Research - Der Pharma Chemica ( 2024) Volume 16, Issue 2
Fabrication and Characterization of Cefixime Containing Micro Emulsion for Parenteral Drug Delivery System
Ashish Jain*, Bhushan Hatwar and Akhlesh Kumar SinghaiAshish Jain, Department of Pharmacy, LNCT University, Bhopal, India, Email: aashish.pharmatech@gmail.com
Received: 01-Apr-2024, Manuscript No. DPC-24-132360; Editor assigned: 04-Apr-2024, Pre QC No. DPC-24-132360 (PQ); Reviewed: 11-Apr-2024, QC No. DPC-24-132360; Revised: 17-Apr-2024, Manuscript No. DPC-24-132360 (R); Published: 30-Apr-2024, DOI: 10.4172/0975-413X.16.2.293-300
Abstract
The development of an injectable or parenteral micro emulsion formulation of the antibiotic cefixime was the aim of this investigation. We identified cefixime, which is easily soluble in methanol and propylene glycol and just slightly soluble in ethanol and acetone. Cefixime is directly dissolved in the aqueous buffer to create organic solvent-free aqueous solutions for use in biological experiments. To define the limits of a micro emulsion’s existence, pseudo ternary phase diagrams of oil, surfactant, cosurfactant (butanol/isopropyl alcohol mixture) and water were created. Cefixime was added to the ideal micro emulsion composition at 3, 6 and 9% w/w. We assessed the conductivity, solution viscosity and particle size of the cefixime micro emulsion. After being diluted in 5% dextrose for injection with 1 mg/ml cefixime, the micro emulsion’s stability and hemolytic activity were tested. The stability of the micro emulsion was evaluated after three months of storage at 4 and 25°C. Based on solubility data, sesameoil/soybean oil/olive oil was chosen as the oil phase for the cefixime micro emulsion. Based on weight percent, the ideal cefixime micro emulsion formulation was 2.0 % cefixime, 9% oil (Soybean oil/sesame oil), 24% Tween 40, 8% butanol, 4% isopropyl alcohol and 52.5% water. The optimized blank and drug-loaded micro emulsions had average particle sizes of 68.7 nm and 71.6 nm, respectively and these values remained constant after being diluted with dextrose 25 times. A three-month stability investigation at 4 and 25°C verified the findings of the cefixime chemical and micro emulsion physical tests. Erythrocytes appeared to tolerate cefixime micro emulsions well according to in vitro hemolysis experiments. Cefixime's unique micro emulsion formulation, which is appropriate for parenteral administration, was created. In comparison to the existing marketable formulation of cefixime based on voxpime® and isopropyl alcohol solution, this novel formulation may have fewer side effects related to vehicles.
Keywords
Cefixime; Tween 40; Micro emulsion; Parenteral; Phase diagram
Introduction
Having vinyl and (2Z)-2-(2-amino-1,3-thiazol-4-yl)-2-[(carboxymethoxy)imino)acetamido] groups at positions 3 and 7, respectively, of the cephem skeleton, cefixime is a third-generation cephalosporin antibiotic. Cefixime is applicable to treat urinary tract infections, tonsillitis, pharyngitis, gonorrhea and tonsillitis. It functions as a medication allergy, antifungal and antibacterial.
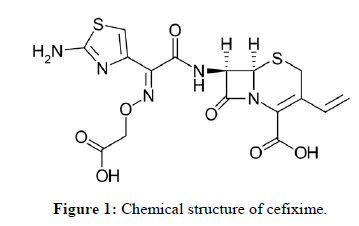
Similar to ceftriaxone and cefotaxime, the antibiotic cefixime is a third-generation cephalosporin. Even in the presence of beta-lactamase enzymes, cefixime is quite stable. Because of the presence of beta-lactamases, many organisms that are resistant to penicillins and some cephalosporins may be sensitive to cefixime. Cefixime's antibacterial action is brought on by the suppression of bacterial cell wall mucopeptide production (Figure 1) [1].
Materials and Methods
Cefixime sample were obtained from sigma aldrich phosphate buffer tablets were also purchased from sigma. Butanol, glycerol monosterate, glycerine and olive oil were purchased from ideal chemical Raipur. Dialysis membrane (Spectro/por dialysis membrane, Spectra/por 4, diameter 16 mm, molecular weight of 12-14 kDa) were purchased from spectrum. All other chemical reagents and solvents were of analytical grade and used as received. Distilled deionized water was used for the preparation of parenteral micro emulsions.
Determination of solubility of cefixime in oils
The solubility of cefixime in sesame oil, soybean oil and olive oil was first determined. An excess amount of cefixime was added to each solvent and the mixture was shaken at 25ºC for 72 h. the suspension was then filtered through a membrane filter (0.45 mm) and the concentration of cefixime in the filtrate was determined by HPLC.
