Research Article - Der Pharma Chemica ( 2023) Volume 15, Issue 6
In Vivo Assessment, Formulation, Characterization and Enhancing Pharmacotherapy of Encapsulated Mini Tablets for Immediate Release Sildenafil Citrate and Sustained Release Bosentan
Deepak Kumar Sarangi1*, Chandra Sekhar Patro2, CH. Niranjan Patra3 and Sanjeeb Kumar Sahoo42Department of Pharmaceutics, Centurion University of Technology and Management, Bhubaneswar, Odisha, India
3Department of Pharmaceutics, Roland Institute of Pharmaceutical Sciences, Brahmapur, Odisha, India
4Department of Pharmaceutics, Centurion University of Technology and Management, Bhubaneswar, Odisha, India
Deepak Kumar Sarangi, Department of Pharmaceutics, Centurion University of Technology and Management, Bhubaneswar, Odisha, India, Email: sarangi.dipu@gmail.com
Received: 28-Oct-2023, Manuscript No. Dpc-23-120632; Editor assigned: 31-Oct-2023, Pre QC No. Dpc-23-120632 (PQ); Reviewed: 14-Nov-2023, QC No. Dpc-23-120632; Revised: 17-Nov-2023, Manuscript No. Dpc-23-120632 (R); Published: 15-Dec-2023, DOI: 10.4172/0975-413X.15.6.127-133
Abstract
The objective of the current study is to formulate encapsulated sildenafil citrate Immediate Release (IR) and Bosentan sustained release Minitablets (MTs) for treating pulmonary arterial hypertension. This piece of research work signifies a targeted dosage form that is designed in the form of encapsulated minitablets taking different super disintegrants and polymers which produces superior product quality. In vivo pharmacokinetic studies of prepared mini tablets are also showed promising results. Using different concentrations of polymers like HPMC K15M, ethyl cellulose and sodium CMC for Bosentan SR tablets, while for sildenafil citrate IR super disintegrants like sodium starch glycollate, magnesium aluminium silicate (Veegum-HV) and crosslinked PVP are taken for the manufacturing of MTs. All the pre compression and post compression parameters are carried out. The investigation showed that formulation S6 for sildenafil citrate IR MTs showed a desirable and optimistic profile for immediate release within 10 minutes, whereas formulation B3 for Bosentan SR MTs showed promising sustained release action for about 24 hours. In vivo pharmacokinetic parameters also show promising results towards the formulation and encapsulation. Therefore, the creation of encapsulated MTs with two active pharmaceutical ingredients could boost patient medication compliance and serve as a platform to get over the technical as well as financial limitations associated with current dosage forms as well as time-consuming and expensive expenditures.
Keywords
Bosentan; Immediate release; Pulmonary artery hypertension; Sustained release ; Sildenafil citrate; Encapsulation; Mini tablets
Introduction
Pulmonary Arterial Hypertension (PAH) has been characterized as when pulmonary artery pressure is more than 30 mmHg while exercising and 25 mmHg while at rest [1]. Dyspnea, exhaustion, chest discomfort and vertigo are all symptoms of PAH, which is characterized by an increase in pulmonary artery pressure as well as pulmonary vascular resistance [2]. PAH is a deadly condition that progresses quickly and is marked by an increase in both pulmonary artery pressure and pulmonary vascular resistance [3].
The endothelin, prostacyclin and nitric oxide pathways, three key mechanistic pathways, are recognized as playing an essential part in the growth of PAH and treatments that target these pathways are readily available [4]. Combination drugs that target multiple pathways are an appealing strategy for treating PAH and could provide better long term results than monotherapy [5]. The first non-peptide Endothelin receptor (ETA and ETB) antagonist created and approved for use with PAH in this particular therapy is called bosentan. Its plasma elimination half-life is 5 hours and its oral bioavailability is 50%. Due to the short half-life, it would be preferable to provide bosentan in a controlled release formulation to maintain therapeutic plasma levels and hence minimize side effects [6].
