Research Article - Der Pharma Chemica ( 2021) Volume 13, Issue 11
Environmentally Green Synthesis of ñ-aminophosphonates
Rahul Patil*Rahul Patil, Department of Chemistry, Yashwantrao Chavan College of Science, Karad, Maharashtra, India, Email: rspatilorg@gmail.com
Abstract
One pot multicomponent condensation of aldehyde, amine and diethylphosphite for the synthesis of α-aminophosphonates catalysed by environmentally green EPZG catalyst was found to be efficient and direct protocol under solvent free condition at room temperature. The green process offers advantages such as simple work up procedure, shorter reaction time, high yield and reusability of the catalyst.
Keywords
α-aminophosphonates; environmentally green; EPZG; diethylphosphit
Introduction
Organic chemistry deals with study of C-C bonds and a few compounds carry C-P bonds. Organo phosphorous compounds are made from the Phosphorous naturally or synthetically [1]. Attraction of the chemists increases towards these compounds due to their antibacterial, antimicrobial, antiviral, enzyme inhibitory properties and plant growth regulators, anti-cancer, [2-6]. The innovation of the amino phosphonic acid and other biologically active compound has wide purpose in agricultural and medicinal field [7-9]. Some organophosphorous compounds are key for pesticides [10], bactericides [11-13], α-pyrones analog of phosphorus act as HIV protease inhibitors [14]. Among the organo phosphorous compound α-aminophosphonic acid is significant motifs due to structural similarities with α-aminoacids [15-16]. The majority of the ester and acid derivatives of α-aminophosphonic acid has demonstrate advanced biological activity such as herbicidal and anticancer [17-22].
Kabachnik M. [23-25] and Fields E. [26] reported primary synthesis of α-amnophosphonic acid by the route of condensation of aldehyde or ketone with amine and dialkyl phosphate, latter on many reported technique used for the synthesis of α-aminophosphonates such as Indium triflate and Ytterbium triflate [27], gallium triiodide [28], VCl3 [29], silica sulfuric acid [30], copper salt [31], tetramethylguanidine [32], samarium di-iodide [33], lithium perchlorate [34], organocatalyst (R)-3,3’[4-fluorophenyl]2-1,1’-bisnaphthol phosphate [44], ionic liquid media [bmim][PF6] [35], Amberlite-IR 120 [36], L-Lactic Acid [37], ZrOCl2.8H2O and ZrO(ClO4)2.6H2O, diterpinic dehydroabietylamine [38] Nickel (II) chloride Hexahydrate, N,N′-dioxide-Sc (III) complex [39], Mg(ClO4)2 or molecular iodine [40], InCl3 [41], Montmorillonite KSF, Amberlyst-15 and Amberlite-IR 120 [42], microwave irradiation [43]. But, one pot multicomponent synthesis of α-aminophosphonates reported methods have drawbacks like long reaction time, use of organic solvent, reactivity with catalyst, completed separation procedure. To overcome this drawback, here in we have reported the synthesis of α-aminophosphonates by the application of environmentally benign inorganic, heterogeneous EPZG as a clay catalyst having Lewis acid and Bronsted acidic property. Envirocat EPZGR synthesized and supplied by Contract Chemicals, UK [44-54].
Materials and Methods
Various substitutedaldehyde (Sigma-aldrich), aniline (Himedia), diethylphosphite (Himedia) were used as received without purification. Envirocat EPZGR synthesized and supplied by Contract Chemicals, UK. Melting point measured by melting point apparatus made by Equiptronics model No. – EQ- 730A IR spectra were recorded on FT-IR -7600 Lambda Scientific Spectrometer.NMR Spectra were recorded on a Bruker AC400 MHz spectrometer in CDCl3 using tetramethylsilane as an internal standard material.
General Experimental Procedure
In a 25mlof round bottom flask mixture of aldehyde (1mmol), aniline (1mmol) and diethylphosphite (1mmol) was stirred with EPZ-GR [48-54] catalyst 20mg (Scheme 1) at room temperature for desired time mentioned in Table 4 (Entry a-v) completion of reaction was monitored by TLC. Upon completion of reaction separation and purification of product was carried out from ethanol. After completion of reaction catalyst was removed washed with water with ethyl acetate and reused for further reaction. All the products were purified by same technique and physical constant were found to be correct. Further structures of the product were confirmed by 1H NMR, 13C NMR, DEPT 135 NMR, HRMS and IR.
Result and Discussion
The use of solid heterogeneous catalyst EPZG catalyst was found to be environmentally green catalyst has attracted much attention in organic synthesis due to their advantages such as acidic property, reusability, inexpensive, low cost, and easy of handling, high yield of the product at room temperature. Model reaction using 20mg EPZGR under neat condition at room temperature agreeably results were obtained with respect to yield and reaction time monitored by TLC (Table1).
| Sr. No. | Catalyst | Amount mg | Time in (min) | Yield% |
|---|---|---|---|---|
| 1 | EPZGR | 10 | 34 | 84 |
| 2 | EPZGR | 20 | 22 | 90 |
| 3 | EPZGR | 30 | 22 | 90 |
| 4 | EPZGR | 40 | 22 | 90 |
| 5 | EPZGR | 40 | 22 | 90 |
Reaction conditions: Anisaldehyde (1mmol); aniline (1mmol); diethylphosphite (1mmol), solvent free, EPZGR catalyst 20mg, room temperature.
