Research Article - Der Pharma Chemica ( 2019) Volume 11, Issue 2
In Vitro Antioxidant Activity and Preliminary Phytochemical Screening of Morus nigra
Sunita Singh1, Alka Tripathi2, VK Lal3 and Dhananjay Singh4*2Department of Applied Sciences, Institute of Engineering & Technology, Lucknow-226021, India
3Lavanya Ayurveda Hospital, Chinhat, Lucknow, Uttar Pradesh-227105, India
4Department of Chemical Engineering, Institute of Engineering & Technology, Lucknow-226021, India
Dhananjay Singh, Department of Chemical Engineering, Institute of Engineering & Technology, Lucknow-226021, India, Email: dhananjay.singh@ietlucknow.ac.in
Abstract
Morus nigra L. (black mulberry) is a medicinal herb belonging to the family Moraceae and found in India and China. It is used as antihyperglycemic, diuretic, antioxidant, antiulcer, antibacterial, brain tonic, laxative, antimicrobial, antihypertensive, antihyperlipidemic, anticancer and antidiabetic. This study involves the extraction of dried leaves power of M. nigra using different organic solvents such as, chloroform, petroleum ether, water and ethanol. Preliminary phytochemical screening for the detection of secondary metabolites like alkaloids, glycosides, tannins, amino acids, flavonoid, resins, saponins, oil, fat, proteins, carbohydrates and phenolic contents. Further used of Thin Layer Chromatography (TLC) for the separation of biologically active constituents and study of in vitro antioxidant activity of leaf extracts.
Keywords
Morus nigra, Medicinal herb, Phytochemical, TLC, Antioxidant.
Introduction
Herbal medicines are the primary health care in several developing countries. Plants are rich source of natural products that is used for preparation of herbal medicine [1-3]. They are used in treatment of various diseases like fever, dysentery, diabetes, cancer, epilepsy, constipation, asthma, cough, cold, diarrhea, malaria, arthritis, piles, jaundice etc. Synthetic drugs are associated with side effects so many people’s are commonly used herbal medicines in India as well as some part of world [4,5].
Morus nigra L. belongs to Moraceae family, it is deciduous tree spread in West Asia Europe. Flowering season is January- February, and fruit mature in March- April. Ripe fruits are sweet and rich flavored, they are eaten fresh and made into jelly, jam and sherbet [6,7]. Wine is prepared from the fruits in Europe. The fruit juice is used as grateful drinks during convalescence; it cools the blood and decrease the thirst [8]. The barks of M. nigra are purgative and vermifuse. Infusion of leaves causes decrease the blood sugar and reduction of karterial pressure [9].
Plants are rich sources of phytochemicals with antioxidant activity. There are so many solvents for the phytochemical analysis of leaf extracts of pants like acetone, ethanol, petroleum ether, chloroform, Methanol and distilled water [10-13]. The composition of these solvents will be changed for different runs during extraction process of active compounds from plants leaf. The extracts will be tested for alkaloids, glycosides, tannins, amino acids, flavonoid, resins, saponins, oil, fat, proteins, carbohydrates and phenolic contents [14]. Isolation of active constituents may be done from different available methods along with the TLC method. Antioxidant activities of these extracts will be explored by using diphenylpicryl-hydrazyl (DPPH) [15]. Over all this effort of extraction of bioactive compounds from leaves of Indian medicinal plant may be a landmark in the area of pharmacognosy & phytochemistry [16-18].
Materials and Methods
Plant collection and authentication
Sample of plant material was collected from Institute of Engineering & Technology campus, sitapur road, Lucknow and it was given to National Botanical Research Institute Lucknow (U.P) for taxonomic authentification. The taxonomic identification of sample conformed that the species as: M. nigra and their accession No. is LWG -100983.