HPLC analysis of cefixime
The cefixime concentration in oil solutions was analyzed using a modified HPLC method as reported previously. A mobile phase containing acetonitrile-aqueous solution of phosphoric acid (0.4%) (85:15, v/v) was delivered at a flow rate of 1.0 mL/min. The detection wavelength was 214 nm and the system temperature was set at 60C. the injection volume was 100 mm. the retention time of cefixime was about 8.0 and no interference from other components was observed.
To determine the cefixime content in the total micro emulsion, methanol was first used to break the micro emulsion with sonication and then the samples were dissolved in mobile phase by dilution to a constant volume, followed by filtration through a membrane filter (0.45 mm) and injection into HPLC.
Construction of pseudoternary phase diagram
Before formulation optimization, the pseudo-ternary phase diagrams of water, oil and surfactant: cosurfactant (Sesame oil: Butanol: Tween40: (16:8:1)) were created. Cosurfactant and tween 40 were combined at set weight ratios. The components of each mixture of surfactant and cosurfactant were then mixed with the lipid phase and subsequently the aqueous phase. To establish equilibrium, mixtures were held at a specified temperature (25°C), gently agitated, or mixed by vortexing. The preceding solution was noticed as having clear and translucent micro emulsions, or rough emulsions or gels, based on visual observation of their appearance.
Preparation of micro emulsion: Cefixime formulation development was dissolved by vortexing in butanol. Oil was combined next and then surfactant. The resulting mixture was vortexed to ensure homogenous mixing [2]. To create a micro emulsion, the precise amount of water was then added and carefully incorporated. The prepared micro emulsion was autoclaved at 121°C and 15 pressure for 15 minutes to sterilize it [3,4].
Characterization of micro emulsion
Optical birefringence: The light transmittance of the cefixime micro emulsion between two polarizing plates was then measured [5] Then, one of the plates was turned 90 degrees with respect to the other (crossed polarizer) and it was looked at [6].
Particle size determination: The size of the particles in micro emulsions of cefixime is frequently determined using photon correlation spectroscopy by laser light scattering [7]. The cefixime micro emulsion's particle size was examined by means of a Beckman N4 Plus submicron Particle Size Analyzer. The apparatus measured spherical particles and computed the mean particle size of micro emulsion and polydispersity as of intensity. The polydispersity parameter, which ranges from 0.0 to 1.0, is a measurement of particle homogeneity [8]. The particles are more homogenous the closer the polydispersity value is to zero. Samples were diluted five times with 0.22 filtered double-distilled water before analysis. At a 90° scattering angle and a temperature of 25°C or 37°C, light scattering was studied. Every measurement was made in triplicate [9].
Physiochemical characterization of microemulsion
Droplet size: The Nicomp 380 ZLS was used to measure the droplet size (Particle sizing systems, Santa Barbara, CA). Micro emulsion formulation samples were put into 6 × 50 mm borosilicate glass tubes made by Kimble Chase in Vineland, New Jersey and placed in the beam of a 100 mW He- Ne laser with a 658 nm wavelength. Until the scattered intensity wavered around 300 kHz, the neutral density filter was modified. A photodiode array detector was used to detect the scattered light after it was gathered at 90 degrees. For Chi-square values greater than 3, nicomp distribution and nicomp software spontaneously adjusted the channel width and base line. Three analyses of each sample were performed and the mean volumeweighted diameter was calculated [10].
Zeta potential: Malvern Zetasizer, nano 90, manufactured by Malvern Instruments in the USA, was used to assess zeta potential via dynamic light scattering [11]. Measurements were made at an angle of 900 while being kept at 25°C. Before the experiment, micro emulsion sample were made by dispersing the nanospheres in an adequate volume of ultrapure water (pH 7) [12]. The Poly Dispersity Index (PDI), a metric for the breadth of the particle size distribution, was used to report the particle size distributions of the nanospheres. To forecast the physical stability of colloidal systems, ZP is a relevant measure. Individually micro emulsion sample was examined three times and the average of the runs was used to calculate the zeta potential [13].
Accelerated stability testing
Centrifugation: The system has been put through centrifugal tests to gauge its accelerated stability [14]. Centrifuging the cefixime micro emulsion at 5,175 g for 30 min allowed us to check for any separation or incompatibility.