Reusability
The catalyst was found to be effective for the present transformation up to five cycles with appreciable loss in yield (Table 2).
| Run | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Yield | 90 | 89 | 88 | 87 | 86 |
Reaction conditions: Anisaldehyde (1mmol); aniline (1mmol); diethylphosphite (1mmol), solvent free, EPZGR catalyst 20mg, room temperature.
Screen of solvent: The present transformation was carried out with different solvents the results obtained predicted that the reaction performed under solvent free condition was effective having high yield at room temperature (Table 3).
| Entry | Solvent | Time Min. | Yield% |
|---|---|---|---|
| 1 | Water | 60 | 64 |
| 2 | Alcohol | 45 | 59 |
| 3 | Solvent free | 22 | 90 |
| 4 | Chloroform | 55 | 56 |
| 5 | Dichloromethane | 63 | 71 |
| 6 | Dimethylsulphoxide | 90 | 78 |
Reaction conditions: Anisaldehyde (1mmol); aniline (1mmol); diethylphosphite (1mmol), solvent free, EPZGR catalyst 20mg, room temperature
Spectral data of Synthesized α-aminophosphonates
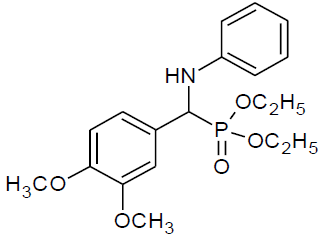
Table 4, Entry a: Diethyl[(4-methoxyphenyl) (phenylamino)] methylphosphonate, Color: White; M. P.: 102-102ºC; IR (KBr) cm-1: 3300, 3150, 3000, 1600, 1500, 1330, 1210, 750,700; 1H NMR(400MHz, CDCl3):δ(ppm):1.14-1.17(t, 3H, -CH3, J= 8 Hz),1.29 - 1.32(t, 3H, -CH3, J = 8 Hz), 3.68-3.74(m, 1H, -OCH2, J= 4 Hz),3.79(s, 3H, -OCH3), 3.93-4.0(m, 1H, -OCH2, J = 4 Hz),4.10-4.17(m, 2H, -OCH2,J= 4 Hz),4.70-4.76(d, 1H, CH, 2JHP = 24 Hz), 6.60-7.42(m, Ar-H); 13C NMR(100 MHz, CDCl3): δ (ppm): 16.28(d, 3Jcp = 6.0 Hz, –CH3-P),16.46(d, 3Jcp = 5.0 Hz, –CH3-P), 55.23(s,-OCH3), 55.35(d, 1Jcp = 151 Hz,-CH-P),63.18(d, 2Jcp = 5.0 Hz, -CH2-P),63.25(d, 2Jcp = 5.0 Hz, -CH2-P ),113.89, 114.04,114.07, 118.34, 127.66, 127.69, 128.93,128.99, 129.15for aromatic carbon, 146.36(d, 2Jcp = 14.00 Hz, Ar-P), 159.29 (d, 3Jcp = 3.00 Hz, Ar-P); DEPT 135 NMR:δ (ppm): 63.19 (d, 2Jcp = 5.0 Hz, -CH2-P), 63.25(d, 2Jcp = 5.0 Hz, -CH2-P).
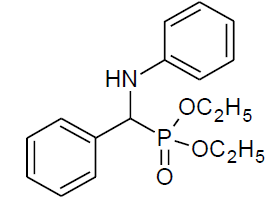
Table 4, Entry b: Diethyl[(phenyl)(phenylamino)] methylphosphonate, Color: White; M. P.: 90-92ºC; IR (KBr) cm-1: 3300, 3000, 1600, 1500,1200, 1010, 750, 700;1H NMR(400MHz, CDCl3): δ (ppm): 1.17-1.20(t, 3H, -CH3, J = 8 Hz), 3.66-3.72(m, 1H, -OCH2, J = 4 Hz),3.91-3.99(m, 1H, -OCH2, J = 4 Hz), 4.08-4.19(m, 2H, -OCH2, J = 4 Hz),4.75-4.81(d, 1H, -CH, 2JHP = 24 Hz), 6.63-7.48(m, Ar-H);13C NMR(100 MHz, CDCl3): δ( ppm):16.21(d, 3Jcp = 6.0 Hz, –CH3-P),16.45(d, 3Jcp = 6.0 Hz, –CH3-P),56.05(d, 1Jcp = 149 Hz,-CH-P), 63.25(d, 2Jcp = 4.0 Hz, –CH2-P), 63.32(d, 2Jcp = 4.0 Hz, –CH2-P),113.85, 118.39, 127.83, 127.88,127.92, 127.95, 128.60, 128.62, 129.18 for aromatic carbon, 135.89(d, 2Jcp = 3.00 Hz, , Ar-P ),146.31(d, 3Jcp = 15.00 Hz, Ar-P);DEPT 135 NMR:δ(ppm): 63.32(d, 2Jcp = 4.0 Hz, –CH2-P),63.25(d, 2Jcp = 4.0 Hz, –CH2-P);HRMS: 319.19;Calculated mass: 319.33.
