Research Article - Der Pharma Chemica ( 2022) Volume 14, Issue 9
Formulation and Characterization of Silver Nanoparticles Using Casiatora and Evaluation of Antimicrobial and Antioxidant Activities
Manoj Bali* and Shalu ShuklaIndia
Manoj Bali, Department of Pharmacy, Bahra University Shimla Hills, Solan, Himachal Pradesh, India, Email: drmanojbali@gmail.com
Received: 10-Dec-2020, Manuscript No. DPC-21-23195; Editor assigned: 15-Dec-2020, Pre QC No. DPC-21-23195; Reviewed: 29-Dec-2020, QC No. DPC-21-23195; Revised: 08-Aug-2022, Manuscript No. DPC-21-23195; Published: 05-Sep-2022, DOI: 10.4172/0975- 413X.14.9.16-23
Abstract
Nanoparticles synthesis and application is broad, active and growing field of research. Nanoparticles have wide variety of novel applications depending on their size, shape, and composition. However, current production techniques are complex and/or may involve hazardous compounds. Using plant metabolites for nanoparticle synthesishas numerous advantages over conventional chemical, physical and biological methods. Silver nanoparticles (AgNPs) have attracted a lot of attention because of their use inpurification and quality management of air, biosensing, imaging and drug delivery system. In the present studies seed extract of cassia tora seeds has been employed for successful green synthesis of AgNPs from cationic silver. The physical properties size shape and morphology of synthesized nanoparticles were investigated by UV visible spectroscopy, fourier transform infrared spectroscopy, scanning electron spectroscopy and Zeta potential analysis. The antibacterial activity of biosynthesized AgNPs was studied against selected bacterial species Staphylococcus aureus, Bacillus subtilis, Pseudomonas aeruginosa, Staphylococcus epidermidis and Escherichia coli. Antioxidant activity was checked using diphenylpicrylhydrazyl scavenging essay. The synthesized AgNPs exhibit great activity against selected bacteria along with the antioxidant activity.
Keywords
Nanoparticles Synthesis; Silver Nanoparticles; Biosynthesis; Antimicrobial; Antioxidant; Green Synthesis
INTRODUCTION Nanoparticles are gaining interest because of their potential for numerous applications which also includes medicinal applications like antioxidants, antibacterial antifungal and antibacterial agents, hence there has been an exponential increase in research on nanoparticles. At the same time a lot of concern has been generated regarding the environmental hazards associated with the commercial scale production on these nanoparticles. The focus has thus shifted towards the ecofriendly ways of production. Green synthesis of nanoparticles is perhaps the fastest growing research area since last decade. Scientists have tried many ways of nanoparticles synthesis which would be free from hazardous environmental effects associated to the conventional methods. It has been established that there exist many biological systems, including plants and algae, diatoms, bacteria, yeast, fungi, and human cells that can transform inorganic metal ions into metal nanoparticles. This has been attributed to the reductive capacities of the proteins and metabolites present in these organisms. Researchers have recently focused their attention to the synthesis of metal nano particles using plant extracts. Phyto chemistry has demonstrated the ability to produce large quantities of nanoparticles without any threats to environment. It has been reported that various plant metabolites, including terpenoids, polyphenols, sugars, alkaloids, phenolic acids, and proteins, play an important role in the bio reduction of metal ions, yielding nanoparticles [1-3].
Nano-materials, in the form of biosensors for pathogen detection and as a therapeutic tool against bacteria, fungi, and viruses have provided a promising results. The properties of nanoparticles are attributed to their high surface area to volume ratio at nano-dimensions. Silver has been known for its antimicrobial activity since the ancient days. It was used for storing drinking water in ancient times. Silver, whether in ionic or nanoparticle form, is highly toxic to microorganisms. It is the most toxic metal known for its activity against microorganisms. Other metals follow silver in respect of antimicrobial activity as shown below: Ag>Hg>Cu>Cd>Cr>Pb>Co> Au>Zn>Fe>Mn>Mo>Sn.