Viscosity measurements: An oswald-type viscometer was used to measure the micro emulsion formulation viscosity. The precise amount of the micro emulsion formulation was added to the viscometer tube. Vacuum was used to raise the meniscus of the liquid solution in the capillary tube to the level of the top graduation mark. The capillary tube's liquid solution flow from the top mark to the lower mark was measured in seconds and reported. It was also reported how long water will take. Three instances of the process were completed and the average value was used to calculate.
pH measurement: For intravenous and intramuscular routes, the acceptable pH range is 2-12, however for subcutaneous routes, the acceptable pH range is reduced to 2.7-9.0 when the rate of in vivo dilution is greatly decreased, increasing the likelihood of discomfort at the injection site. Using a systronic digital pH meter 335 and standard buffers of pH 4.0 and 7.0, the pH of the micro emulsion was determined [15,16].
Dilutability: The stability of cefixime micro emulsion was examined upon dilution to 1 mg/ml cefixime concentration used for clinical administration. The cefixime micro emulsion was diluted 25 times (v/v) with 5% dextrose solution for injection. The solution appearance and droplet size before and after dilution were evaluated.
Electric conductivity measurements: The conductivity of micro emulsion was determined using a DDS11C conductance meter at 25C.
Clarity: At a wavelength of 400 nm, a UV-Vis Spectrophotometer was used to gauge the samples' clarity. For the analysis, deionized water was utilized as a blank. The clarity was measured in triplicate and expressed as a percent transmittance.
Differential Scanning Calorimetry (DSC): Utilizing a Differential Scanning Calorimeter (DSC) (822e Mettler Toledo) outfitted with a TS0801RO sample robot and TS0800GCI gas flow system, the thermal and physical state parameters of Cefixime (CEF-ME) were evaluated. In a 100 l aluminum pan, samples weighing 5 mg-12 mg were weighed with a Mettler MT5 microbalance before being immediately sealed with a mechanical crimper. The trial was conducted between 10°C and 300°C at a heating rate of 10°C/min. To avoid any oxidation of the materials, nitrogen was utilized as a standard purging gas at a rate of 20 ml/min. The DSC scans were acquired using star-e software version 8.10.
Transmission Electron Microscopy (TEM): Transmission Electron Microscopy (TEM) (model TECNAI 200 Kv TEM-Fei, electron optics, Japan) was used to analyze the surface morphology of the Cefixime (CEF-ME) loaded micro emulsion. On a copper grid that had been coated with carbon, a drop of diluted samples was applied and it was then stained with a drop of a 2% (w/w) aqueous solution of phosphotungstic acid for 30 seconds. Filter paper removed extra staining solution, leaving a thin watery coating on the surface. Samples were stained and then dried for ten minutes at room temperature for the investigation [17]. Quartz PCI version 8 software was used to take TEM pictures after the copper grids had cured overnight.
Compatibility assessment with different injectable diluents
The developed micro emulsion formulations' dilutability and compatibility with injections of 0.9% NaCl or 5% dextrose are listed in Table 1. Solubility cefixime data in oils, surfactants and solvents oils/surfactants/solubilizes saturation (mg/mL) soybean oil and Miglyol-812 epikuron 135 F, 1.5 capmul MCM, 5.5 PEG-400 30 tween-20, 35 tween-80 voxpime®. The created micro emulsion formulation was diluted in different concentration ranges (0.2-1 mg/ml) with 0.9% NaCl injection and 5% dextrose injection and they were then stored for 3 hours for visual inspection with respect to phase separation or precipitation.
| Sample | Particle size (nm) | Polydispersity Index (PI) |
|---|---|---|
| Preparation before autoclaving | 56.7 | 0.792 |
| Preparation after autoclaving | 65.1 | 0.87 |
Table 1: Globule size and polydispersity index of developed formulation.
Evaluation of in vitro drug release
Three millilitres of cefixime-loaded microemulsion formulations were placed inside a synthetic membrane (Spectro/por dialysis mebrane, spectra/por 4, diameter 16 mm, molecular weight 12-14 kDa). Alcohol made up the receiver compartment (37 mL), according to Acta Pharmaceutica Sciencia (APS). To achieve sink condition, use PBS pH 7.4 and 55 No. 4, 2017 31 in a 20:80 ratio. In order to prevent evaporation, the receptor compartment was left exposed to room temperature and covered with parafilm. The buffer solution was continually swirled at 600 rpm with a magnetic bar while the temperature of the receptor compartment was kept at 37ºC. At predefined intervals (0, 2, 4, 8, 12, 16, 20 and 24 hours), samples (1 mL) were taken out of the release medium [18]. By using a UV-Visible spectrophotometer (UV-1800, Shimadzu, Japan) set at 261 nm, the samples were examined. The analytical approach was approved. An alignment curve was created [19].