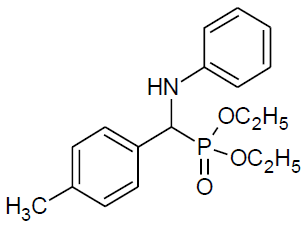
Table 4, Entry d :Diethyl[(4-methylphenyl) (phenylamino)] methylphosphonate,Color: White; M. P.: 65ºC; IR (KBr) cm-1: 3300, 3100, 3000, 2900, 1600, 1500, 1220, 1010, 700, 650;1H NMR(400MHz, CDCl3): δ(ppm): 1.12-1.15(t, 3H, -CH3, J = 8 Hz),1.27-1.31(t, 3H, -CH3, J = 8 Hz), 2.31 (s, 3H, -CH3-Ar),3.66-3.72(m, 1H, -OCH2, J= 8 Hz),3.92-3.98(m, 1H, -OCH2, J = 8 Hz),4.08-4.15(m, 2H, -OCH2, J= 8 Hz),4.71-4.77(m, 1H, 2JHP= 24 Hz), 6.58-7.59(m, Ar-H) ;13C NMR (100 MHz, CDCl3 ):δ (ppm):16.23(d, 3Jcp = 6.0 Hz, -CH3-P), 16.4(d, 3Jcp = 6.0 Hz, -CH3-P),21.15(s,-CH3),55.07 (d, 1Jcp = 150 Hz,-CH-P), 63.20(d, 2Jcp = 3.0 Hz, -OCH2- P), 63.27(d, 2Jcp = 4.0 Hz, -OCH2-P),113.84, 118.31, 127.72,129.14, 129.31, 129.33,132.64, 132.67,137.59 for aromatic carbon,137.61(d, 2Jcp = 4.00 Hz , Ar-P),146.03(d, 3Jcp = 14.00 Hz, Ar-P);DEPT 135 NMR:δ(ppm): 63.21(d, 2Jcp = 4.0 Hz, -OCH2-P), 63.27(d, 2Jcp = 3.0 Hz, -OCH2-P).
Table 4, Entry f :Diethyl[(4-chlorophenyl)(phenylamino)] methylphosphonate, Color: White; M. P.: 58-59ºC; IR (KBr) cm-1:3300, 3100, 3000, 2950, 1590, 1250, 1000, 750, 600.1H NMR(400MHz, CDCl3):δ(ppm): 1.16-1.20(t, 3H, -CH3, J = 8 Hz),1.29-1.33(t, 3H, -CH3, J = 8 Hz), 3.76-3.83(m, 1H, -OCH2, J = 14 Hz),3.98-4.01(m, 1H, -OCH2, J= 12 Hz), 4.10-4.17(m, 2H, -OCH2, J = 12 Hz), 4.72-4.78(d, 1H, -CH, 2JHP= 24 Hz), 6.57-7.45(m, Ar-H);13C NMR (100 MHz, CDCl3):δ(ppm):16.29(d, 3Jcp = 6.0 Hz, -CH3-P),16.44(d, 3Jcp = 6.0 Hz, -CH3-P ),55.54(d, 1Jcp = 150 Hz,-CH-P), 63.36(d, 2Jcp = 6.0 Hz, -OCH2-P), 63.45(d, 2Jcp = 6.0 Hz, -OCH2-P),113.85, 118.68,128.80, 128.82, 129.12, 129.17, 129.24,133.71,133.75,for aromatic carbon, 134.58(d, 2Jcp = 3.00 Hz , Ar-P), 146.01(d, 3Jcp = 14.00 Hz, Ar-P); DEPT 135 NMR:δ(ppm): 63.36(d, 2Jcp = 6.0 Hz, -OCH2-P), 63.45(d, 2Jcp = 8.0 Hz, -OCH2-P).
Table 4, Entry h :Diethyl[(2-nitrophenylphenyl)(phenylamino)] methylphosphonate ,Color:brown; M. P.: 148-150ºC; IR (KBr) cm-1: 3300, 3000, 2900, 1600, 1500, 1220, 1000, 750, 700; 1H NMR(400MHz, CDCl3): δ(ppm): 1.09-1.13(t, 3H, -CH3, J = 4 Hz, ), 1.31-1.33(t, 3H, -CH3, J = 4 Hz), 3.79-3.85(m, 1H, -OCH2, J = 8 Hz),3.93-3.98(m, 1H, -OCH2, J = 4 Hz),4.16-4.21(m, 2H, -OCH2, J = 10 Hz),6.17-6.22(d, 1H, -CH, 2JHP= 24 Hz),6.68-8.02(m, Ar-H); 13C NMR (100 MHz, CDCl3): δ (ppm): 15.96(d, 3Jcp = 6.0 Hz, -CH3-P),16.33(d, 3Jcp = 6.0 Hz, -CH3-P), 49.93(d, 1Jcp = 150 Hz,-CH-P ), 63.39(d, 2Jcp= 7.0 Hz, -OCH2-P),63.87(d, 2Jcp = 7.0 Hz, -OCH2-P), 113.57, 118.85,125.27, 125.29, 128.53,128.56, 128.75, 128.79,129.45, 131.95, 131.97, 133.52, 133.55 for aromatic carbon, 145.33(d, 2Jcp = 13.00 Hz , Ar-P), 149.44(d, 3Jcp = 6.00 Hz, Ar-P);DEPT 135 NMR: δ(ppm): 63.39(d, 2Jcp = 7.0 Hz, -OCH2-P), 63.87(d, 2Jcp = 7.0 Hz, -OCH2-P);
Table 4, Entry j :Diethyl[(4-nitrophenyl) (phenylamino)] methylphosphonate, Color: Yellow; M. P.: 185-160ºC; IR (KBr) cm-1: 3300, 3050, 3000, 2900, 1600, 1500, 1350, 1210, 1020, 750, 700;1H NMR(400MHz, CDCl3): δ(ppm): 1.19-1.23(t, 3H, -CH3,J = 8Hz), 2.45-2.49(t, 3H, -CH3, J =8Hz),3.87-4.05(m, 1H, -OCH2, J = 4Hz),4.07-4.21(m,3H, from two -OCH2,J = 4Hz), 4.85-4.91(d, 1H, -CH, 2JHP = 24Hz),6.54-8.23(m, Ar-H); 13C NMR (100 MHz, CDCl3): δ(ppm):16.27(d, 3Jcp = 6.0 Hz, -CH3-P),16.44(d, 3Jcp = 6.0 Hz, -CH3-P), 56.01(d, 1Jcp = 147 Hz,-CH-P), 63.49(d, 2Jcp = 7.0 Hz, -OCH2-P), 63.76(d, 2Jcp = 7.0 Hz, -OCH2-P),113.80, 119.11, 123.77, 123.79, 128.63, 128.68, 129.37, 144.06, 144.10, for aromatic carbon,145.64(d, 2Jcp = 14.00 Hz, Ar-P),147.61(d, 3Jcp = 4.00 Hz, Ar-P);DEPT 135 NMR:δ(ppm): 63.49(d, 2Jcp = 7.0 Hz, -OCH2-P),63.76(d, 2Jcp = 7.0 Hz, -OCH2-P) ; HRMS: 364.19; Calculated mass:364.61.