The unique optical, electrical, and thermal properties of silver nanoparticles have made it useful for products that range from photovoltaics to biological and chemical sensors. Examples include conductive inks, pastes and fillers which utilize silver nanoparticles for their high electrical conductivity, stability, and low sintering temperatures. An increasingly common application is the use of silver nanoparticles for antimicrobial coatings and wound dressings. Biomedical devices now contain silver nanoparticles that continuously release a low level of silver ions to provide protection against bacteria [4]. The present studies focused on the synthesis of silver nanoparticles using extract of cassia tora seeds. Cassia tora Linn. (Family: Leguminosae) is well known plant widely distributed in India and other tropical countries. It is an annual under shrub and grows in wild waste land. Different parts of the plant (Leaves, seed, and root) are known for their medicinal value. The chemical constituents include Roots: 1,3,5-trihydroxy-6-7-dimethoxy-2-methylanthroquinone and beta-sitosterol. Seeds: Naptho-alpha-pyrone-toralactune, chrysophanol, physcion, emodin, rubrofusarin, cchrysophonic acid-9-anthrone. Leaves: Emodin, tricontan-1-0l, stigmasterol, b-sitosteral-b-D-glucoside, freindlen, palmitic, stearic, succinic and d-tartaric acids uridine, quercitrin and isoquercitrin. The seed extract of cassia tora has been utilized for green synthesis of biologically active silver nanoparticles [5-7].
MATERIALS AND METHODS
Synthesis of Silver Nano Particles
Cassia tora seeds extract was prepared by using methanol as solvent. Extraction was done by hot extraction method. The powdered plant material was stored in well closed container until further use. Analytical grade silver nitrate (AgNO3) was used for the preparation of different concentrations of silver nitrate i.e. 0.1 M, 0.01 M, 0.001 M in chlorine free water. All the solutions were kept away from light (the containers were wrapped with brown papers) and kept in dark. About 250 mg of Cassia tora seeds extract (semi-solid mass) was taken in a wide neck borosil flask proceeded by addition of 50 ml of double distilled water. In order to subdue the enzymes and proteins which interferes the reduction process this flask was subjected to microwave heating for 3 min. The clear filtrate obtained from the above procedure was used for nanoparticle synthesis. This extract was added to 50 ml of different concentrations of silver nitrate solution (ie. 0.1 M, 0.01 M, 0.001 M) in ratios 1:1, 1:2, 2:1 respectively for each and exposed to microwave radiations. Immediately after the addition of plant extract solution to AgNO3 aqueous solution, a light yellowish color was observed which changed to dark brown color on subjecting to microwave irradiation which further enhanced on increased exposure. This change of color indicates that the formation of AgNPs has taken place. The various components of the mixture were separated using centrifugation. The supernatant obtained from centrifugation (4000 rpm, 20 min) of the above solution was further centrifuged (20000 rpm, 30 min). The resultant residue was carefully washed with 7 ml of double distilled water and 100 ml of double distilled water was added to it. The resultant suspension was divided into two portions of 25 ml and 75 ml. Smaller portion was vacuum dried to check the weight of the separated AgNPs (along with cassia tora component). The weight of the solid was found to be 16mg. The 75 ml portion was stabilized with 0.75 ml of twin 80 and used to study antimicrobial and antioxidant activities [8,9].
Characterization
The reduction of silver ions to nanoparticle was monitored by measuring the UV-visible spectra of the solutions after diluting the sample with double distilled water 20 times. The spectra were recorded on UV-visible double beam spectrophotometer from 200 nm to 800 nm. Scanning electron microscopic analysis was done by preparing a thin film of the sample on a carbon coated copper grid by just dropping a very small amount of sample on the grid, extra solution was removed using blotting paper and sample on grid was allowed to dry by putting it under mercury lamp for 5 minutes [10].
The FT-IR spectra were scanned in the range of 4000 cm-400 cm at a resolution of 4 cm-1. The sample was prepared by dispersing the AgNPs uniformly in a matrix of dry KBr (potassium bromide), compressed to form an almost transparent disc. KBr was used as a standard. FT-IR measurements were carried out to identify the possible biomolecules responsible for the reduction of the Ag+ ions and the capping of the bioreduced AgNPs synthesized by Cassia tora seeds extract. For comparison, the Cassia tora seeds extract filtrate was mixed with KBr powder and pelletized after drying properly and subjected to measurement. The zeta potential of the synthesized nanoparticles was determined by means of zeta potential analyzer [11].