Test for sterility
Utilizing nutritional broth medium and a micro emulsion formulation, the sterility test was carried out for seven days at 37°C. Groups of created cefixime micro emulsion, negative control medium and positive control medium cultured with Bacillus subtilis were observed for the sterility testing study assessment [20].
Stability study
Any pharmaceutical product must undergo stability testing before it can be approved since it ensures the product's quality, safety and efficacy throughout the product's shelf life [21]. Micro emulsion samples were kept at various temperatures (4 degrees celsius, 25 degrees celsius and 40 degrees celsius) for a period of three months in order to evaluate the physical and chemical stability of the developed formulation [22]. At regular intervals of 0, 30 and 90 days, samples were taken out and tested for pH, particle size and clarity/transmittance and drug concentration.
Statistical analysis
All of the data were presented as mean SD. Analysis of Variance (ANOVA) was used to determine which samples' differences were statistically significant. P 0.05 was regarded as significant in each case.
Hemolytic study
In vitro erythrocyte toxicity study: We investigated the erythrocyte toxicity assay according to Bock, et al. A vial of freshly drawn blood serum was filled with the anticoagulant EDTA [23]. The red blood cells were centrifuged at 5,000 rpm for five minutes to isolate the RBCs, and they were then rinsed three times in isotonic phosphate buffer pH 7.4 before being diluted with buffer to make an erythrocyte stock dispersion (three parts centrifuged erythrocytes plus 11 parts buffer). Na2PO410H2O (7.95 g), KH2PO4 (0.76 g), NaCl (7.2 g) and distilled water (add 1,000 ml) make up the buffer solution [24]. To get rid of dirt and serum protein, the washing of the RBCs stage was repeated. Per millilitre of the test sample, a 100-l component stock dispersion was applied. For one hour, the resulting solution was incubated at 37°C. Debris and intact erythrocytes were separated by centrifugation following in HH cubation with shaking. 100 millilitres of the resultant supernatant were mixed to 1 millilitre of HCl (37% w/v) and 39 millilitres of ethanol (99% v/v). All of the components were dissolved by this mixture, which also prevented hemoglobin from precipitating. Using a spectrometer to evaluate the generated mixture's absorbance at 398 nm in comparison to a control sample. The experiment used a 100% hemolysis control sample. The following calculation was used to calculate the percentage of lysis brought on by the test sample:
Hemolysis caused by sample (%)=(Abs of the test sample/Abs at 100% lysis) × 100
In vitro cytotoxicity analysis on vero cell line: 10% fetal bovine serum was added to DMEM media to maintain the Vero cell line. Vero cells (105 cells/mL) were planted in 96-well plates and allowed to adhere to the plate by being incubated at 37°C with 5% CO2 for 24 hours. P60+CAF was applied to cells in both aqueous and ME formulations at optimal doses (matching to their respective MIC values), along with a placebo. Each well received 20 L of MTT (3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide) produced in D-PBSA (5 mg/mL) after 24 hours of incubation. This time, the incubation period was 4 hours. 200 L of DMSO was added to the medium to end the test. An ELISA plate reader was used to measure absorbance at 570 nm. The viability (%) was estimated using the formula, where and are the absorbance obtained for the positive control (cells not treated with the test formulation) and the cells treated with the formulation, respectively.
Results and Discussion
Solubility of cefixime in oils
Micro emulsion components like oil, surfactant (s), and cosurfactant (s) must be biocompatible, secure and efficient for parenteral administration. Because they are less toxic and less sensitive to variations in pH and ionic strength, nonionic or zwitter ionic surfactants are utilized to find an acceptable pharmaceutical dosage form for this purpose.
Additionally, the main goal of a formulation for a medicine that is lipid soluble is to get the molecule to dissolve in the liquid solvent. It is possible to reduce or completely avoid medication precipitation by choosing the ideal liquid vehicle composition. Soybean oil was chosen as the oil component out of the small selection of excipients, as shown in Table 1. Tween 40 was chosen as the surfactant and solubilizes components, in that sequence, because the medicine's 20 mg dosage made the oil component insufficient for solubilizing the drug. It was discovered throughout the formulation process that the majority of the micro emulsion formulations created by altering the mixes of the aforementioned excipient precipitated the medication. Epikuron®135 F, an amphiphilic surfactant with oil miscibility, was selected because complex interfacial films of combinations of surfactants are advantageous for the creation of stable cefixime micro emulsion.