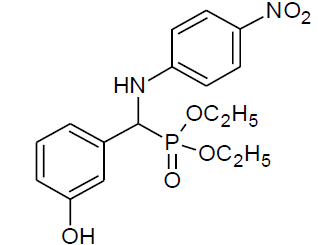
Table 4, Entry l : Diethyl[(4-hydroxyphenyl)(phenylamino)] methylphosphonate
Color: White; M. P.: 102-104ºC; IR (KBr) cm-1:3350, 3250, 3000, 1600, 1510, 1230, 1010, 730, 700; 1H NMR( 400MHz, CDCl3 ): δ(ppm): 1.15-1.19 (t, 3H, -CH3, J = 8 Hz), 1.27-1.30(t, 3H, -CH3, J = 8 Hz), 3.74-3.81(m, 1H, -OCH2, J = 12 Hz), 3.95-4.01 (m, 1H, -OCH2,J= 4 Hz), 4.06-4.18(m, 2H, -OCH2, J = 4 Hz),4.69-4.75(d, 1H, -CH, 2JHP = 24 Hz), 6.60-7.27(m, Ar-H), 7.64(bs, Ar-OH); 13C NMR (100 MHz, CDCl3 ): δ(ppm): 16.25(d, 3Jcp = 5.0 Hz, -CH3-P ), 16.42(d, 3Jcp =5.0 Hz, -CH3-P),55.21(d, 1Jcp = 153 Hz,-CH-P), 63.47(d, 2Jcp = 7.0 Hz, -OCH2-P),63.65(d, 2Jcp = 7.0 Hz, -OCH2-P),113.94, 115.97, 116.00, 118.46, 125.89, 125.92, 128.87, 128.92, 129.17for aromatic carbon, 146.21(d, 2Jcp = 14.00 Hz , Ar-P), 156.52(d, 3Jcp = 3.00 Hz, Ar-P );DEPT 135 NMR:δ(ppm):63.47(d, 2Jcp = 7.0 Hz, -OCH2-P ),63.65(d, 2Jcp = 7.0 Hz, -OCH2-P).
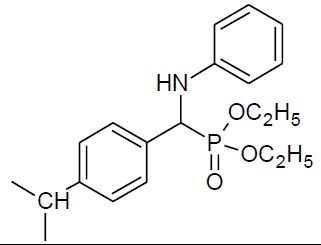
Table 4, Entry n :Diethyl[(4-isopropylphenyl)(4-methoxyphenylamino)] methylphosphonate, Color: White; M. P.: 82-84ºC; IR (KBr) cm-1:3301, 2973, 1500, 1234, 1035, 932, 828; 1H NMR(400MHz, CDCl3):δ(ppm):1.10-1.12(t, 3H, -CH3, J = 8 Hz), 1.20-1.22(t, 3H, -CH3, J = 8 Hz), 3.65-3.71 (m, 3H, -OCH3), 3.90-3.96(m, 1H, -OCH2, J = 4 Hz), 4.08-4.14(m, 3H, -OCH2, J = 4 Hz), 4.64-4.70(d, 1H, -CH, 2JHP = 28 Hz), 6.55-7.37(m, Ar-H); 13C NMR (100 MHz, CDCl3):δ(ppm): 16.14(d, 3Jcp = 5.0 Hz, -CH3-P),16.43(d, 3Jcp = 5.0 Hz, -CH3-P),55.63 (s, -OCH3), 56.59(d, 1Jcp = 151 Hz,- CH-P),63.14(d, 2Jcp = 8.0 Hz, -OCH2-P),63.21(d, 2Jcp = 6.0 Hz, -OCH2-P),114.69, 115.15,126.64, 126.66, 127.69,127.75,133.11, 133.13for aromatic carbon, 140.45(d, 2Jcp = 16.00 Hz , Ar-P), 148.52(d, 3Jcp = 4.00 Hz, Ar-P), 152.85(s, Ar-O); DEPT 135 NMR:δ(ppm): 63.15(d, 2Jcp = 6.0 Hz, -OCH2-P).