The Phosphodiesterase type 5 (PDE5) inhibitor sildenafil is orally active, strong and selective. It is distributed in high concentrations throughout the body, particularly in the lungs [7]. Because PDE5 inhibits the breakdown of Cyclic Guanosine Monophosphate (cGMP), which fosters vascular smooth muscle relaxation and stimulates blood flow, by virtue of which nitric oxide's vasodilator effects in pulmonary hypertension are improved. Sildenafil has been found to provide a relatively selective decrease in pulmonary artery pressure without having adverse systemic hemodynamic effects [8]. Patients with metabolic syndrome typically need to take two or more drugs at therapeutic dosages [9]. The sequential administration of medications in accordance with a set schedule is necessary for some desired pharmacological therapies in order for this combination therapy to be effective [10]. Fixed dose combos are administered when two or more medicatio ns are produced in a single dosage form with a predetermined ratio of Fixed-Dose Combinations (FDC). By encouraging comfortable medication administration, this FDC has developed into an efficient method for raising patient compliance [11]. In the field of pharmaceutical technology, MTs with sustained release have made possible new developments in novel drug delivery systems [12]. Bosentan has been taken in the form of sustained release MTs for this research and sildenafil citrate has been administered in the form of Immediate Release (IR) MTs. Size 1 gelatine capsule shells are used to encapsulate both MTs [13].
Materials and Methods
Bosentan was received from Care Formulations Limited (CFL) as a gift sample (Delhi, India). Also, Kay Biotech provided a free sample of sildenafil citrate (Delhi, India). Starch, microcrystalline cellulose, sodium starch glycollate, lactose, magnesium aluminum silicate and crosslinked PVP were acquired from BASF. Methocel (HPMC K15M) was bought from Dow Chemical (USA). Colorcon provided sunset yellow FCF and PVP as film coating materials. Shin-Etsu chemical (Japan), which provides ethyl cellulose and sodium CMC. The supplier of the magnesium stearate and purified talc was Asia Pacific lab (Merlimau, Singapore). A milli-Q system was employed to create ultrapure water (Millipore, Bedford, MA, USA). The other compounds were all of the reagent variety.
Pre-formulation study
Fourier transform infrared spectroscopy: Bosentan and Sildenafil citrate were analyzed using FT-IR in their pure forms as well as in mixtures with their corresponding polymers and a super disintegrating agent utilizing KBr discs to assess drug-polymer compatibility (Shimadzu, Japan). During operation, dry air was purge pumped into the equipment and resolution scans were performed over the range of 4000-400 cm-1.
Differential Scanning Calorimetry (DSC): With the aid of a differential scanning calorimeter, the thermal analyses were carried out (DSC-TA-60, Shimadzu, Japan). A sealed aluminum pan containing 2 milligrams of drug along with its physical mixture combined with polymers and super disintegrates were heated from 30°C to 300°C at a rate of 10°C/min. Referencing was done using an unfilled aluminum pan.
Preparation of MTs: Bosentan and Sildenafil citrate were passed through screen 40 along with polymers, super disintegrating agents and other excipients. The necessary amounts of each ingredient were then weighed and using multi-tip flat punches, the resulting powder mixtures were compacted into MTs with a 3 mm diameter (Karnavati, Ahmedabad, India).
Film coating of Bosentan MTs: Prepared Bosentan MTs are film coated using a 60:40 ratio of isopropyl alcohol and dichloro methane, with 2% polyvinayl pyrrolidone as plasticizer and 5% sunset yellow FCF as coating material. Dip coating technology has been employed to coat all the Bosentan MTs.
Quality control tests for MTs: By using a mortar and pestle to crush ten MTs, the amount of the drug's content was determined. A portable digital hardness tester (Electrolab, India) was used to measure the hardness of six MTs and the average of the six MTs was computed. A Roche friability tester was used to assess the friability of 20 MTs. Prior to and following the friability test, ten MTs were weighed. The weight reduction in percent was then computed. Taking the weights of 20 MTs individually and collectively, the weight variation test with its percentage of deviation was computed.
Preparation of Bosentan SR and sildenafil citrate IR encapsulated MTs
The direct compression method was used to compress the drugs along with their excipients, which had a punch size of 3 mm according to the formulation table and all the prepared MTs were filled with size 1 hard gelatine capsule shells.
Study of in vitro dissolution
The prepared Sildenafil citrate IR and Bosentan SR MTs were subjected to an in vitro dissolution study using USP type 2 equipment. Time stamps for sildenafil citrate MTs were 2, 4, 6, 8 and 10 minutes and for Bosentan SR, it was 1, 3, 6, 9, 12, 15, 18 and 24 hours. Exactly 5 mL aliquots were taken from the dissolution jar as the testing samples. The sink condition was maintained by replenishing the dissolution jar with 5 mL of fresh media.