Biological Activity
Disc-diffusion method was used to determine the antibacterial activity of biologically prepared silver nanoparticles. For performing the experiment, cultures of test bacterial strains- Staphylococcus aureus, Staphylococcus epidermidis, Bacillus subtilis (Gram- positive bacterium) and Pseudomonas aeruginosa, Eschirichia coli (Gram- negative bacterium) were collected. The sterile discs were dipped in silver nanoparticles solution and placed in nutrient agar plate and kept for incubation at 370°C for 24 hours. Standard discs of amoxicillin, discs dipped in stock solutions of Cassia tora seeds extract prepared using DMSO (Dimethyl Sulfoxide) were used for comparison of the silver nanoparticles. Zone of inhibition for Cassia tora seeds extract, standard, silver nanoparticles and silver nitrate were measured. After 24 hours of incubation at 37°C, distinct zone of bacterial growth inhibition was observed around all the discs. The antioxidant activity was measured as per the standard DPPH assay procedure [12].
RESULTS
Ultraviolet-Visible Spectral Analysis
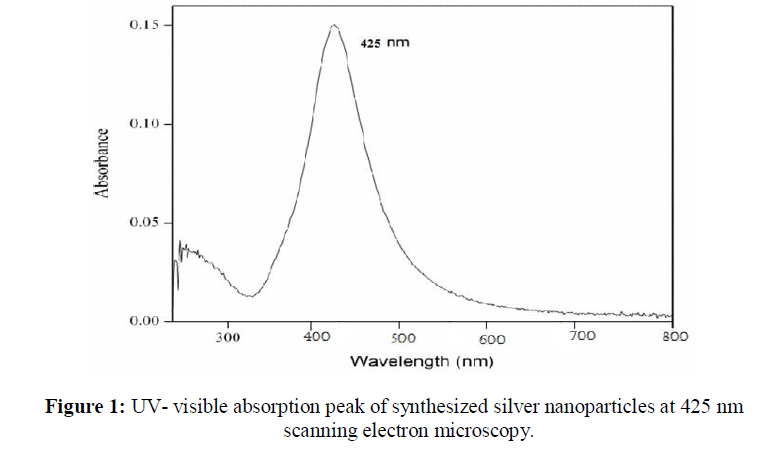
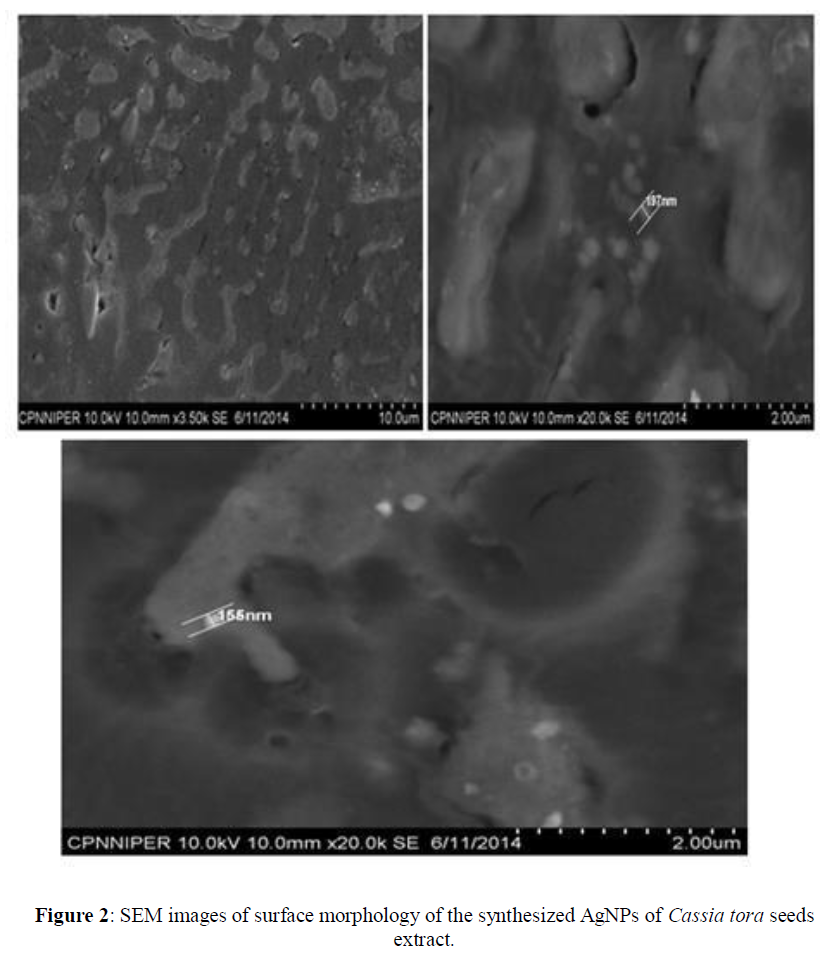
UV-visible absorption spectra of reduction of synthesized silver ions to silver nanoparticles was recorded, with three different concentrations of silver nitrate (0.1 M, 0.01 M, 0.001 M) in ratios 1:1, 1:2, 2:1 respectively for each. The silver nanoparticles exhibit a unique peak in the range of 400 nm- 460 nm. From graphical representation of UV- visible absorption spectra, it is clear that formation of silver nanoparticles take place. The best peak is shown at 425 nm when Cassia tora seed extract solution and 0.1 M concentration of AgNO3 mixed at 1:2 ratio. Broadening of peak, indicates that the particles are dispersed (Figure 1). SEM analysis illustrates synthesize of silver nanoparticles which were roughly spherical in shape with the diameter range of 120 nm- 200 nm (Figure 2).
FT-IR Analysis
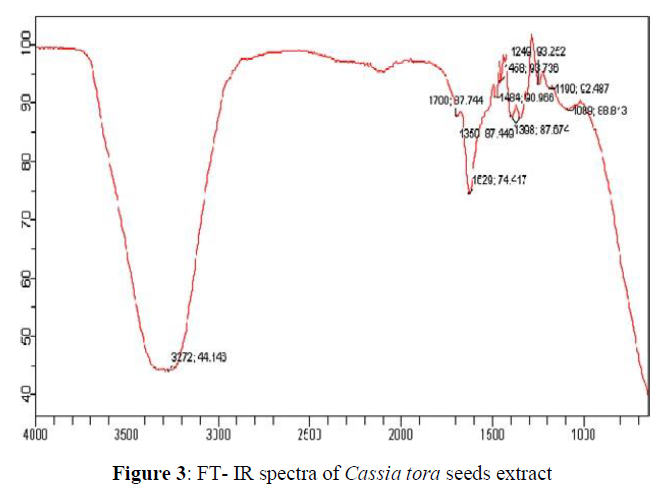
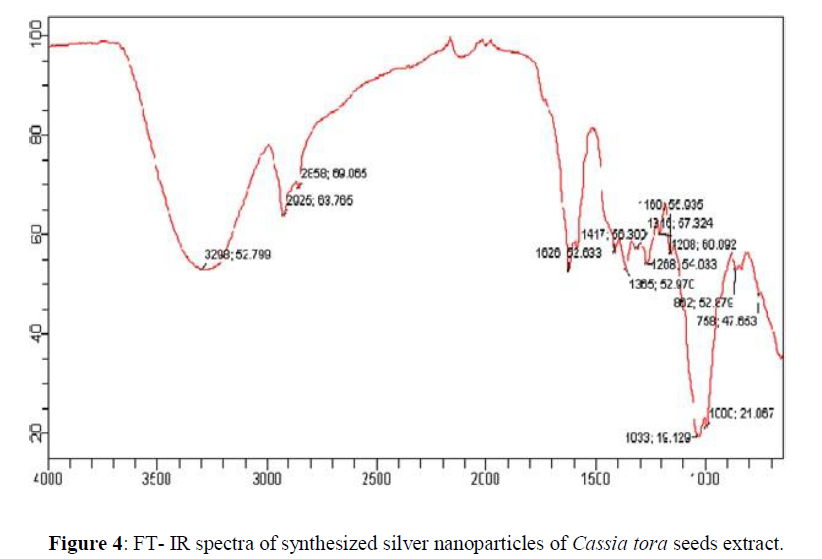