Table 4, Entry p: Diethyl[(4-methylphenyl)(4-methoxyphenylamino)] methylphosphonate, Color: White; M. P.: 78-80ºC; IR (KBr) cm-1:3291, 2976, 1506, 1236, 1446, 1008, 807, 755;1H NMR(400MHz, CDCl3): δ(ppm):1.05-1.09(t, 3H, -CH3, J = 8 Hz), 1.20-1.24(t, 3H, -CH3, J = 4 Hz), 2.24(s, 3H, -CH3), 3.61-3.67(m, 3H, -OCH3), 3.85-3.91(m, 1H, -OCH2, J = 4 Hz), 4.00-4.08(m, 3H, 2-OCH2, J = 4 Hz), 4.57-4.63(d, 1H, -CH, 2JHP = 24 Hz), 6.74-7.27(m, Ar-H); 13C NMR (100 MHz, CDCl3): δ(ppm): 16.19(d, 3Jcp = 6.0 Hz, -CH3-P ),16.40(d, 3Jcp = 6.0 Hz, -CH3-P), 21.10(s,CH3-Aromatic), 55.56(s, -OCH3),56.52(d, 1Jcp = 151 Hz,-CH-P ),63.10(d, 2Jcp = 5.0 Hz, -OCH2-P),63.16(d, 2Jcp = 7.0 Hz, -OCH2- P),114.63, 115.16, 127.67,127.73, 129.22, 129.25,132.78, 132.81 for aromatic carbon, 137.47(d, 2Jcp = 3.00 Hz , Ar-P), 140.36(d, 3Jcp = 16.00 Hz, Ar-P), 152.49(s, Ar-O); DEPT 135 NMR:δ(ppm):63.11(d, 2Jcp = 6.0 Hz, -OCH2-P), 63.17(d, 2Jcp = 5.0 Hz, -OCH2-P).
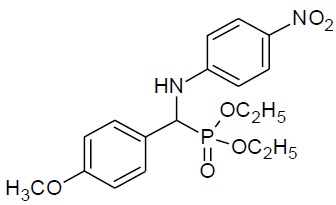
Table 4, Entry r :Diethyl[(4-methoxyphenyl)(4-nitrophenylamino)] methylphosphonate, Color: White; M. P.: 128-130ºC; IR (KBr) cm-1: 3291, 2985, 1594, 1498, 1287, 1218, 999, 834, 763 cm-1; 1H NMR(400MHz, CDCl3): δ(ppm):1.11-1.15(t, 3H, -CH3, J = 8 Hz), 1.29-1.32(t, 3H, -CH3, J = 8 Hz), 3.61-3.67(m, 1H, -OCH2, J = 8 Hz), 3.78(s, 3H, -OCH3), 3.90-3.96(m, 1H, -OCH2, J = 8 Hz),4.104.17(m, 2H, -OCH2, J = 4 Hz), 4.73-4.79(d, 1H, -CH, 2JHP = 24 Hz), 6.57-8.02(m, Ar-H): 13C NMR (100 MHz, CDCl3): δ (ppm):16.24(d, 3Jcp = 6.0 Hz, -CH3-P),16.43(d, 3Jcp = 6.0 Hz, - CH3-P ),54.81(d, 1Jcp = 153 Hz,-CH-P),55.27(s, -OCH3),63.46(d, 2Jcp = 7.0 Hz, -OCH2-P),63.90(d, 2Jcp = 7.0 Hz, -OCH2-P),112.42, 114.33, 114.35,126.05, 126.14, 126.18, 128.89, 128.89, 138.96for aromatic carbon, 151.89(d, 2Jcp = 14.00 Hz , Ar-P),159.69(d, 3Jcp = 3.00 Hz, Ar-P ); DEPT 135 NMR:δ(ppm):63.33(d, 2Jcp = 8.0 Hz, -OCH2-P), 63.90(d, 2Jcp = 7.0 Hz, -OCH2-P).
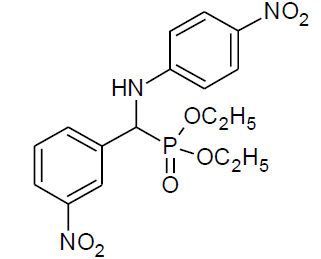
Table 4, Entry t :Diethyl[(3-nitrophenyl)(4-nitrophenylamino)] methylphosphonate, Color: Yellow green; M. P.: 172-174ºC; IR (KBr) cm-1: 3264, 3072, 2985, 1621, 1542, 1305, 1209, 1017, 843, 755, 659; 1H NMR(400MHz, CDCl3): δ (ppm): 1.18-1.22(t, 3H, -CH3, J = 8 Hz), 1.31- 1.34(t, 3H, -CH3, J = 8 Hz), 3.86-3.93(m, 1H, -OCH2,J= 4 Hz), 4.03-4.09(m, 1H, -OCH2, J = 4 Hz), 4.16-4.25(m, 2H, -OCH2, J = 8 Hz), 4.94- 5.00(d, 1H, -CH, 2JHP = 24 Hz), 6.59-8.36(m, Ar-H); 13C NMR (100 MHz, CDCl3 ): δ (ppm): 16.24(d, 3Jcp = 6.0 Hz, -CH3-P), 16.30(d, 3Jcp = 6.0 Hz, -CH3-P),54.98(d, 1Jcp = 150 Hz,-CH-P),63.95(d, 2Jcp = 8.0 Hz, -OCH2-P),64.03(d, 2Jcp = 8.0 Hz, -OCH2-P),112.43, 122.55, 122.60, 123.42, 123.44, 126.12, 129.84,129.87, 133.67, 133.72,137.48, 137.50, 139.45for aromatic carbon, 148.54(d, 2Jcp = 2.00 Hz , Ar-P), 151.36(d, 3Jcp = 13.00 Hz, Ar-P); DEPT 135 NMR:δ(ppm):63.95(d, 2Jcp = 8.0 Hz, -OCH2-P).