HPLC analysis of cefixime
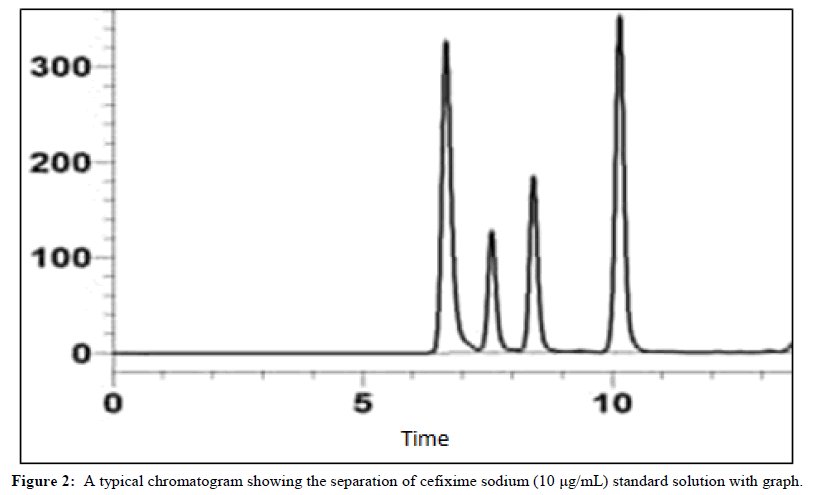
Cefixime (CEF) was analyzed using HPLC (waters alliance e2695 separation module, Milford, MA), equipped with a 2998 PDA detector and a reverse-phase C8 column (5 μm, 100°A, Luna, Torrance, CA, USA) utilizing a mobile phase of ammonium acetate buffer (pH 6.8) and acetonitrile (85:15 v/v) with UV detection at wavelength 252 nm at the flow rate 0 .8 ml/min. was pumped. The absorbance of Cefixime (CEF) was measured at 252 nm and the drug content in the samples was determined by plotting a calibration curve. A stock solution of 1000 μg/ml of Cefixime (CEF) in methanol was prepared and calibration standards ranging from 0.39-50 μg/ml were prepared in the mobile phase. Each calibration standard was analyzed in triplicate and the average peak area was plotted against the amount of Cefixime (CEF) to obtain the calibration curve (Figure 2).
Preparation of pseudoternary phase diagram
To determine the impact of the ratio of surfactant to co-surfactant on the size of the stable o/w micro emulsion area, pseudoternary phase diagram studies were undertaken. When their mixtures came into contact, the cefixime-containing micro emulsions in the current study formed on their own at room temperature. As the ratio of surfactant and cosurfactant was raised, the phase of the micro emulsion and the isotropic areas increased (Figure 3). It demonstrates that when the ratio of surfactant and cosurfactant to oil grew, the maximum amount of oil employed in micro emulsions also climbed significantly. Cefixime should be supplied slowly over a period of 30 to 60 minutes through intravenous infusion. The best component ratios for a micro emulsion that would remain stable and prevent drug precipitation over infinite dilution were therefore chosen utilizing a developed pseudoternary phase diagram.
Characterization of micro emulsion
Optical birefringence: A principle of light scattering is called birefringence. It also goes by the name "double refraction" and it has its roots in anisotropic and liquid crystal systems. In this type of light-scattering system, light that is travelling through a substance is split into two components that have different speeds and as a result, different refractive indices. As a result, birefringence, often known as the appearance of vivid color bands when a liquid crystal is seen between crossed polarizers, is produced. Micro emulsion, on the other hand, appears to be entirely black. When the formulated micro emulsion was seen between cross-polarizing plates, it was entirely black, confirming that the formulation was an isotropic, colloidal dispersion.
Determination of particle size
It is acknowledged that one of the most crucial aspects of an emulsion for determining its stability and in vivo fate is the distribution of its particle sizes. As a result, it follows that size has a significant impact on how long particle carriers circulate. According to a variety of publications, tiny particle carriers can avoid MPS detection and hence circulate in the bloodstream for extended periods of time. Additionally, the smaller size avoids capillary obstruction, which reduces the negative effects commonly connected to the intravenous administration of particle carriers. As a result, the cefixime micro emulsion's particle size was evaluated in three separate samples. The particle size before and after autoclaving is presented in Table 2. The cefixime micro emulsion globule's particle size did slightly rise, but it was still less than 100 nm.
| % Drug content | |||||
|---|---|---|---|---|---|
| Conditions | Initial | 7 days | 1 month | 2 months | 3 months |
| Room tempreture | 100.01 ± 0.56 | 99.91 ± 0.23 | 98.50 ± 0.67 | 98.01 ± 0.79 | 97.20 ± 0.91 |
| 3°C/60% RH | - | 99.03 ± 0.51 | 99.60 ± 0.32 | 99.56 ± 0.89 | 101.89 ± 0.40 |
| 4°C/75% RH | - | 102.71 ± 0.22 | 101.31 ± 0.49 | 99.11 ± 0.79 | 101.91 ± 0.783 |
Note: RT= Room Temperature; RH = Relative Humidity.