Extraction of leaves of M. nigra
Leaves of black mulberry were collected in bulk and washed thoroughly with running tap water to remove dust particle. It was dried under shade at room temperature, then crushed in to powder with a mechanical pulverizer and sieved (sieve no. 35). About 50 g of powdered leaves (Table 1) were uniformly packed in a thimble and plant extract was derived with different solvents (organic) such as, chloroform, petroleum ether, ethanol and H2O (from non polar to polar grade) in Soxhlet apparatus, reflux at 40ºC. It was run up to 24 h about 15 cycles until the solvent in the siphon tube of Soxhlet apparatus became colorless. Extracts were filtered with the help of whatmann (no.1) paper used for filtration and obtained plant extracts (crude) were dried through evaporation, in a evaporator (rotary vacuum) and stored in desiccator until further use. Various components are further derived from different residues.
| Extracts | Morus nigra |
|---|---|
| Petroleum ether | Yellowish green |
| Chloroform | Light green |
| Ethanol | Dark green |
| Water | Blackish green |
Table 1: Physical appearance of extracts of leaves of Morus nigra.
Phytochemical screening of different extracts
The plant is a rich source of various phytochemical compounds like alkaloids, glycosides, carbohydrates, tannins, amino acids, flavonoid, resins, saponins, proteins, oil, fat etc. The secondary metabolites are responsible for physiological and therapeutic effects. Therapeutic effects depend upon the type of chemical constituents present in drugs. Different organic solvents such as petroleum ether, chloroform, alcohol and water (non polar to polar) were used for determination of different groups of compounds like alkaloids, glycosides, carbohydrates, steroids, proteins, tannins, resins etc. (Table 2) [19-22].
| S. No. | Different Test Components | Name of various tests |
|---|---|---|
| 1 | Test for alkaloids | Mayer’s test, Dragendroff’s test, Wagner’s test, Hager’s test |
| 2 | Test for glycosides | Borntrager’s test, Keller kiliani’s test, Legal’s test, Baljet test |
| 3 | Test for sugars | Molish test, Fehling’s test, Benedict test, Barford’s test |
| 4 | Test for proteins and amino acids | Biurate test, Xanthoproteic test, Millon’s Test, Ninhydrin test |
| 5 | Test for sterols | Salkowaski test, Liebermann’s test, Liebermann’s Burchard test |
| 6 | Test for phenolic compounds | Ferric Chloride test, Lead Acetate test |
| 7 | Test for tannins | Ferric Chloride test, Lead Acetate test, Bromine water test, Potassium dicromate test, Gelatin test, Match stick test |
| 8 | Test for flavanoids | Ferric Chloride test, Lead Acetate test, Shinoda test |
| 9 | Test for saponins | Foam test, Confirmatory test |
| 10 | Test for fixed oil and fats | Bromine water test, Spot test, Saponification test |
Table 2: Different tests and their components in the extracts of the leaves of Morus nigra
Results and Discussions
Thin Layer Chromatography
The adsorbent used for thin layer chromatography is silica gel G. About 25 g of silica gel G was taken in a glass mortar and about 20-25 ml of distilled water was added slowly to it. The mixture was stirred continuously with gloss rod until it become uniform. The slurry is placed in an applicator. The adsorbent layer is activated by drying the thin layer in an oven for 30 min at 110°C. Capillary tube may be used for sample application. 1 ml/mg of the standard (Rutin, quekrcetin, gallic acid) was prepared with methanol. 10 mg of the sample was diluted with 1 ml of methanol (Table 3).
| Tests | Petroleum Ether | Chloroform | Ethanol | Aqueous |
|---|---|---|---|---|
| Test for alkaloids | ||||
| Mayer’ test | + | + | + | - |
| Dragendroff’ test | + | - | - | + |
| Wagner’s test | - | - | - | - |
| Hager’s test | + | + | + | + |
| Test for glycosides | ||||
| Borntrager’s test | + | - | - | - |
| Keller kiliani’ s test | - | - | + | + |
| Legal’s test | + | + | - | - |
| Baljet’s test | + | - | - | - |
| Test for sugars | ||||
| Molish’s test | + | + | + | + |
| Fehlig’s test | - | + | + | - |
| Benedict’s test | + | + | - | - |
| Barford’s test | - | - | - | - |
| Tollen’s test | + | + | + | - |
| Test for proteins and amino acids | ||||
| Biuret test | - | - | - | + |
| Xanthoproteic test | - | - | + | + |
| Millon’s test | + | + | + | - |
| Ninhydrin test | + | + | - | - |
| Test for sterols | ||||
| Salkowaski’s test | + | - | - | + |
| Liebermann’s test | + | + | + | - |
| Liebermann- Burchard’s test | + | - | - | - |
| Test for phenols | ||||
| Ferric chloride test | + | + | + | + |
| Lead acetate test | _ | + | + | + |
| Test for tannins | ||||
| Ferric chloride test | + | + | + | + |
| Lead acetate test | - | + | + | + |
| Bromine water test | + | + | + | - |
| Potassium dichromate test | - | - | - | - |
| Gelatin test | - | - | + | + |
| Match stick test | + | + | - | - |
| Test for flavonoids | ||||
| Ferric chloride test | + | + | + | + |
| Lead acetate test | - | + | + | + |
| Shinoda test | + | - | - | - |
| Test for saponin | ||||
| Foam test | + | + | + | + |
| Test for oil and fats | ||||
| Bromine water test | + | + | + | - |
| Spot test | + | + | - | - |
| Saponification test | - | + | + | + |
| Test for coumarin | - | + | - | - |
| Test for gums and mucilage | - | - | - | - |
Table 3: Preliminary phytochemical screening of different extracts of Morus nigra leaves
Good separation was achieved by using of several mobile phase to identify the compounds. Plate is kept at 45º angle in a development chamber. The solvent system is filled in the chamber about one millimeter from the bottom. All sides (except upper side) of tank are covered with paper and upper side is covered with lid. The saturated condition of solvent in the development chamber is necessary to avoid evaporative losses.
Visualization: Observe the plate under UV 366 nm & spray the plate with 1% sulphuric acid and note the Rf value (Table 4).
| S. No. | Extracts | Solvent system | Spot visible in UV light (366 nm) | Rf value |
|---|---|---|---|---|
| 1 | Petroleum ether | Toluene: ethyl acetate: diethyl amine (70:20:10 v/v/v) | 4 | 0.57, 0.4 |
| 2 | Chloroform | Methanol: glacial acetic acid: formic acid: water (3:0.9:0.9:.0.5 v/v/v/v) | 2 | 0.7, 0.38 |
| 3 | Ethanol | Formic acid: methanol: water (3:40:57 v/v/v) | 3 | 0.8, 0.55, 0.93 |
| 4 | Water | Ethyl acetate: dichloromethane: formic acid: glacial acetic acid: Water (10:2.5:1:1:0.1 v/v/v/v/v) | 4 | 0.32, 0.15, 0.56, 0.9 |
Table 4: Different extracts of Morus nigra species and their Rf values
Rf value of standard rutin, quercetine and gallic acid were 0.58, 0.95 and 0.35. Petroleum ether and water has more no. of spots. Extract obtained from petroleum ether, show the occurrence of rutin and gallic acid, extract obtained from chloroform show the occurance of gallic acid, ethanol extract show the occurrence of rutin and quercetine, water extract shows the presence of rutin, quercetine and gallic acid (Table 5).
| S. No. | Extracts | % Scavenging activity | IC50 Values (µg/ml) |
|---|---|---|---|
| 1 | Petroleum ether | 56.53 ± 4.32 | 321.55 ± 1.38 |
| 2 | Chloroform | 42.80 ± 2.58 | 286.62 ± 3.19 |
| 3 | Ethanol | 77.45 ± 1.26 | 358.41 ± 2.51 |
| 4 | Water | 82.76 ± 3.61 | 392.76 ± 2.11 |
Table 5: Various extracts and their scavenging activity, along with IC50 values
In vitro antioxidant activity
DPPH free radical scavenging assay
The plant extracts was analyzed for scavenging activity to estimate the free radical. The antioxidant compounds are characterized by DPPH, which is extensively used to estimate the scavenging activity for determination of free radicals. It is a stable free radical. By the action of an antioxidant compound or a free radical scavenging species, the DPPH which are purple in colour is reduced to form yellow colour diphenylpicrylhydrazine, due to the decrease of the absorption of DPPH.