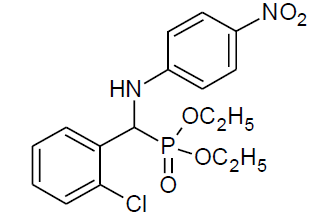
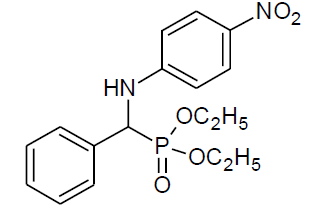
Table 4, Entry v:Diethyl[(phenyl)(4-nitrophenylamino)] methylphosphonate, Color: Yellow; M. P.: 145-146ºC ; IR (KBr) cm-1: 3255, 3089, 2958, 1594, 1489, 1340, 1227, 1017, 965, 834, 675; 1H NMR(400MHz, CDCl3): δ (ppm):1.09-1.12(t, 3H, -CH3, J = 8 Hz), 1.14-1.16(t, 3H, -CH3, J = 8 Hz), 3.60-3.64(m, 1H, -OCH2, J = 8 Hz), 3.89-3.95(m, 1H, -OCH2, J = 4 Hz), 4.09-4.19(m, 2H, -OCH2, J = 4 Hz), 4.79-4.85(d, 1H, -CH, 2JHP = 24 Hz), 6.57-8.08(m, Ar-H); 13C NMR (100 MHz, CDCl3 ):δ (ppm):16.15 (d, 3Jcp = 6.0 Hz, -CH3-P ),16.42(d, 3Jcp = 6.0 Hz, -CH3-P),55.54(d, 1Jcp = 151 Hz,-CH-P),63.33(d, 2Jcp = 7.0 Hz, -OCH2-P),63.88(d, 2Jcp = 7.0 Hz, -OCH2-P), 112.43, 126.08, 127.68, 127.74, 128.51, 128.55, 128.91, 128.93, 139.04 for aromatic carbon, 134.51(d, 2Jcp = 3.00 Hz , Ar-P), 151.78(d, 3Jcp = 13.00 Hz, Ar-P);DEPT 135 NMR:δ(ppm):63.32(d, 2Jcp =7.0 Hz, -OCH2-P), 63.88(d, 2Jcp = 7.0 Hz, -OCH2-P).
| Entry | Aldehyde | Amine | Product | Time min. | Yield % |
|---|---|---|---|---|---|
| a | 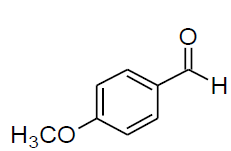 |
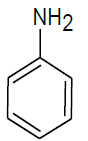 |
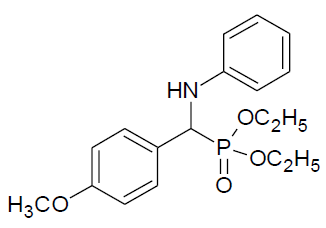 |
22 | 90 |
| b | 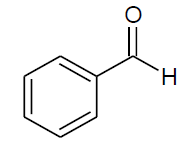 |
 |
 |
18 | 92 |
| c |  |
 |
 |
30 | 89 |
| d | 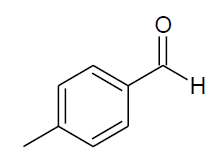 |
 |
 |
22 | 91 |
| e | 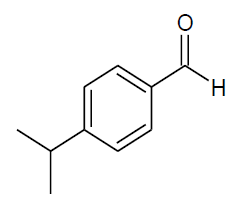 |
 |
 |
25 | 90 |
| f | 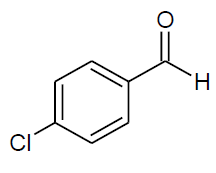 |
 |
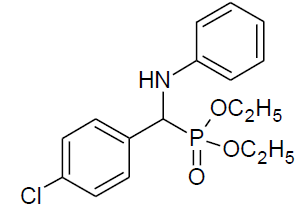 |
20 | 93 |
| g | 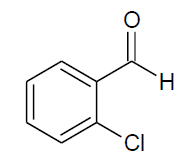 |
 |
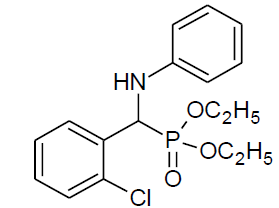 |
23 | 92 |
| h | 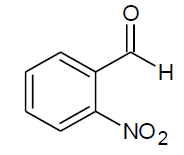 |
 |
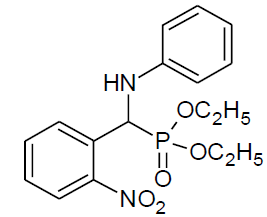 |
20 | 92 |
| I | 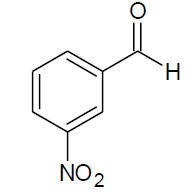 |
 |
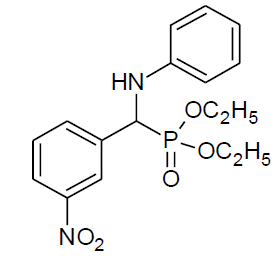 |
22 | 91 |
| j | 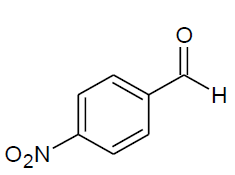 |
 |
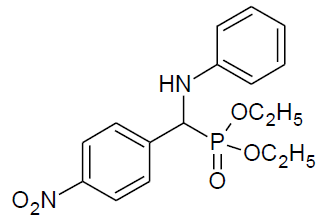 |
15 | 94 |
| k | 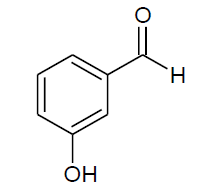 |
 |
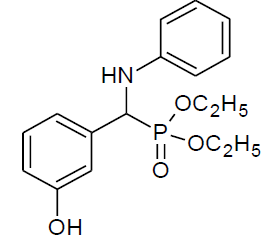 |
23 | 89 |
| l | 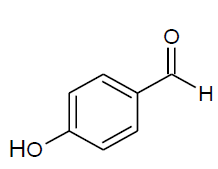 |
 |
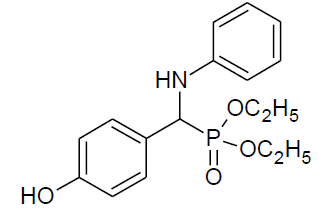 |
24 | 91 |
| m | 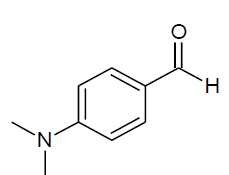 |
 |
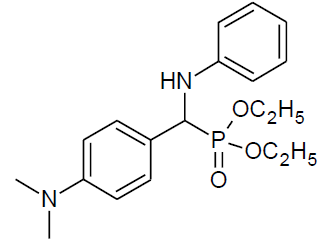 |
25 | 90 |
| n | 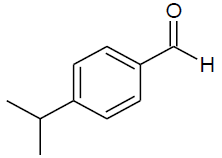 |
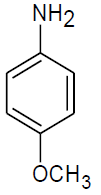 |
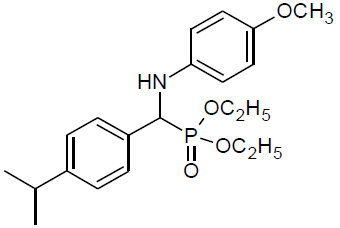 |
34 | 91 |
| o | 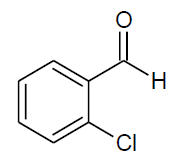 |
 |
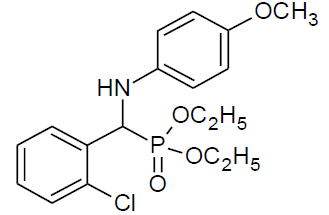 |
22 | 92 |
| p | 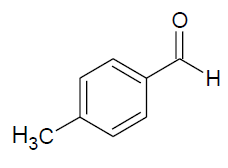 |
 |
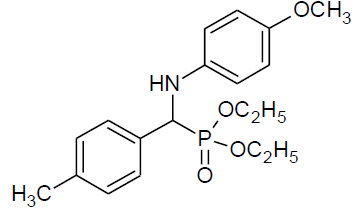 |
29 | 91 |
| q | 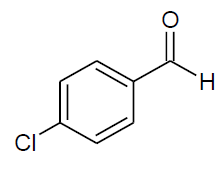 |
 |
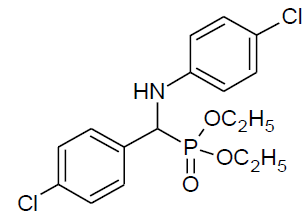 |
27 | 89 |
| r |  |
 |
 |
33 | 90 |
| s |  |
 |
 |
53 | 90 |
| t |  |
 |
 |
17 | 91 |
| u |  |
 |
 |
18 | 91 |
| v |  |
 |
 |
21 | 92 |
Reaction conditions: Anisaldehyde (1mmol); aniline (1mmol); diethylphosphite (1mmol), solvent free, EPZGR catalyst 20mg, room temperature.
Conclusion
A convenient methodology for the synthesis of α –aminophosphonates derivatives is demonstrated employing EPZG a green, efficient and Lewis acid catalyst promoter. Solvent free and room temperature makes this protocol green one.
References
- Horiguchi M and Kandatsu M. Nature, 1959, 184: p. 901.
- Naydenova E, Topashka- Ancheva M., Todorov P et al., Bioorg Med Chem. 2006, 14: p. 2190-2196.
- Kafarski P and Lejczak B. Wiley, New York. 2000, p. 407.
- Kafarski P and Lejczak B. Curr Med Chem. 2001, 1: p. 301-312.
- Goding J. Academic Press. NY, 1986.
- Bigge C, Ortwine D, Drummond J et al., J Med Chem. 1992, 35: p. 1371-138.