FT-IR spectrum clearly indicates the bio-fabrication of silver nanoparticles. In the Cassia tora seeds extract peaks were observed at 3272; 44.146 cm-1, 1700; 87.744 cm-1, 1629; 74.417 cm-1, 1484; 90.966 cm-1, 1458; 93.736 cm-1, 1398; 87.674 cm-1, 1350; 87.674 cm-1, 1249; 93.252 cm-1, 1190; 92.487 cm-1, 1089; 88.813 cm-1 which are associated OH stretching, CH stretching, C=N stretching, N-H stretching, CH stretching, CN stretching, C- Cl stretching. In the synthesized AgNPs from Cassia tora seeds peaks (Figure 4) were observed at 3298; 52.799 cm-1, 2925; 63.765 cm-1, 2859; 69.065 cm-1, 1626; 52.633 cm-1, 1417; 56.300 cm-1, 1365; 52.970 cm-1, 1316; 57.324 cm-1, 1268; 54.033 cm-1, 1208; 60.092 cm-1, 1160; 55.935 cm-1, 1033; 19.129 cm-1, 1000; 21.067 cm-1 , 862; 52.879 cm-1, 758; 47.653 cm-1, which are associated with NH stretching, C=O stretching, N-O stretching, CH2 and CH3 deformation and C-O stretching and halogen group presence. The Cassia tora seeds extract shows broad peak at 3272; 44.146 cm-1 which indicate the presence of OH group and carboxyl groups and after synthesis of AgNPs there is a shift in the broad peak to the right at 3298; 52.799 cm-1 indicating the NH stretching. A new peak is shown at 2925; 