In vivo pharmacokinetic study
The institutional animal ethical committee approved for the bio analytical estimation of sildenafil citrate and bosentan in wister rats serum samples, the reported UFLC method was used . The wistar rats (150 g-200 g) rats were housed in clean polypropylene or corrugated paper cages at controlled room temperature (25°C ± 2°C) and humidity at 50%-60% with 12h light and dark cycle throughout the experiment. All rats had free access to pelleted food and tap water ad libitum before the experiments. The experimental procedures were approved by the institutional ethical committee. Two minitablets to be administered at a time. One mini tablet of sildenafil containing 0.3 mg/rat and one minitablet of bosentan 0.97 mg/rat to two gropus of animals each containing 6 animals.
Blood samples (150 μl) were collected via the tail vein 0.5, 1, 2, 4 and 8 hrs after the drug administration. After the oral administration of sildenafil citrate. The blood samples were immediately transferred to heparinised micro centrifuge tubes and centrifuged at 4000 for 20 min at 4°C. The separated plasma samples were transferred to Eppendorf tubes and stored at -70°C until further use. Each sample was mixed for 10 seconds by vortexing and centrifuged at 9000 rpm for 10min. The supernatant was transferred to another Eppendorf tube for further analysis. The plasma concentrations of sildenafil were determined using a liquid chromatography method accordingly.
The HPLC system consisted of a solvent delivery pump equipped with an inline degasser. The mobile phase used was 75% acetonitrile plus 25% phosphate buffer (20 mM) which was first filtered and degassed using a 0.22 μm nylon membrane filter before use. The flow rate was 0.9 μL/min (isocratic) and the injection volume was 20 μL.
Stability studies: The stability studies had been conducted for prepared encapsulated MTs as per the ICH guidelines.
Results and Discussion
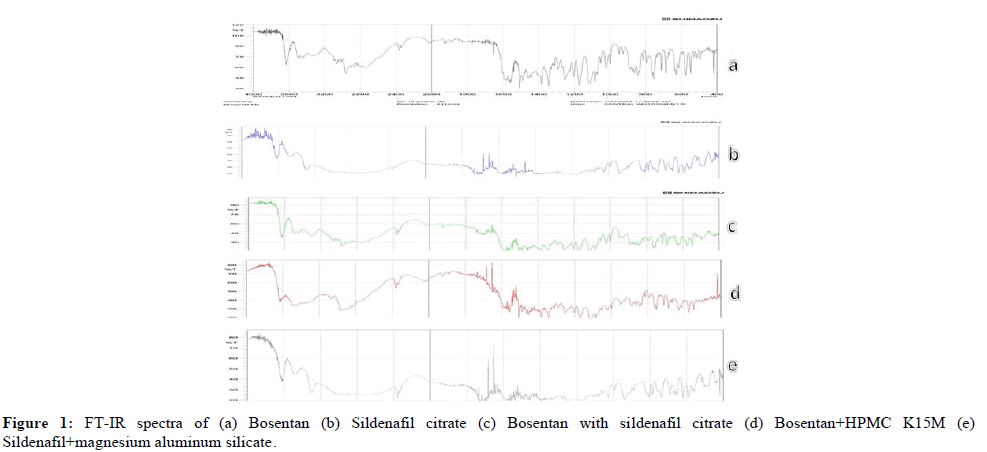
Fourier transform infrared spectroscopy (FT‑IR): Bosentan pure drug revealed two carbonyl absorption bands at 1671 cm-1, which were described as amide carbonyl stretching. There were two absorption peaks at 3484 cm-1 and 1779 cm-1 in the physical mixing of Bosentan with HPMCK15M. Similarly, an absorption band appeared in Sildenafil citrate pure drug, which was attributed to amide stretching. When sildenafil is mixed with magnesium aluminum silicate, no interaction is observed between the peak of sildenafil and the physical mixture. Figure 1 clearly shows that the FT-IR spectra of the drug in its purest form and its designed formulations showed no variation in peak features, demonstrating unequivocally that there were no interactions between the drug itself and the excipients in the current study.
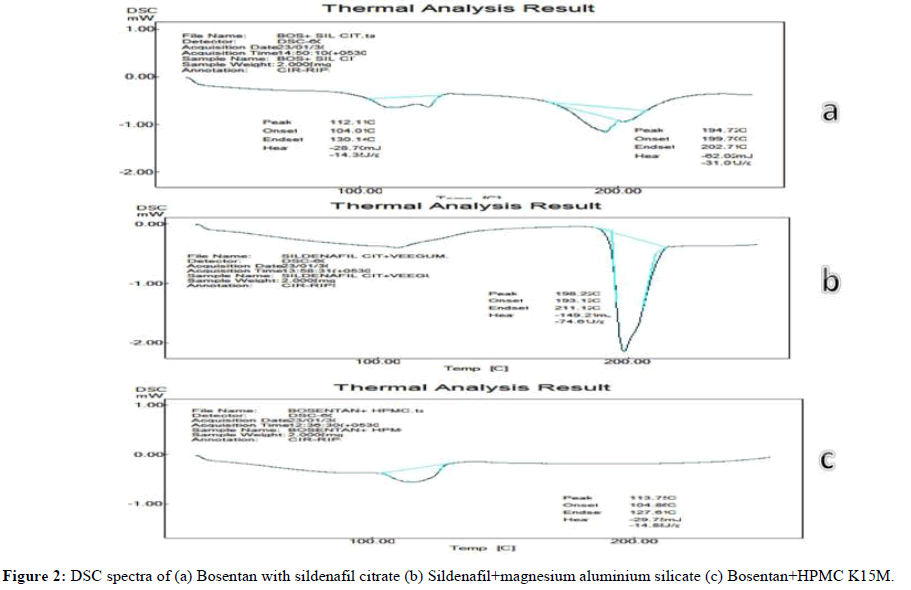
Differential Scanning Calorimetry (DSC): The physical mixture of Bosentan and Sildenafil citrate in the DSC thermogram displayed an onset and endset temperature between 199.71°C and 202.71°C and an endothermic peak at 194.71°C. It was determined that the latent heat of fusion (H) is 62.02 mJ. As the thermo gram of DSC revealed that Bosentan and HPMC K15M, the endothermic peak was recorded at temperatures of 104.86°C and 127.62°C and the latent heat of fusion was determined to be -29.75 mJ.
Similarly, the DSC thermo gram for sildenafil citrate with magnesium aluminum silicate revealed an endothermic peak at 192.22°C; however, the start and end temperatures were both at 193.12°C and 211.12°C. It was discovered that the latent heat of fusion was -149.2 mJ. In order to extrapolate the results, the medicine and excipients employed in this investigation must be compatible. There are no discernible changes in the melting point. The endothermic peak characteristics between the API and the formulation were identical, as demonstrated by the DSC spectra in Figure 2, which emphasize that there will be no interactions observed between the pure drug and excipients.
Preparation of MTs: The necessary amounts of each ingredient were then weighed in accordance with Tables 1 and 2, which show 18 formulations using three different super disintegrating agents for sildenafil citrate Immediate Release (IR) MTs and three different polymers for Bosentan Sustained Release (SR) MTs.
| S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | |
|---|---|---|---|---|---|---|---|---|---|
| Sildenafil citrate | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Starch | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Microcrystalline cellulose | 3.9 | 3.7 | 3.5 | 3.9 | 3.7 | 3.5 | 3.9 | 3.7 | 3.5 |
| Lactose | 3.9 | 3.7 | 3.5 | 3.9 | 3.7 | 3.5 | 3.9 | 3.7 | 3.5 |
| Sodium starch glycollate | 0.2 | 0.6 | 1 | ||||||
| Magnesium aluminum silicate (veegum HV) | 0.2 | 0.6 | 1 | ||||||
| Crosslinked PVP | 0.2 | 0.6 | 1 | ||||||
| Magnesium stearate | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Purified talc | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Total weight (mg) | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 |
Table 1: Formulation and composition of immediate release minitablets of sildenafil citrate.
| Formulation code | B1 | B2 | B3 | B4 | B5 | B6 | B7 | B8 | B9 |
|---|---|---|---|---|---|---|---|---|---|
| Bosentan | 12.5 | 12.5 | 12.5 | 12.5 | 12.5 | 12.5 | 12.5 | 12.5 | 12.5 |
| Starch | 1.7 | 1.5 | 1.1 | 1.7 | 1.5 | 1.1 | 1.7 | 1.5 | 1.1 |
| Microcrystalline cellulose | 1.7 | 1.7 | 1.7 | 1.7 | 1.7 | 1.7 | 1.7 | 1.7 | 1.7 |
| Lactose | 1.7 | 1.7 | 1.7 | 1.7 | 1.7 | 1.7 | 1.7 | 1.7 | 1.7 |
| HPMC K15M | 0.2 | 0.6 | 1 | ||||||
| Ethyl cellulose | 0.2 | 0.6 | 1 | ||||||
| Sodium CMC | 0.2 | 0.6 | 1 | ||||||
| Magnesium stearate | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Purified talc | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Total weight (mg) | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 |
Table 2: Formulation and composition of immediate release minitablets of sildenafil citrate.
Encapsulation of Bosentan monohydrate MTs: Prepared encapsulated sildenafil IR MTs and Bosentan SR MTs are produced in size 1 hard gelatine capsule shell depicted in Figure 3.
Evaluation tests for MTs: All of the MT formulations had drug content percentages between 95% and 99.5%. All MTs have a high drug content, which can be attributed to their uniform size and optimal drug and excipient combination. The MTs ranged in hardness from 3 to 5 kg/cm2. This guarantee the MTs have the desired physical strength to keep their integrity while being processed and stored. It was found that the weight loss of mini tablets during friability testing was less than 0.35%, which falls below the 1% limit allowed. The MT formulations of both the drugs, i.e., sildenafil citrate IR MTs (S1 to S9) and Bosentan SR MTs (B1 to B9), passed the weight variation test within the deviation limit of 10%. The results of post compression parameters for both Bosentan and Sildenafil citrate MTs are given in Table 3.
| Formulation code | Hardness* kg/cm2 | Friability** % | Uniformity of content*** (mg) | Uniformity of weight**** (mg) |
|---|---|---|---|---|
| S1 | 3.4 ± 0.021 | 0.42 ± 0.002 | 5.01 ± 1.11 | 20.12 ± 1.23 |
| S2 | 4.6 ± 0.028 | 0.49 ± 0.003 | 5.12 ± 1.13 | 20.41 ± 1.23 |
| S3 | 3.8 ± 0.025 | 0.41 ± 0.001 | 5.13 ± 1.15 | 20.75 ± 1.23 |
| S4 | 4.4 ± 0.026 | 0.38 ± 0.002 | 4.98 ± 1.12 | 20.59 ± 1.23 |
| S5 | 4.2 ± 0.021 | 0.25 ± 0.004 | 5.01 ± 0.99 | 20.16 ± 1.23 |
| S6 | 6.4 ± 0.022 | 0.31 ± 0.002 | 4.99 ± 0.89 | 20.06 ± 1.38 |
| S7 | 4.4 ± 0.023 | 0.29 ± 0.004 | 5.08 ± 0.97 | 20.18 ± 1.29 |
| S8 | 3.9 ± 0.022 | 0.39 ± 0.006 | 5.09 ± 1.12 | 20.83 ± 0.99 |
| S9 | 4.4 ± 0.029 | 0.23 ± 0.002 | 5.11 ± 1.23 | 20.56 ± 1.43 |
| B1 | 4.4 ± 0.024 | 0.44±0.003 | 12.51 ± 0.86 | 20.23 ± 1.23 |
| B2 | 5.1 ± 0.026 | 0.37 ± 0.006 | 12.45 ± 1.13 | 20.33 ± 1.29 |
| B3 | 4.4 ± 0.022 | 0.23 ± 0.007 | 12.53 ± 0.99 | 20.39 ± 1.11 |
| B4 | 4.4 ± 0.029 | 0.24 ± 0.002 | 12.48 ± 0.93 | 20.66 ± 1.33 |
| B5 | 5.5 ± 0.028 | 0.33 ± 0.005 | 12.59±0.89 | 20.25 ± 1.98 |
| B6 | 5.2 ± 0.028 | 0.41 ± 0.005 | 12.49 ± 0.91 | 20.14 ± 1.34 |
| B7 | 5.1 ± 0.027 | 0.27 ± 0.009 | 12.53 ± 1.11 | 20.09 ± 1.65 |
| B8 | 4.9 ± 0.025 | 0.36 ± 0.001 | 12.59 ± 0.98 | 20.87 ± 1.99 |
| B9 | 5.3 ± 0.024 | 0.41 ± 0.002 | 12.56 ± 0.97 | 20.76 ± 1.76 |
Table 3: Post compression parameters of the prepared sildenafil citrate IR MTs and Bosentan SR MTs. Note: *n=5, **n=20, ***n=10, ****n=20.
In vitro dissolution study
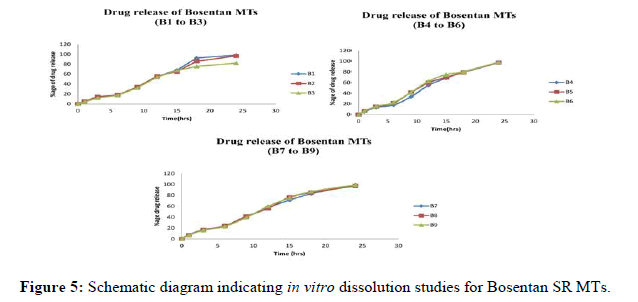
The in vitro dissolution study was conducted for encapsulated sildenafil citrate IR MTs for 10 minutes and Bosentan SR MTs prepared for 24 hrs. The release profile data is given in Figure 4. The disintegration rate of sildenafil citrate MTs made with magnesium aluminum silicate that are encapsulated with Bosentan and HPMC K15M shows immediate release as well as sustained release patterns.