- Moonen K, Laureyn I and Stevens C. Chem Rev. 2004, 104: p. 6177.
- Demmer C, Krogsgaard-Larsen N and Bunch L. Chem Rev. 2011, 111: p. 7981-8006.
- Popa A, Ene R, Visinescu D et al., J Mol Catal A: Chem. 2015, 408: p. 262.
- Fest C and Schmidt K. The Chemistry of Organophosphorus Pesticides, 1982, 12.
- Ali N, Zakir S, Patel M et al., Eur J Med Chem. 2012, 50: p. 39.
- Sampath C, Harika P and Revaprasadu N. Sulfur Silicon Relat Elem. 2016, 191: p. 1081.
- Sivala M, Devineni S, Golla M et al., J Chem Sci. 2016, 128: p. 1303.
- Bhattacharya AK, Raut DS, Rana KC et al., Eur J Med Chem. 2013, 66: p. 146-15.
- Mulla S, Pathan M, Chavan S et al., RSC Adv. 2014, 4: p. 7666.
- Subhedar D, Shaikh M, Nawale L et al., Res Chem Intermed. 2016, 42: p. 6607.
- Stowasser B, Budt K, Jian-Qi L et al., Tetrahedron Lett. 1992, 33: p. 6625.
- Lacbay C, Mancuso J, Lin Y et al., J Med Chem. 2014, 57(17): p. 7435.
- Xiao L, Li K and Shi D. Phosphorus Sulfur silicon Related Elements. 2008, 183: p. 3156-3165.
- Ye M, Yao G, Pan Y et al., Eur J Med Chem. 2014, 83: p. 116.
- Huang X, Wang M, Pan Y et al., Eur J Med Chem. 2013, 69: p. 508.
- Huang K, Chen Z, Liu Y et al., Eur J Med Chem. 2013. 63: p. 76.
- Kabachnik M, Medved T and Dokl Akad. Nauk SSSR. 1952, 83: p. 689 .
- Kabachnik M and Medved T. Bull. Acad. Sci USSR. 1953, 2: p. 769-777.
- Medved T and Kabachnik M. Bull Acad Sci USSR. 1954, 6: p. 893-900.
- Fields E. J Am Chem Soc.1952, 74: p. 1528-1531.
- Qian C and Huang J. J Org Chem. 1998, 63: p. 4125.
- Sun P, Hu Z and Huang Z. Synth Commun. 2004, 34: p. 4293.
- Sunil Kumar B, Thirupathi Reddy Y, Narsimha Reddy P et al., Chemistry of Heterocyclic Compounds. 2005, 11: p. 1634-1636.
- Maghsoodlou M, Khorassani S and Hazeri N. Heteroatom Chem. 2009, 20: p. 316.
- Ramkrishna K, Thomas J and Sivasankar C. J Org Chem. 2016, 81: p. 9826-9835.
- Narayanaeddy M, Siva Kumar B and Balkrishna A et al., Arkivoc. 2007, p. 246-254.
- Xu F, Luo Y, Deng M et al., Euro J Org Chem. 2003, p. 4728 – 4730.
- Heydari A, Zarei M, Alijanianzadeh R et al., Tetrahedron lett. 2001, 42: p. 3629-3631.
- Xu W, Zhang S, Yang S et al., Molecules. 2010, 15: p. 5782-5796.
- Bhattacharya A and Rana K. TetrahedronLett. 2008, 49: p. 2598–2601.
- Hou J, Gao J and Zhang Z. Appl Organometal Chem. 2011, 25: p. 47–53.
- Rao X, Song Z and He L. Heteroatom Chem. 2008, 19: p. 512-516.
- Zhou X, Shang D, Zhang Q et al., Org Lett. 2009, 11: p. 1401-1404.
- Wu J, SunW, Xia H-G et al., Org Biomol Chem. 2006, 4: p. 1663-1666.
- Ranu B, Hajra A and Jana U. Org Lett. 1999, 1: p. 1141–1143.
- Bhattacharya A and Rana A. Tetrahedron Lett. 2008, 49: p. 2598–2601.
- Ranu B and Hajra A. Green Chem. 2002, 2002, p. 551–554.
- Envirocats-Supported Reagents: Product information, Contract Chemicals, UK, 1994.
- Upadhya T, Daniel T, Sudlai A et al., Synth Commun. 1996, 26: p. 4539.
- Janeshwara G, Barhate N, Sudlai A et al., J Chem Soc. 1996, p. 965.
- Shaikh N, Gajare A, Deshpande V et al., Tetrahedron Lett. 2000, 41: p. 385-387.
- Bandgar BP, Uppalla LS and Sadavarte VS. Green Chem. 2001, 3: p. 39-41.
- Bandgar BP, Kasture SP, Tidake K et al., Green Chem. 2000, 2: p. 152.
- Sonali Ghandande and Supriya Mahajan. Indian J Chem. 2005, 44B: p. 188-192.
- Bandgar BP, Uppalla LS and Sadavarte VS, Green Chemistry, 2001, 3: p. 39-41.
- Bandgar BP, Zirange MB and Wadgaonkar PP. Synlett, 1996, 2: p. 149-150.
- Bandgar BP and Wadgaonkar PP. Synthetic Communication. 1997, 27: p. 2069.
- Bandgar BP, Jagtap SR, Ghodeshwar SB et al., Synthetic Communication, 1995, 25: p. 2993.



