiency reaches 90% at a concentration 10-3 M of (HTADP). The effect of biocide, namely cetyltrimethylammonium bromide (CTAB), on the inhibition efficiency of (HTADP) has been studied, too.
Keywords
Corrosion, Brass, EIS, Polarization, Yeast, Inhibition
Introduction
Brass has been widely used as tubing material for condensers and heat exchangers in various cooling water systems because of their resistance to corrosion. Brass materials are extensively used to fabricate structures and components exposed to sea water and other marine environments [1-4]. Nowadays, Microorganisms may present several problems, corrosion, and scale. These problems lead to significant economic repercussions. The presence of microorganisms in various cooling water non-sterile is an important factor that accelerates the corrosion process. These microorganisms can modify the electrochemical reactions and changing the corrosion mechanism. One of the most important methods in corrosion protection is the utilization of organic inhibitors [5-10]. In general, the organic compounds have demonstrated a great effectiveness in inhibiting the aqueous corrosion of many metals and alloys [11-16]. The inhibiting action of those organic compounds is usually attributed to interactions with metallic surface by adsorption. The adsorption of inhibitors takes place through heteroatoms such as oxygen, phosphorus and sulphur or aromatic rings containing polar groups and п electrons [17]. Subsisted triazole has desirable characteristic for a corrosion inhibitor. It has been extensively studied in several media and for different metals and alloys [18,19]. In this paper, we have studied the effect of the addition of HTADP on the corrosion inhibition of Cu-40Zn alloy in NaCl 3% medium pure and polluted by the yeast using polarization curves, electrochemical impedance spectroscopy, and scanning electron microscopy. The impact of both adition of HTADP and biocide, namely Cetyltrimethylammonium Bromide (CTAB) was also investigated. EXPERIMENTAL DETAILS Specimens Brass strips Cu-40Zn having chemical compositions (wt%) Cu (61.61%) Zn (38.19%), Al (0.12%) and Si (0.08%). The brass specimens, prior to all measurements. Were polished mechanically with different grades of silicon carbide papers (120-1200), rinsed with doubly distilled water, degreased ultrasonically in acetone for 5 min and dried with hot air. Electrolytes. To evaluate the resistance corrosion of HTADP. We used two corrosive mediums: pure 3% NaCl and 3% NaCl polluted by 100 ppm of the yeast. The yeast used is mark germa, product of company SOMADIR Morocco. Inhibitor Molecular formula Figure 1 shows the molecular structure of 1H-1,2,4-Triazol-4-amine,3,5-diphenyl-N-(phenyl methylene) (HTADP).
Synthesis of HTADP A mixture of 3,5-diphenyl-4-amino-1,2,4-triazole (1 mole), and benzaldehyde (1 mole) in ethanol, with a small amount of l’APTS (acidic paratoluene sulfonic) was heated in a steel autoclave for 1.5 to 2 h. After cooling, the reaction mixture was diluted with a small amount of ethanol, which separated, was filtered off, the filtrate was evaporated under reduce pressure and the residue crystallised from ethanol. Biocide Cetyltrimethylammonium bromide (CTAB) is well known as a potential biocide and cationic surfactant. Indeed several authors have studied the influence of CTAB on the corrosion control of mild steel [17,20]. Besides corrosion inhibitors (surfactants) are widely employed in the petroleum industry to protect iron, alloys and steel equipment used in drilling, production, and transport and refining of hydrocarbons [21-24]. Electrochemical measurement Electrochemical experiments were conducted using impedance equipment (Voltalab model PGZ 100) and controlled with Tacussel corrosion analysis software model Voltamaster 4. A conventional three electrode cylindrical glass and thermostated cell. The working electrode was Brass strips with surface area of 0.8 cm2. A platinum plate of surface area of 2 cm2 as the auxiliary electrode (CE) and Ag/AgCl were used as the reference electrodes, The working electrode was immersed in test solution for 60 min until a steady state open circuit potential (Eocp) was obtained. The rotating sweep rate was maintained at 1000 rpm. The impedance measurements (EIS) were carried-out at Ecorr after immersion in a solution without bubbling, After the determination of steady state current at a given potential, sine wave voltage (10 mV) peak to peak, at the frequencies between 100 KHz and 10 mHz was superimposed on the open circuit potential. The impedance diagrams are given in the Nyquist representation. Values of Rp and Cdl were obtained from Nyquist plots. For polarisation curves, Potentiodynamic polarisation studies was performed with a scan rate of 1 mv.s-1 in the potential range from -1200 mv to +400 mv relative to the corrosion potential. The polarization curves were corrected for the ohmique drop measured by electrochemical impedance spectroscopy. RESULTS AND DISCUSSION Polarization curves The working electrode was immersed in test solution during 1 h until steady-state open circuit potential (Eocp) was obtained. Polarisation curves of Cu-40Zn alloy in 3% Nacl solution pure and polluted by yeast. The effect of 10-3 M HTADP at different media was studied. The cathodic polarization curves was recorded by polarization form Eocp to negative direction and the anodic polarization curves was recorded from the Eocp to positive direction. Cathodic curves The cathodic curves obtained in 3% Nacl pure and polluted by yeast and in the presence of HTADP are shown in Figure 2.
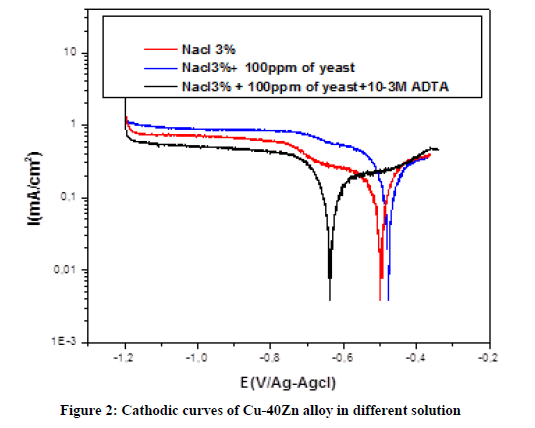 Examination of this figure shows that. In Nacl 3% pure; the two distinct regions appearing. From Ecorr to -650 mv/Ag-Agcl the active region (apparent tafel region) and from -650 mv to -1200 mv/Ag-Agcl. This result reveals that the mechanism of reduction dissolved oxygen is a mixed process (diffusion/activation).
Examination of this figure shows that. In Nacl 3% pure; the two distinct regions appearing. From Ecorr to -650 mv/Ag-Agcl the active region (apparent tafel region) and from -650 mv to -1200 mv/Ag-Agcl. This result reveals that the mechanism of reduction dissolved oxygen is a mixed process (diffusion/activation).
In 3% Nacl polluted by 100 ppm of yeast. The cathodic current density increases from 0.6 mA.cm-2 without yeast to 0.7 mA.cm-2 with yeast. The addition of 100 ppm of yeast accelerates the corrosion process by formation the biofilm. We note also a decrease the current densities with addition of HTADP to Nacl 3% solution polluted by yeast. Indicating that the addition of HTADP can influence in cathodic charge transfer. The cathodic reaction is a mixed process (diffusion/activation). The inhibition effeciencies were calculated from polarisation curves using relation.
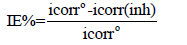
Were the icorr° (inh) and icorr are the corrosion current density (μA.cm-2) values without and with inhibitor respectively. Anodic curves Figure 3 shows the anodic curves of Brass in Nacl 3%, without and with yeast. In the absence and in the presence of yeast.