Table 2: Drug content stability data.
Accelerated stability testing
Centrifugation: Emulsion scientists have long employed centrifugal techniques to create and speed up instability using gravitational force. The separation of the dispersion phase during centrifugation of the micro emulsion can be used to quickly estimate the shelf life under normal storage conditions.
This technique allows for a quick and accurate identification of the systems as micro emulsions by determining the behavior of small particles in the gravitational field, i.e., their separation rates. Smaller than 0.5 m particles exhibit brownian motion. The micro particles in this size range are small enough to pass through the molecules of the dispersion medium and absorb kinetic energy from bombardment. Such a particle is thought to change direction 1024 times per second as a result. This system prevents the dispersed droplets from settling in the gravitational field by maintaining them in a state of furious motion. Because of brownian mobility, the cefixime micro emulsion droplets do not settle or cream and as a result, they do not consolidate. The surface free energy of a micro emulsion system is what prevents cefixime micro emulsion droplets from coalescing. The system now possesses negative free-surface energy as two droplets combine to form a single, larger droplet. At this point, the interfacial tension of the new droplet turns negative. Two droplets of the original size are formed as a result of the bigger droplet size's abrupt rise in curvature, which results in zero interfacial tension once more. The bombardment of droplets by molecules of the dispersion medium is a continual occurrence as well. The stability of the cefixime micro emulsion systems is maintained by this dynamic equilibrium. After 30 minutes, centrifugation at 3,000 rpm on the generated cefixime micro emulsion revealed no phase separation or drug precipitation, demonstrating the formulation's stability.
Viscosity measurements: The micro emulsion's stability is also influenced by its viscosity, which expresses flow resistance. The tendency of the system to aggregate is what is meant by viscosity. The produced cefixime micro emulsion also required diluting with infusion fluids before delivery; hence the viscosity measurement was crucial. It is generally known that the syringe ability parameter can be impacted by the parenteral formulation's viscosity. The formulated micro emulsion's viscosity was determined to be 106.92 cP using the equation below. The equation-was used to determine the viscosity.
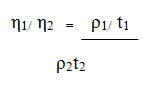
Where: η1 and η2=Viscosities of the test and the standard sample, ρ1 and ρ2=densities of the liquids t1 and t2= respective flow times in seconds. The created cefixime micro emulsions low viscosity ensures easy syringe ability and simplicity of mixing with intravenous fluids with little mechanical agitation.
pH measurement: The pH of the following micro emulsion formulation was repeatedly measured over the course of ten days, and the results indicated that the pH was within an acceptable pH range for intravenous administration. When it comes to cefixime, pH is crucial to the drug's stability. Cefixime's pH for stability was discovered to be pH 5.2, which is thought to be the most beneficial for preventing drug breakdown. The medication content was found to be within permissible bounds during a three-month period of time because of the constant pH range.
Compatibility assessment with different injectable diluents
Cefixime for injection had to be diluted as directed in order to be administered intravenously through an infusion of either 5% dextrose or 0.9% sodium chloride, yielding a solution that contained 200 to 400 g (0.2 to 0.4 mg) of cefixime per millilitre. As can be shown in Tables 3 and 4, the diluted solution was stable enough to allow for a slow intravenous infusion of cefixime at concentrations up to 1.1 mg/ml for 1.5 and 2 hours in injections of 0.9% sodium chloride and 5% dextrose, respectively. However, it should be mentioned that with injections of 0.9% sodium chloride and 5% dextrose, respectively, there was no drug precipitation for 3 hours at the acceptable concentration of 0.5 mg/ml. This demonstrates the new formulation's superiority over the currently available cefixime injectable, which lists drug precipitation as one of its drawbacks.
|
Time in hours | |||||
|---|---|---|---|---|---|---|
| Concentration of drug mg/ml | 0.5 | 1 | 1.5 | 2 | 2.5 | 3 |
| 0.2 | C | C | C | C | C | C |
| 0.5 | C | C | C | C | C | C |
| 0.7 | C | C | C | C | C | C |
| 0.9 | C | C | C | C | C | P |
| 1.1 | C | C | C | P | - | - |
Note: C: Clear; P: Precipitation.
Table 3: Compatibility of micro emulsion with 0.9% sodium chloride injection.
| Time in Hours | ||||||
|---|---|---|---|---|---|---|
| concentration of drug mg/ml | 0.5 | 1 | 1.5 | 2 | 2.5 | 3 |
| 0.3 | C | C | C | C | C | C |
| 0.6 | C | C | C | C | C | C |
| 0.9 | C | C | C | C | C | C |
Note: C: Clear; P: Precipitation.