63.765 cm-1 which is corresponds to asymmetric stretching of methylene groups. The carboxyl and amide group indicate the presence of secondary amines which confirming the bio fabrication of the nanoparticles (Figures 3 and 4).
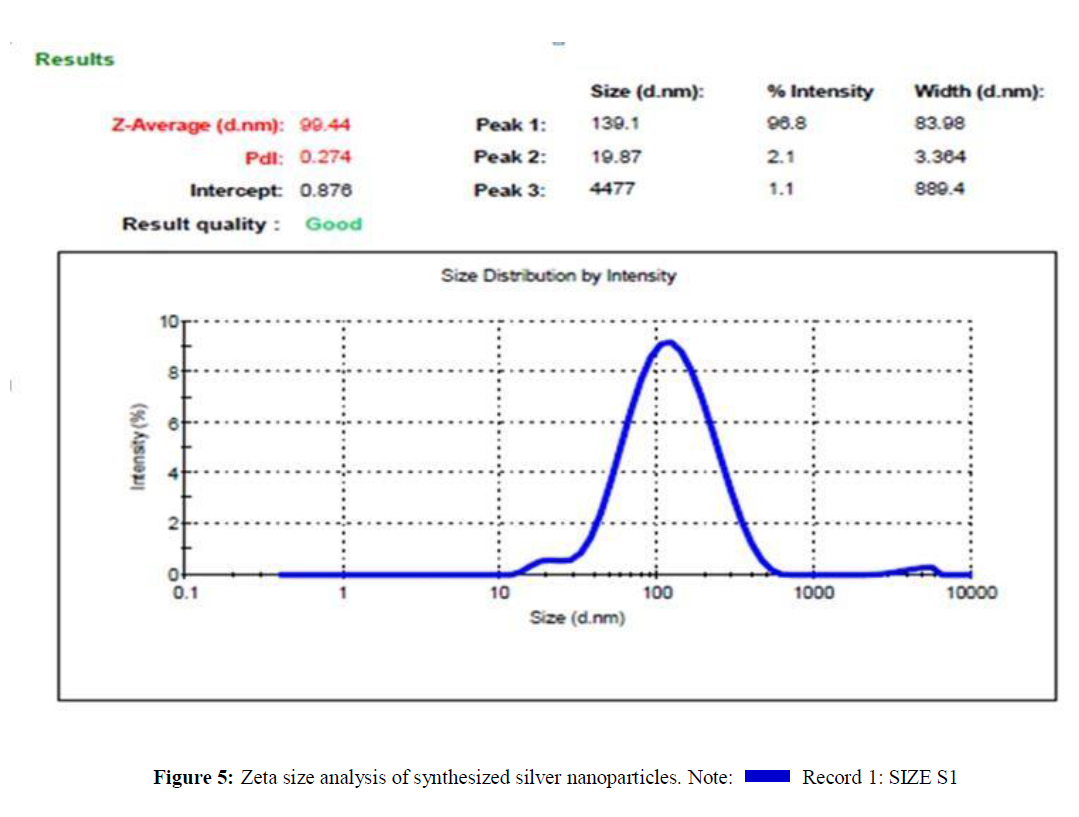
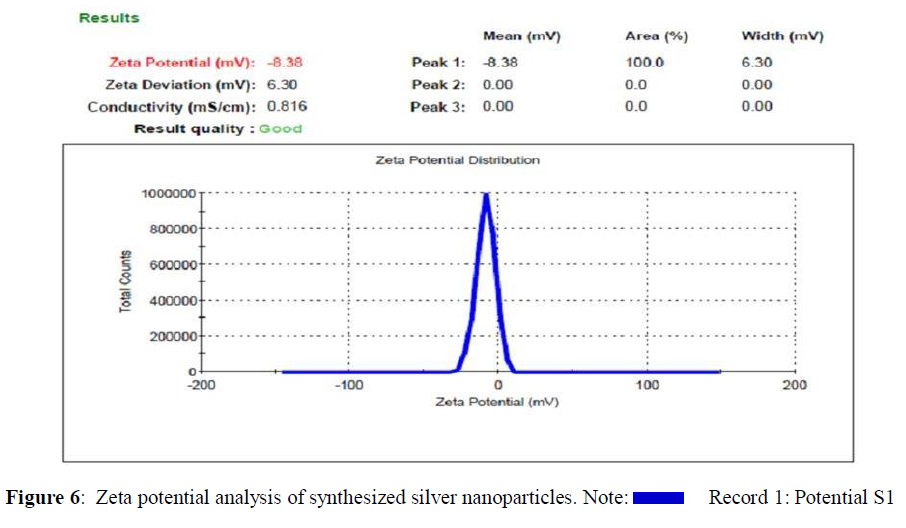
Particle Size and Zeta Potential Analysis
Zeta size and zeta potential analysis of synthesized silver nanoparticles has an average size of 99.44 nm and the particles carry a charge of -8.38 mV. Poly Disparity Index (PDI) is a measurement for distribution of silver nanoparticle from 0.000 to 0.5. PDI greater than 0.5 values indicates the aggregation of particles. From Figure 5, it was clear that all the AgNPs synthesized from Cassia tora seeds extracts does not aggregate at all. Zeta potential measures the potential stability of the particles in the colloidal suspension. Silver nanoparticles generally carry a negative charge. Silver nanoparticles synthesized from the Cassia tora seeds extracts showed negative charge and were stable at room temperature (Figures 5 and 6) [13].