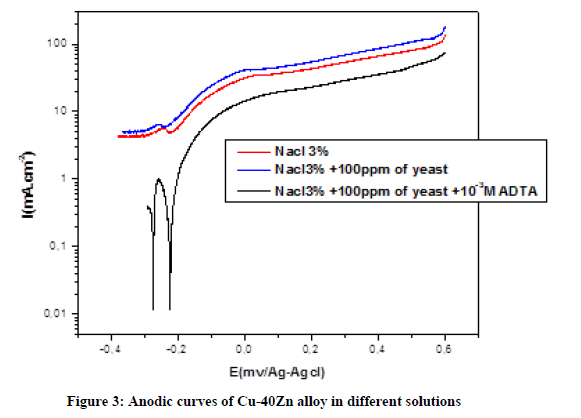
In Nacl 3% pure; the two distinct regions appearing in the anodic polarization curve were the active dissolution region (apparent tafel region), the active-to-passive transition region. The addition of 100 ppm yeast caused the increase the anodic current indicating that microorganisms accelerate the dissolution Brass by formation a biofilm. The mechanism of anodic dissolution of Brass in presence the yeast. In initial stage, zinc forms ZnO [25-27]: Zn+H2O → ZnO+2H++2 e
And copper form Cu2O: 2 Cu+H2O → Cu2O+2H++2e- After this stage, the yeast covered surface and act to barrier to the oxygen diffusion on the surface. This result caused the acceleration of reaction [28,29]: Cu++Cl- → CuCl 2CuCl → Cu+CuCl2 And Zn2++2 Cl- → ZnCl2 On adding HTADP to the 3% NaCl polluted by 100 ppm of yeast decrease the anodic current density by adsorption of the triazole derivative on the Brass by interaction of inhibitor with already adsorbed chloride ions in the presence of inhibition the anodic curves were shifted towards positive potential region (Table 1).
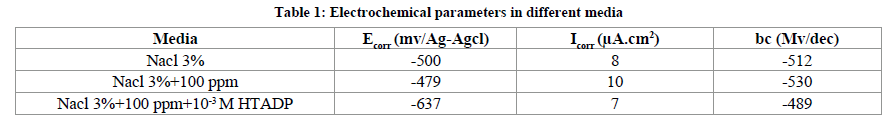
Electrochemical impedance spectroscopy (EIS) Effect of the yeast The corrosion behaviour of Brass in 3% Nacl and 3% Nacl+100 ppm of yeast were investigated by EIS at 30C°. Nyquist plots displayed tow capacitive loop are not perfect semicircles and this difference has been attributed to frequency dispersion. The diameter of the two capacitive loop decreases from 1465 Ω cm2 in the absence of yeast to 1230 Ω cm2 in the presence of yeast (Figure 4). These values of resistance were attributed to the presence of microorganisms in Nacl 3% accelerates corrosion process by formation the biofilm.
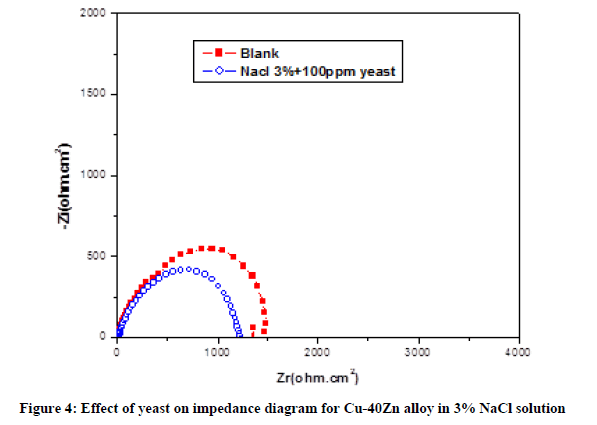
Effect of the HTADP Nyquist plot of impedance spectra of Cu-40Zn alloy in Nacl 3% without yeast, in the absence and the presence of HTADP are shown in Figure 5. Examination of this figure reveals the following points:
- In Nacl 3% pure, the diameter of the tow capacitive loop increases in the presence of 10-3 M HTADP. The increase is due to the adsorption of HTADP on the metal surface. This adsorption depends on the charge of the metallic surface, the charge or the dipole moment or the inhibitor molecule and the adsorption of other ionic species present on solution.
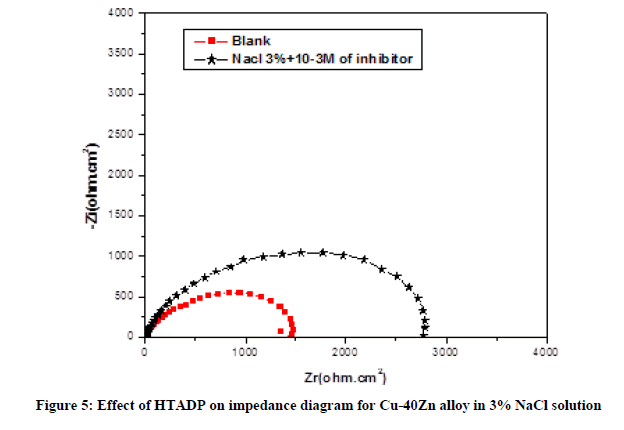
- In Nacl 3% polluted by the yeast. The diameter of the two capacitive loop increases in the presence of 10-3 M HTADP can be attributed to the adsorption of HTADP before formation of primer biofilm by microorganisms (Figure 6).
The inhibition efficiency is calculated by polarisation resistance as follows:
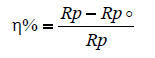
The efficiency of the inhibitor in Nacl 3% pure is greater than that obtained in Nacl 3% +100 ppm of yeast.
Effect of CTAB
To understand the influence of HTADP with 10 ppm CTAB, the inhibitor and biocide were added simultaneously to study corrosion inhibition efficiency. Figure 7 shows the impedance spectra in 3% Nacl polluted by yeast in presence of inhibitor only and inhibitor+CTAB.
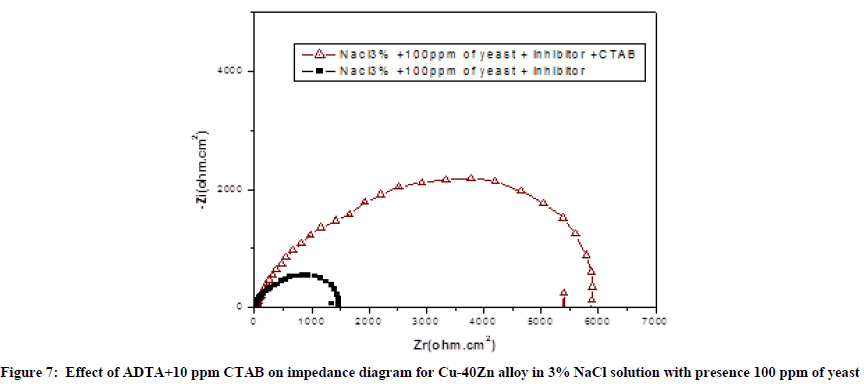
In the presence of 10 ppm CTAB. The diameter of the capacitive loop increases from 1474Ω.cm2 to 5902 Ω.cm2. This variation confirmed that a synergetic effect is to exist between CTAB and triazole molecule [30].
In the case of CTAB the bromide ions are first chemisorbed on the metal surface, replacing the OH− ions and eliminate the microorganisms. Second the positive cationic part of the CTAB group is held by physical (electrostatic) adsorption [31-33]. This type of adsorption blocks the active sites vulnerable for corrosion and thus reduces corrosion.
Conclusions
- Triazole HTADP is a good inhibitor for the corrosion of Brass in Nacl 3% solution.
- The addition of yeast accelerates the dissolution of Brass by formation a biofilm.
- The first passive layer region is attributed to the adsorbed layer consisting of Cu2O and ZnO.
- The HTADP as a mixed inhibitor type.
- The efficiency of the inhibitor increase with presence of biocide confirmed a synergetic effect between CTAB and triazole molecule.
References
[1] H.C. Shih, R.J. Tzou, J. Electrochem. Soc., 1991, 138, 958.
[2] M.I. Abbas, B.J. Corros, 1991, 26, 273.
[3] G. Quartarone, G. Moretti, T. Bellomi, Corros., 1998, 54, 606.
[4] A.G. Gad-Allah, M.M. Abou-Romia, M.W. Badawy, H.H. Rehan, J. Appl. Electrochem., 1991, 21, 829.
[5] W. Qafasowi, C.H. Blanc, N. Pe´be`re, A. Srhiri, J. Appl. Electrochem., 2000, 30, 959-966.
[6] H.L. Wang, R.B. Liu, J. Xin, Corros. Sci., 2004, 46, 2455-2466.
[7] B.D. Mert, M.E. Mert, G. Kardas, B. Yazıcı, Corros. Sci., 2011, 53, 4265-4272.
[8] W. Li, X. Zhao, F. Liu, B. Hou, Corros. Sci., 2008, 50, 3261-3266.
[9] F. Xu, J. Duan, S. Zhang, B. Hou, Mater. Lett., 2008, 62, 4072-4074.
[10] R. Francis, Br. Corros. J., 1985, 20, 4, 167-173.
[11] A.Y. El-Etre, M. Abdallah, Corros. Sci., 2000, 42, 731.
[12] H. Shokry, M. Yuasa, I. Sekine, R.M. Issa, H.Y. El-Baradie, G.K. Gomma, Corros. Sci., 1998, 40, 2173.
[13] Y. Gonzalez, M.C. Lafont, N. Pebere, G. Chatainier, J. Roy, T. Bouissou, Corros. Sci., 1995, 37, 1823.
[14] R. Touir, N. Dkhireche, M. Ebn Touhami, M. Lakhrissi, B. Lakhrissi, M. Sfaira, Desalination., 2009, 249, 922.
[15] A. Ghazoui, R. Saddik, N. Benchat, B. Hammouti, M. Guenbour, A. Zarrouk, M. Ramdani, Der. Pharm. Chem., 2012, 4, 352.
[16] A. Zarrouk, B. Hammouti, H. Zarrok, R. Salghi, A. Dafali, Lh Bazzi, L. Bammou, S.S. Al-Deyab, Der. Pharm. Chem., 2012, 4, 337.
[17] Y. Abboud, A. Abourriche, T. Saffaj, M. Berrada, M. Charrouf, A. Bennamar, N. Al Himidi, H. Hannache, Mater. Chem. Phys., 2007, 105, 1-5.
[18] S. Ramesh, S. Rajeswari, Electrochim. Acta., 2004, 49, 811.
[19] K. Wippermann, J. Schultze, R. Kessel, J. Penninger, Corros. Sci., 1991, 26, 257.
[20] R.C.B. da Aliva, T.M.C. Nogueira, M.D. Capelato, Corros. Prevent. Contr., 1997, 44, 183.
[21] M.M. Osman, Anti-Corros. Methods. Mater., 1998, 45, 176.
[22] J.H. Clint, Surfactant Aggregation, Blackie, Glasgow, 1992.
[23] A.J. McMahon, Colloids. Surf., 1991, 59, 187.
[24] S. Ramesh, S. Rajeswari, S. Maruthamuthu, Mater. Lett., 2003, 57, 4547.
[25] H.W. Pickering, Corros. Sci., 1983, 23, 1107.
[26] R. Ravichandran, N. Rajendran, App. Surf. Sci., 2005, 239, 182.
[27] R. Ravichandran, S. Nanjunden, N. Rajendran, App. Surf. Sci., 2004, 236, 241.
[28] El-Warraky, H.A. El-Shayeb, E.M. Sherif, Anti-Corrosion Methods Materials., 2004, 51, 1.
[29] M. Abdallah, M. Al- Agez, A.S. Fouda, Int. J. Electrochem. Sci., 2009, 4, 336.
[30] M. Kabasakaloglu, T. Kiyak, O. Sendil, A. Asan, Appl. Surf. Sci., 2002, 193, 167.
[31] S.S. Abd. El-Reuim, F. H.Assaf, A. El-Sayed, A.M. Zaky, Br. Corros. J., 1995, 30, 297.
[32] R. Touir, N. Dkhirechea, M. Ebn Touhamia, M. Sfaira b, O. Senhajic, J.J. Robind, B. Boutevind, M. Cherkaouia, Materials Chemistry Physics., 2010, 122, 1-9.
[33] H. Ma, S. Chen, B. Yin, S. Zhao, X. Liu, Corros. Sci., 2003, 45, 867.