In vivo pharmacokinetic study
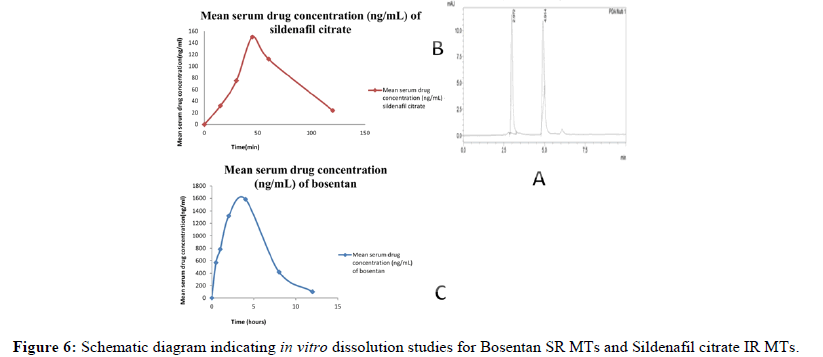
RP-UFLC measured serum drug concentrations at various time intervals during the pharmacokinetic investigation using mini tablets of sildenafil citrate and bosentan are depicted in Table 4 and area under the curve are shown in Figures 5 and 6.
| PK parameters | For sildenafil citrate MTs | For Bosentan MTs |
|---|---|---|
| Cmax (ng/mL) | 150.4 ± 13.12 | 1317.3 ± 112.16 |
| Tmax (hr) | 0.75 ± 0.03 | 3.11 ± 1.21 |
| AUC (ng h)/mL | 5103.2 ± 211.13 | 2340.5 ± 109.13 |
| AUMC (ng/h2)/mL | 4123.2 ± 113.21 | 1262.7 ± 102.65 |
| MRT (h) | 1.12±0.34 | 5.04±1.11 |
Table 4: Estimation of the pharmacokinetic parameters for encapsulated sildenafil citrate and bosentan mini tablets.
Stability study: The stability testing for the chosen encapsulated MTs revealed no appreciable changes in the homogeneity of the content or drug release. As a result, the formulations are stable in accordance with ICH requirements. Table 5 illustrates the stability study findings. As a result, the formulations are stable in accordance with ICH requirements.
| No. of months | Drug content (%) | Hardness* kg/cm2 | Uniformity of weight**(mg) | |
|---|---|---|---|---|
| 1 | Sildenafil citrate IR MTs | 99.76 ± 1.231 | 3 ± 0.021 | 20.56 ± 1.43 |
| Bosentan SR MTs | 99.86 ± 1.271 | 4 ± 0.023 | 20.12 ± 1.25 | |
| 3 | Sildenafil citrate IR MTs | 99.57 ± 1.131 | 3 ± 0.018 | 20.34 ± 1.35 |
| Bosentan SR MTs | 99.37 ± 1.071 | 4 ± 0.011 | 20.09 ± 1.11 | |
| 6 | Sildenafil citrate IR MTs | 99.17 ± 1.101 | 3 ± 0.012 | 20.21 ± 1.12 |
| Bosentan SR MTs | 99.06 ± 1.031 | 4 ± 0.001 | 20.04 ± 1.09 | |
Table 5: Six month stability studies of the optimized formulation. Note: *n=5, **n=10.
The post compression evaluation test for all the 9 formulations of immediate release mini tablets of sildenafil were performed as per the standard procedure. The content uniformity for all the formulations was more than 95% which are depicted to uniform particle size, optimum mixing time and uniform filling of die cavity because of good flow ability [14]. The hardness for all IR MTs was nearly 4 kg/cm2. The hardness of MTs in lower side of acceptable range 4 to 6 kg/cm2 may because the smaller size 3 mm of MTs and use of multi tip punches. The permissible limit for weight variation is ± 10% and all the nine IR MTs passed weight variation test. This is attributed to desirable flow ability of pre compression powder mixture as evident from angle of repose values less than 25 degree for all formulations. Friability for all the IR MTs formulations was less than 1% which meets the official requirements [15]. In case of sodium starch glycollate based IR MTs (S1 to S3), the disintegration time decreased from 19.5 to 8.6 seconds. The quick disintegration was because of the rapid uptake of water followed by rapid and enormous swelling [16]. In case of magnesium aluminum silicate based IR MTs when the percentage of disintegrate was increased from 1 to 3% a slight decrease in disintegration time from 14.6 seconds to 11.5 seconds was observed [17]. However further increase in the proportion of magnesium aluminum silicate from 3 to 5% (S4 to S6) a sudden decrease or very quick disintegration time i.e., DT 1.4 second was observed [18]. Magnesium aluminium silicate has been studied by strain recovery mechanisms and swelling mechanistic theories [19]. In strain recovery mechanism, the stored energy present in the tablet is released in terms of heat and therefore the disintegrates present in the tablet consequently break due to the irreversible and unidirectional compaction force released from the tablet, which leads to strain recovery [20]. Similarly, in the swelling mechanism, magnesium aluminium silicate creates pressure when it comes in contact with liquid, which leads to stresses of exertion that break the tablet [21]. Totea AM, et al., used cryogenic calorimetry to better understand how different drugs are attached to the magnesium aluminium silicate [22]. It was additionally utilized as a super disintegrating agent and to enhance the physical properties of pharmaceuticals. One alumina or magnesia octahedral sheet is positioned among two tetrahedral silicate sheets to create the compound's layered silicate structure [23]. In case of crosslinked PVP based IR MTs the disintegration time was in the range of 11.5 to 5.3 mean seconds for formulations (S7 to S9). The decrease in the disintegration time was attributed to the moisture sorption and hydration capacity of crosslinked PVP [24]. The loss in weight after friability test was very less which is less than 0.3%. This can be attributed to unique property of cross linked PVP which produce tablets with low percentage of friability. Disintegration test performed for all the nine IR MTs exhibited that as the concentration of super disintegrates increased the disintegration time also decreased [25]. The Bosentan MTs ranged in hardness from 3 to 5 kg/cm2. In the formulation B1 to B3; HPMC K15M works by forming a gelling network, which acts as a barrier and delays the release of the drug. Industrial varieties of HPMC were moulded into compacts and a study evaluated their hydration behavior and surface hydrophilicity to assess the in vitro muco adhesive performance, their tensile strength between the compacts and various mucosal membrane regions was measured [26]. As the viscosity of HPMC compacts increased their hydration also increased mucus adhesion was found to be significantly influenced by the polar lipid concentration of the mucosa [27]. A lower polar lipid content in the mucosal membrane facilitated the development of muco adhesive force with increasing viscosity of HPMC [28]. It was found that an ample, desirable sustained release profile of 24 hours was achieved with a substantially higher amount of HPMC K15M polymer in the case of Bosentan MTs when compared to ethyl cellulose and sodium CMC [29]. Similarly, magnesium aluminium silicate was found to be the most important ingredient, which indicates that the desired disintegrating time of the drug is less than 2 minutes with a concentration of 2% of the total formulation [30-32].
Conclusion
The current investigation indicated that the best encapsulated mini tablet S6 and B3 formulations were taken into consideration for the encapsulation. The in vitro pharmacokinetic shows promising results towards the development and implementation of encapsulated MTs. Both of the formulations exhibit a high drug content percentage, good compressibility and a promising drug release pattern. As a result, it can be said that the formulation created is appropriate for MTs that are encapsulated in order to manage PAH effectively.
Acknowledgment
The authors would like to thank all the staff members of Centurion university of technology and management, Bhubaneswar and Roland institute of pharmaceutical sciences, Brahmapur for their discussions and technical support in preparing this research paper.
Ethical Approval
This study involves experiments on animals and taken permission from the institutional ethical committee.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of medical Journal Editors (ICMJE) requirements/guidelines.
Statements and Declaration
The authors have no competing interests to declare that are relevant to the content of this article and no funding was received to assist with the preparation of this manuscript.
References
- Cui Z, Geng L. Trop J Pharm Res. 2021; 20(12): p. 2619-2624.
- Aras MA, Psotka MA, De Marco T. Curr Cardiol Rep. 2019; 21(7): p. 62-64.
[Crossref] [Google Scholar] [PubMed]
- Dighe PP, Tank HM. Int J App Pharm. 2019; 11(2): p. 239-246.
- De Haro J, Bleda S, Gonzalez-Hidalgo C, et al. Am J Cardiovasc. 2019; 19: p. 203-209.
[Crossref] [Google Scholar] [PubMed]
- Mandras SA, Mehta HS, Vaidya A. In Mayo Clinic Proceedings 2020; 95(9): p. 78-88.
[Crossref] [Google Scholar] [PubMed]
- Verlinden NJ, Benza RL, Raina A. Pulm Circ. 2020; 10(4): p.159-523.
[Crossref] [Google Scholar] [PubMed]
- Bhogal S, Khraisha O, Al Madani M, et al. Am J Ther. 2019; 26(4): p. 520-526.
[Crossref] [Google Scholar] [PubMed]
- Vizza CD, Jansa P, Teal S, et al. BMC Musculoskelet Disord. 2017; 17: p. 1-3.
[Crossref] [Google Scholar] [PubMed]
- Bhusari S, Ansari I, Chaudhary A. Drug Dev Ind Pharm. 2020; 46(5): p. 732-743.
[Crossref] [Google Scholar] [PubMed]
- Fatima N, Arshad S, Quddusi AI, et al. J Ayub Med Coll Abbottabad. 2018; 30(3): p. 333-336.
[Google Scholar] [PubMed]
- Maheshwari R, Todke P, Kuche K, et al. Academia. 2016; 23(7): p.15-20.
- Özyılmaz ED, Comoglu T. Drug Dev Ind Pharm. 2022; 48(11): p. 667-681.
[Crossref] [Google Scholar] [PubMed]
- Sarangi D, Patro CS, Patra CN, et al. Int J Pharm Sci Nanotechnol. 2023; 16(1): p. 6325-6336.
- Jawed S, Cs S. Drug Dev Ind Pharm. 2023; 49(1): p. 115-128.
[Crossref] [Google Scholar] [PubMed]
- Panda SK, Sahu M, Panigrahi KC, et al. Drug Deliv Lett. 2021; 11(2): p. 179-194.
- Wiedey R, Kokott M, Breitkreutz J. Expert Opin Drug Deliv. 2021; 18(12): p. 1873-1890.
[Crossref] [Google Scholar] [PubMed]
- Chen L, Lin Y, Irdam E, et al. Pharm Res. 2022; 39(12): p. 3185-3195.
[Crossref] [Google Scholar] [PubMed]
- Comoglu T, Ozyilmaz D. Pharm Dev Technol. 2019; 24(7): p. 902-214.
[Crossref] [Google Scholar] [PubMed]
- Tran DT, Komínová P, Kulaviak L, et al. Int J Pharm. 2021; 592: p. 120-154.
[Crossref] [Google Scholar] [PubMed]
- Siriwachirachai C, Pongjanyakul T. J Drug Deliv Sci Technol. 2023; 85: p. 594-594.
- Kaliyaperumal V, Kandasamy AK, Ramalingam V, et al. IET Nanobiotechnol. 2020; 14(9): p. 750-755.
[Crossref] [Google Scholar] [PubMed]
- Totea AM, Dorin I, Laity PR, et al. Eur J Pharm Biopharm. 2020; 154: p. 270-282.
[Crossref] [Google Scholar] [PubMed]
- Zhang Q, Li X, Jasti BR. Int J Pharm. 2021; 67(34): p. 609-611.
[Crossref] [Google Scholar] [PubMed]
- Sharma A, Thakur M, Bhattacharya M, et al. Biotech Rep. 2019; 21 (8): p.316-320.
- Bayan MF, Sbaih HM, Saadh MJ. Indian J Forensic Med Toxicol. 2021; 15(1): p. 1291-1285.
- Ly HH, Daniel S, Soriano SK, et al. Mol Pharmaceutics. 2022; 19(6): P. 1892-1905.
[Crossref] [Google Scholar] [PubMed]
- Bhise JJ, Bhusnure OG, Mule ST, et al. J Drug Deliv Ther. 2023; 12(6): p. 23-25.
- Shoaib MH, Ahmed FR, Yousuf RI, et al. Front pharmacol. 2023; 14 (4): p.106-108.
[Crossref] [Google Scholar] [PubMed]
- Panda J, Rao ME, Swain S, et al. Beni-Suef univ J Basic Appl Sci. 2022; 11(1): p. 1-5.
- Alalaiwe A, Alsenaidy MA, Almalki ZS, et al. Pharmaceutics. 2022; 15 (8): p. 923-925.
[Crossref] [Google Scholar] [PubMed]
- Markl D, Zeitler JA. Pharm Res. 2017; 34(5): p. 890-899.
[Crossref] [Google Scholar] [PubMed]
- Liu W, Huo Q, Wang Y, et al. Open Chem. 2018; 16(1): p. 333-339.