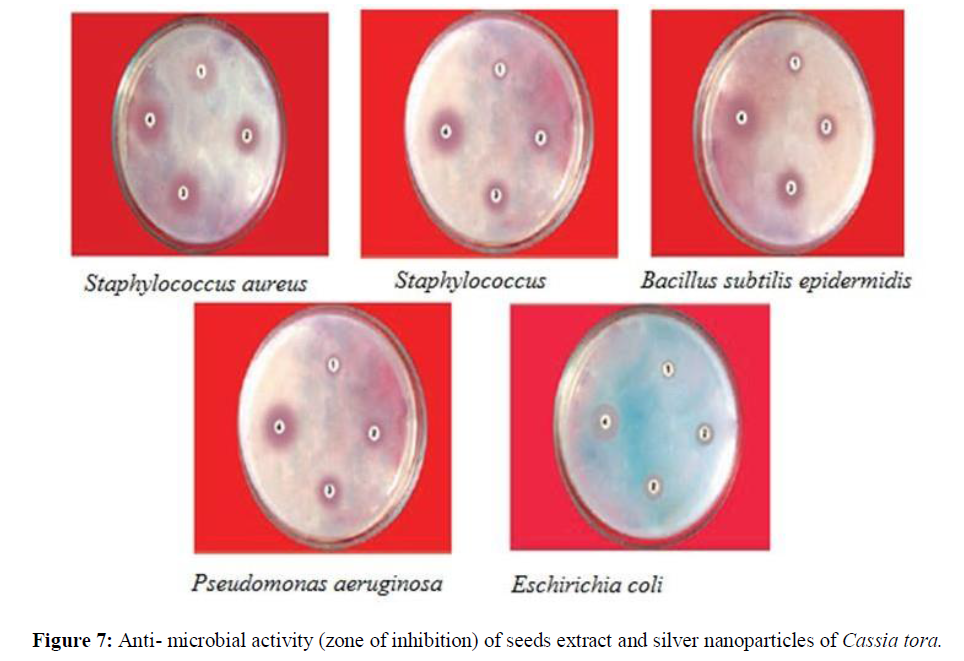
Antimicrobial Activity of AgNP’s
The distinct zones of inhibition were observed from silver nanoparticles, Cassia tora seeds extract, silver nitrate and amoxicillin as shown in figure 7.
The synthesized silver nanoparticles were administered in 0.2 mM concentration to investigate the growth behavior of different bacterial species. It was noticed that at this concentration the growth was reduced (Table 1) and (Figure 7).
This clearly indicates that AgNP’sare toxic to bacterial species and therefore, the growth was inhibited. The synthesized silver nanoparticles exhibited inhibitory effect against all the tested bacterial strains but good inhibitory effect was shown against Staphylococcus aureus, Bacillus subtilis and E. coli with inhibition zones of 10.80, 8.90 and 9.85 mm respectively. At same concentration Cassia tora seeds extract exhibited inhibitory effect against all strains of bacteria but to less extent. Silver nitrate (AgNO3) also exhibited inhibitory effect against all the tested strains. Finally, amoxicillin (standard drug) showed the highest inhibitory effect against all the micro-organisms with inhibition zone of 27.25 mm for S. aureus, 25.33 mm for S. epidermidis, 22.40 mm for B. subtilis, 20.0 mm for P. aeruginosa and 21.56 mm for E. coli. At the end of this antimicrobial screening test, it was Confirmed that the biologically synthesized silver nanoparticles possess antibacterial property [14].
| Bioactive agent | Zone of inhibition (mm) | ||||
|---|---|---|---|---|---|
| S. aureus | S. epidermidis | B. subtilis | P. aeruginosa | E. coli | |
| Silver nanoparticles (64 µg/ml) | 10.8 | 9.74 | 8.9 | 12 | 9.85 |
| Cassia tora seeds extract (2.5 mg/ml) | 4.2 | 4.5 | 6.5 | 6.8 | 7.33 |
| Silver nitrate (0.01M) | 9.4 | 16.66 | 8.54 | 15.43 | 7.3 |
| Amoxicillin (standard drug) (50 µg/ml) | 27.25 | 25.33 | 22.4 | 20 | 21.56 |
Anti-Oxidant Activity of AgNP’s:
The effect of antioxidants on DPPH is thought to be due to their hydrogen donating activity (Table 2).
| S. No | Concentration of the sample | Cassia Tora seeds extract | AgNP’s synthesized from Cassia tora seeds | Ascorbic acid (standard) |
|---|---|---|---|---|
| 1 | 50 μg | 0.161 | 0.292 | 0.115 |
| 2 | 100 μg | 0.139 | 0.246 | 0.109 |
| 3 | 150 μg | 0.104 | 0.22 | 0.095 |
DISCUSSION
The tested samples showed good activity when compared with ascorbic acid (standard). There was a decrease in absorption at 517 nm indicating that Cassia tora seeds extract and synthesized AgNPs have hydrogen donating ability or can scavenge free radicals. It was observed that Cassia tora seeds extract and AgNPs exhibited good activity as free radical scavenger (Table 3).