Change of colour of DPPH from purple to yellow is measured at 517 nm spectrophotometrically (UV-visible spectrophotometer). Quercetin is used as standard. Concentrations of quercetin 20, 40, 60 and 80 μg/ml were made by using methanol. Concentration of plant extract 100 μg/ml was also made by using methanol. The solution (stock) of DPPH was prepared by 0.2 mg DPPH dissolved in 15.3 ml methanol. The mixture of 1.8 ml of DPPH in methanol solution, 0.1 ml of the plant extract and 0.1 ml of methanol. It was kept for 30 min at room temperature in the absence of light and the measurement of absorbance was done against a blank (DPPH in methanol) at 517 nm. All determinations were taken three times and the results are shown as mean % scavenging activity (% inhibition). The scavenging activity of plant extracts against DPPH radicals was calculated by using the following equation:
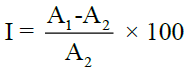
Where, I=% inhibition,
A1= Ablank, absorbance of control (DPPH in methanol)
A2= Asample, absorbance of test sample
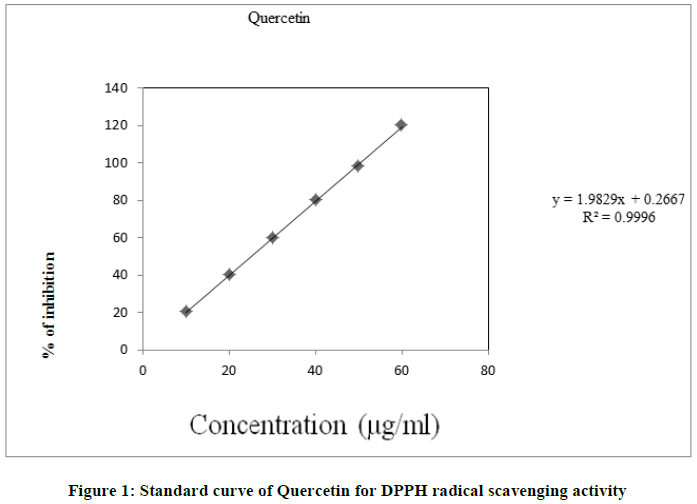
The antioxidant activity is depending on the DPPH scavenging capacity of the test sample. The antioxidant activity of leaf extracts was evaluated by monitoring its ability in the free radical DPPH. The ability of DPPH was expressed as IC50. The IC50 value is the concentration of the test extract necessary to reduce 50% of the DPPH in the mixture. IC50 was calculated by plotting a graph between the % inhibition and concentration of standard. IC50 values were expressed as g/ml (Figure 1) [23,24].
Mean and standard deviation calculated as follows
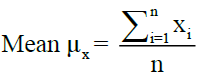
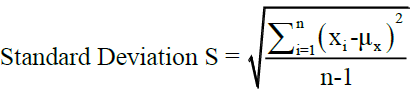
Where, i= 1,…., n, denote the number of samples, X=Variable parameters.
The present paper shows that the leaf of M. nigra is a significant source of natural antioxidants. Graph shoes that the scavenging effects increased with their concentrations.
In the table scavenging activity of DPPH radicals increased in the following order: 42.80 ± 2.58 % in Chloroform extract, 56.53 ± 4.32% in Petroleum ether extract, 77.45 ± 1.26 % in ethanol extract, 82.76 ± 3.61% in water extract. The IC50 values indicated that the antioxidant capacity of water extract was the greatest (392.76 ± 2.11 μg/ml) than Chloroform extract, Petroleum ether extract and ethanol extract.
Conclusion
M. nigra species has various secondary metabolites like alkaloids, glycosides, carbohydrates, tannins, amino acids, flavonoid, resins, saponins, proteins, oil, fat etc. The secondary metabolites are responsible for physiological and therapeutic effects. Therapeutic effects depend up on the type of chemical constituents present in drugs. This study shows the major biologically active compounds present in the chloroform, petroleum ether, water and ethanol extracts of the leaves of M. nigra. This paper shows that the leaf of M. nigra is major source of natural antioxidants. These results contribute significantly and motivate us to explore deeply its phytochemical outcomes.
References
- P. Supritha, K.V. Radha, Int. J. Pharma. Edu. Res., 2018, 52(2), 321-326.
- M. Savitree, P. Isara, S.L. Nittaya, S. Worapan, J. Pharm. Sci., 2004, 9(1), 32-35.
- E. Pinar, K. Sevda, Int. J. Nature Life Sci., 2017, 1(1), 22-31.
- H. Chen, J. Pu, D. Liu, W. Yu, Y. Shao, G. Yang, Plos One., 2016, 11(4), E0153080.
- G.T. Volpato, I.M.P. Calderon, S. Sinzato, K.E. Campos, M.V.C. Rudge, D.C. Damasceno, J. Ethnopharma., 2011, 138, 691-696.
- Y. Jiang, W.J. Nie, Food Chem., 2015, 174, 460.
- H.M. Tag, BMC Complement Altern Med., 2015, 15, 252.
- Z.P. Zheng, K.W. Cheng, Q. Zhu, X.C. Wang, Z.X. Lin, M. Wang, J. Agric. Food Chem., 2010, 58(9), 5368-73.
- N. Muharrem, B. Lutfi, Int. J. Nature Life Sci., 2018, 2(2), 57-71
- S. Zhang, Y. Lin, H. Zhou, S. Wei, G. Lin, G. Ye, Molecules, 2010, 15(8), 5658-70.
- G.A. Naderi, S. Asgary, Z.N. Sarraf, H. Oroojy, Phytother. Res., 2004, 18(5), 365.
- L.A. Margareth, Molecules, 2013, 18, 8342-8357.
- S. Kaur, P. Mondal, J. Microbiol Exp., 2014, 1(1), 00005.
- E. Figen, D. Guldan, K. Yasar, E. Harun, Int. J. Nature Life Sci., 2017, 1(1), 1-11.
- O. Mazimba, R.R.T. Majinda, D. Motlhanka, Afri. J. Pharm. Pharmacology., 2011, 5(6), 751-754.
- S.E.M. Sanchez, M. Tassotti, R.D. Del, F. Hernandez, J.J. Martanez, P. Mena, Food Chem., 2016, 212, 250-255.
- D.A. Kostic, D.S. Dimitrijevic, S.S. Mitic, M.N. Mitic, G.S. Stojanovic, A.V. Zivanovic, Trop. J. Pharmaceut. Res., 2013, 12(1), 105-110.
- B. Ebru Yuce, V. Ernst, C. Ugur, Int. J. Nature Life Sci., 2017, 1(2), 39-66.
- R. Uddin, M.R. Saha, N. Subhan, H. Hossain, I.A. Jahan, R. Akter, A. Alam, Adv. Pharma. Bull., 2014, 4(3), 273-281.
- A.M.A. Mawla, K.M. Mohamed, A.M. Mostafa, Sci. Pharm., 2011, 79, 951-961.
- Q.V. Vuong, S. Hirun, P.D. Roach, M.C. Bowyer, P.A. Phillips, C.J. Scarlett, J. Herbal Med., 2013, 3, 104-111.
- S. Aydan Acar, E. Ibrahim, A. Senol, P. Nur Munevver, Int. J. Nature. Life Sci., 2017, 1(2), 83-88.
- P. Sunitha, N. Sathyanarayana, V.C. Suresh, Indian J. Pharm. Sci., 2018, 80(1), 192-198.
- H.N.T. Pham, Q.V. Vuong, M.C. Bowyer, C.J. Scarlett, Chemical Papers, 2017, 1-10.