Table 4: Compatibility of micro emulsion with 5% dextrose injection.
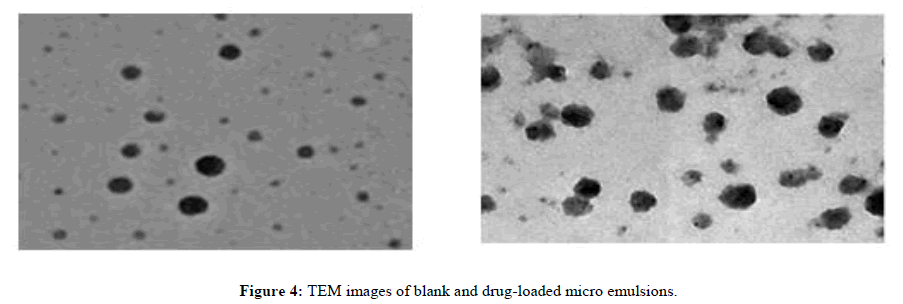
TEM: TEM images of blank and drug-loaded micro emulsions are shown in Figure 4. The observed droplets were roughly spherical in shape and the droplet size in both cases was found to be slightly higher than that determined using DLS.
In vitro hemolytic studies
To demonstrate the parenteral micro emulsions' safety for blood components, their hemolytic potential should be assessed. On long-term contact with the blood, it has been shown that commonly used parenteral cosurfactants like glycerol or propylene glycol can significantly cause hemolysis. When the formulation is to be given as a continuous infusion over an extended period of time, it is crucial to determine the parenteral micro emulsion's hemolytic potential. All of the Cefixime (CFX-ME) loaded micro emulsions induced little (1%) hemolysis when in contact with human blood for two hours, despite the fact that they were all based on ingredients suitable for parenteral delivery (Figure 5).
In vitro cytotoxicity analysis on vero cell line
Using the vero cell line maintained in DMEM media containing 10% foetal bovine serum, the cytotoxicity of the micro emulsions was compared to that of Voxpime®. In the presence of 20% DMSO, 10.88% of the cells were still viable. At lower concentrations (0.01%, 0.1%, 1% and 5%) for both formulations, cell viability was greater than 100%; at higher concentrations (50 and 100%), cell viability was less than 15%. The results are shown as a percentage of the control (100%), which omitted both antibiotics. The same effects as on day one persisted after five days, although on the final day of testing there was no discernible change in viability compared to control.
In vitro release studies
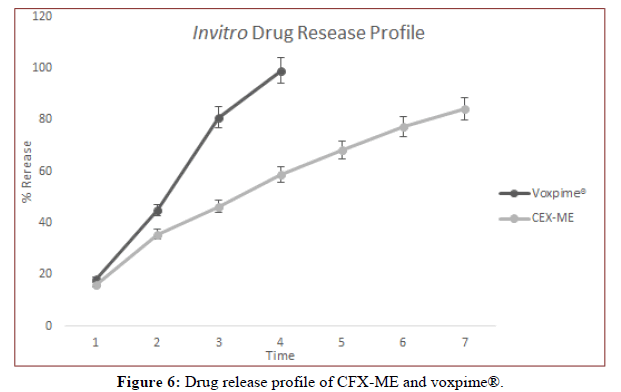
By using a UV-visible spectrophotometer (UV-1800, Shimadzu) set at 261 nm, the samples were examined. The samples were examined using the analytical technique. For the standard solution of bulk cefixime, a calibration curve was produced using an eight-point calibration concentration with a range of 0.001 mg/mL-0.2 mg/mL. Where in three separate assessments at each concentration were made. Cefixime concentration and absorption were shown to be linearly related. The slope's and intercept's standard deviations were displayed. For the conventional solution of cefixime, the regression line's determination coefficient R2 is 0.99854, with a slope of 20.624x and a y+intercept of+0.0149. In order to evaluate and compare the performances of formulations, cefixime loaded micro emulsion formulations and CFX-ME and CFX2-ME solution component were tested for in vitro release across synthetic membrane. Graphics for in vitro release are shown in Figure 6. At the end of the 24th hour, the CFXME and CFX2-ME solutions show 100% release.
Test for sterility
One of the critical parameters for parenteral formulations is sterility. Phase separation may occur at appropriate temperatures, but the micro emulsions spontaneously revert to their initial state upon cooling to room temperature and after sufficient mixing. Cefixime micro emulsions can be sterilized by autoclaving as they are established; the created micro emulsion was sterilized for 15 minutes at 121°C and 15 pressures. Even though phase separation may have occurred after autoclaving, shaking produced a uniform micro emulsion. This sterile micro emulsion's sterility tests revealed no microbiological growth, demonstrating the efficiency of autoclaving. Additionally, this was supported by the created micro emulsion's stability during a three month period.
Conclusion
Particles smaller than 100 nm were successfully created for the parenteral cefixime micro emulsion. The created micro emulsion formulation was found to be robust when diluted with IV fluids and was susceptible to autoclave sterilization. The formulation's suitability for parenteral administration was confirmed by the in vitro erythrocyte toxicity testing. Too far, it has been demonstrated that micro emulsions can protect labile medications, regulate medication release, increase medication solubility, boost bioavailability and decrease patient variability. Additionally, it has been demonstrated that preparations that work well for the majority of administration methods can be created. Before they may fully realize their potential as versatile drug delivery systems, however, a sizable amount of fundamental research defining the physicochemical behavior of micro emulsions needs to be done. A thermodynamically stable isotropically transparent dispersion of two immiscible liquids, such as oil and oil, stabilized by an interfacial coating of surfactant molecules is referred to as a "oil-in-oil emulsion." A liquid dispersion of an oil phase and a water phase that is thermodynamically or kinetically stable is referred to as an "oil-in-oil emulsion.
References
- Shahbaz K. Pharm Biol Eval. 2017; 10(2): p. 99-102.
- Wu HR, Wang CQ, Wang JX, et al. Int J Nanomedicine. 2020; 19(11): p. 2391-2402.
[Crossref] [Google Scholar] [PubMed]
- Jain J, Fernandes C, Patravale V. Aaps Pharm Sci Tech. 2010; 11: p. 826-831.
[Crossref] [Google Scholar] [PubMed]
- Noreen S, Sumrra SH. ACS Omega. 2021; 6(48): p. 33085-33099.
- Sato M, Grasser W. J Bone Miner Res. 1990; 5(1): p. 31-40.
[Crossref] [Google Scholar] [PubMed]
- Full AP, Kaler EW, Arellano J, et al. Macromolecules. 1996; 29(8): p. 2764-2775.
- Pathak L, Kanwal A, Agrawal Y. J Food Sci Technol. 2015; 52: p. 6143-6156.
- Das S, Ng WK, Tan RB. Eur J Pharm Sci. 2012; 47(1): p. 139-151.
[Crossref] [Google Scholar] [PubMed]
- Algharib SA, Dawood A, Zhou K, et al. J Mol Struct. 2022; 1252: p. 132-133.
- Fligner KL, Fligner MA, Mangino ME. Food Hydrocoll. 1991; 5(3): p. 269-280.
- Strickley RG. Pharm Res. 2004; 21: p. 201-230.
[Crossref] [Google Scholar] [PubMed]
- Mahmoud DB, Shukr MH, Bendas ER. Int J Pharm. 2014; 476(1-2): p. 60-69.
[Crossref] [Google Scholar] [PubMed]
- Bozzi A, Yuranova T, Kiwi J. J Photochem Photobiol: A Chem. 2005; 172(1): p. 27-34.
- Bhavsar D, Gajjar J, Sawant K. Mesoporous Mater. 2019; 279: p. 107-116.
- Javed R, Rais F, Fatima H, et al. Mater Sci Eng C. 2020; 116: p. 111-184.
[Crossref] [Google Scholar] [PubMed]
- Bajaj S, Singla D, Sakhuja N. J Appl Pharm Sci. 2012: 13(6): p. 129-138.
- Constantinides PP, Scalart JP. Int J Pharm. 1997; 158(1): p. 57-68.
- Biswas J, Sinha D, Mukherjee S, et al. Hum Exp Toxicol. 2010; 29(6): p. 513-524.
[Crossref] [Google Scholar] [PubMed]
- Thakkar V, Dhankecha R, Gohel M, et al. Int J Drug Deliv. 2016; 8 (3): p. 77-88.
- Joshi M, Pathak S, Sharma S, et al. Int J Pharm. 2008; 364(1): p. 119-126.
[Crossref] [Google Scholar] [PubMed]
- Arifin MA, Mel M, Abdul Karim MI. Biomed Res Int. 2010; 105(56): 201-210.
[Crossref] [Google Scholar] [PubMed]
- Gupta S, Bansal R, Ali J, et al. Biomed Res Int. 2014; 34(18): 99-101.
[Crossref] [Google Scholar] [PubMed]
- Kaur A, Gabrani R, Dang S. Adv Pharm Bull. 2019; 9(3): p. 401-410.
[Crossref] [Google Scholar] [PubMed]
- Mady MS, Mohyeldin MM, Ebrahim HY, et al. Bioorg Med Chem. 2016; 24(2): p. 113-122.
[Crossref] [Google Scholar] [PubMed]