| S. No. | Percentage (%) of inhibition | |
|---|---|---|
| Cassia tora seeds extract | AgNP’s synthesized from Cassia tora seeds |
|
| 1 | 67.92 | 41.83 |
| 2 | 72.31 | 50.99 |
| 3 | 79.28 | 56.17 |
The Cassia tora seeds extract gave percentage inhibition of 79.28% while that of synthesized AgNP’s was 56%. The percentage of inhibition increases with increase in concentration of Cassia tora seeds extract and synthesized silver nanoparticles (i.e. 50 μg, 100 μg, 150 μg) [15].
CONCLUSION
The environmental limitations of physical and chemical methods of nanoparticle synthesis motivate progress in biosynthetic green route. Though there exist many biological routes, the use of plants for facile robust synthesis has tremendous potential. The green synthesis of AgNPs using cassia tora seed extract is quick, easy and environmentally benign. The synthesized nanoparticles were of uniform shape. The best peak is shown at 425 nm when Cassia tora seed extract solution and 0.1 M concentration of AgNO3 mixed at 1:2 ratio. SEM analysis shows that the Cassia tora seeds have tremendous capability to synthesize silver nanoparticles which were roughly spherical in shape with the diameter range of 120 nm-200 nm. Further in FT-IR spectrum a new peak is shown at 2925; 63.765 cm-1 which corresponds to asymmetric stretching of methylene groups. The carboxyl and amide group indicate the presence of secondary amines which confirms the synthesis of the nanoparticles. Silver nanoparticles synthesized from the Cassia tora seeds extracts showed negative charge in Zeta potential analysis and were stable at room temperature. The nanoparticles also possess significant antioxidant and antibacterial activities. So, it is summarized that, the above plant based green synthesis is effective and eco-friendly process for synthesizing biologically active silver nanoparticles.
ACKNOWLEDGEMENT
Authors are thankful to for providing Bahra University Shimla Hills, Waknaghat, Solan, Himachal Pardesh the necessary facilities for research.
REFERENCES
- Wang L, Hu C, Shao L. Int J Nanomedicine. 2017, 12: p. 1227.
- Kuppusamy P, Yusoff MM, Maniam GP, et al. Saudi Pharm J. 2016, 24: p. 473-484.
- Makarov VV, Love AJ, Sinitsyna OV, et al. Acta Naturae. 2014, 6: p. 35-44.
- Mittal AK, Chisti Y, Banerjee UC. Biotechnol Adv. 2013, 31: p. 346-356.
- Khezerlou A, Alizadeh-Sani M, Azizi-Lalabadi M, et al. Microb Pathog. 2018, 123: p. 505-526.
- Bhardwaj N, Bhardwaj SK, Bhatt D, et al., Trends Anal Chem. 2019, 113: p. 280-300.
- Zhang XF, Liu ZG, Shen W, et al. Int J Mol Sci. 2016, 17: p. 1534.
- Mehta D, Singh UK, Sharma AK. Rev Med power house.
- Raghunandan D, Mahesh BD, Basavaraja S, et al. J Nano Res. 2011, 13: p. 2021-2028.
- Elumalai EK, Kayalvizhi K, Silvan S. J Pharm Bioallied Sci. 2014, 6: p. 241.
- Prathna TC, Chandrasekaran N, Raichur AM, et al. Colloids Surf B: Biointerfaces. 2011, 82: p. 152-159.
- Saranraj P, Stella D, Sathiyaseelan K, et al. J Ecobiol. 2010, 2.
- Samadi N, Hosseini SV, Fazeli A, et al. Daru J Pharma Sc. 2010,18:p. 168.
- Martínez-Robles ÁM, Loyola-Rodríguez JP, Zavala-Alonso NV, et al. J Nanomater. 2016, 6: p.136.
- Maqsood M, Qureshi R, Arshad M, et al. Pak J Bot. 2017, 49: p. 353-359.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref