Research Article - Der Pharma Chemica ( 2018) Volume 0, Issue 0
Green Approach to Corrosion Inhibition of Mild Steel in Acid Medium by Aqueous Extract of Pedalium murex L. Leaves
Riaz Ahamed K1, Mushira Banu A1*, Jameel1, Joany RM2 and Susai Rajendran3,4
1Department of Chemistry, PG and Research, Jamal Mohamed College (Autonomous), Tiruchirappalli, India
2Sathyabama University, Chennai, India
3Department of Chemistry, St. Antony’s College of Arts and Sciences for Women, Dindigul-624 005, Tamil Nadu, India
4AMET University, 135, East Coast Road, Kanathur-603 112, Chennai, India
- *Corresponding Author:
- Mushira Banu A
Department of Chemistry
PG and Research, Jamal Mohamed College (Autonomous)
Tiruchirappalli, India
Abstract
The corrosion inhibition of mild steel in 0.5 M H2SO4 by the Pedalium murex leaves extract has been studied using weight loss methods, potentiodynamic polarization and electrochemical impedance spectroscopy techniques. The results show that the inhibition efficiency increases with the increase of the extract concentration. The adsorption of the extract molecules on the steel surface obeys Langmuir adsorption isotherm and occurs spontaneously. The activation energy as well as other thermodynamic parameters for the inhibition process were calculated. These thermodynamic parameters show strong interaction between inhibitor and mild steel surface.
Keywords
Pedalium murex L. extract, Inhibitor, Weight loss, Polarization, Impedance study, Adsorption isotherm
Introduction
Corrosion of metals is a spontaneous natural process. To control corrosion rate in cooling water systems many inhibitors are used. To avoid environmental toxicity, extract of natural products are used. For this many natural products are used by many researchers. The use of plant extracts as corrosion inhibitors has gained prominence as replacement for synthetic organic compounds. The plant natural products have been found to be effective, cheap and eco-friendly anticorrosion agents. Corrosion inhibitions of essential oils of Alpinia galanga were investigated on mild steel in hydrochloric acid solution using weight loss method by Ajeigbe et al., [1]. Sanaei et al. have used an effective green corrosion inhibitive hybrid pigment based on zinc acetate-Cichorium intybus L leaves extract to control corrosion of mild steel in aqueous chloride solutions [2]. A comparative study on the inhibitory action of some green inhibitors on the corrosion of mild steel in hydrochloric acid medium has been reported by Shyamala and Arulanantham [3]. The use of morinda citrifolia as a green corrosion inhibitor for low carbon steel in 3.5% NaCl solution has been by Kusumastuti, et al. [4]. Evaluation of Thymus vulgaris plant extract as an eco-friendly corrosion inhibitor for stainless steel 304 in acidic solution by means of electrochemical studies has been done by Ehsani et al. [5]. They have used density functional theory also, Rajendran et al. have used extracts of various plant materials such as henna leaves, curcumin, caffeine, spirulina to control of corrosion of metals [6-31].
The present work is undertaken to evaluate the inhibition efficiency of an aqueous extract of Pedalium murex leaves in controlling corrosion of mild steel in sulphuric acid medium. Weight loss method and electrochemical methods such as polarization study and AC impedance spectra have been used.
Materials and Methods
Extract of P. murex leaves is used as corrosion inhibitor in the present study. The leaves and flowers of P. murex are shown in Figure 1.
The botanical details of P. murex are given below: Botanical name: Pedalium murex; Tamil name: Yaanai nerunjil; Kingdom: Plantae; Division: Mangnoliophyta; Class: Mangnoliopsida; Order: Lamiales; Family: Pedaliaceae; Genus: Pedalium; Species: P. murex Linns; Traditional uses: Plant pacifies vitiated vata, kidney stone, urinary retention, inflammation, Flatulence and fever. Phytochemical screening of P. murex leaves extract is given in Table 1 [32].
| Phytochemical Constituents | Petroleum ether | Chloroform | Acetone | Methanol |
|---|---|---|---|---|
| Flavonoids | - | - | - | +++ |
| Alkaloids | - | - | - | + |
| Glycosides | - | ++ | +++ | +++ |
| Steroids | - | - | - | +++ |
| Phenols | - | - | ++ | +++ |
| Terphenoids | - | - | - | +++ |
| Saponins | - | + | + | +++ |
| Resins | - | - | - | - |
| Tannins (FeCl3 test) | - | - | ++ | +++ |
| Lead | ||||
| acetate test | - | - | - | +++ |
| Cardiac glycosides | - | - | - | - |
- = Absent; + = Present; ++ = Moderately Present; +++ = Appreciable amount
Table 1: Phytochemical screening of Pedalium murex leaves extract
From the preliminary phytochemical screening may revealed the presence of saponins, steroids, phenols, flavonoids, alkaloids, tannins and glycosides in aqueous extract also.
Preparation of extract
An aqueous extract of P. murex was prepared by grinding 10 g of P. murex boiled with distilled water, filtering the suspending impurities, and making up to 100 ml. The extract was used as corrosion inhibitor in the present study.
Materials and chemicals used
For polarization cylindrical mild steel rod embedded in Teflon with an exposed area of 1 cm2 was used. The electrodes were polished with emery papers of 0/0, 2/0, 3/0, and 4/0 grades and degreased with acetone, dried and used.
Weight loss method
Weight loss measurements were performed in 0.5 M H2SO4 with and without the presence of inhibitor of various concentrations. Inhibition efficiency was calculated using the formula
Inhibition efficiency (%) = W0-Wi/W0 × 100
Where, W0=Weight loss in blank, Wi=Weight loss in presence of inhibitor
Electrochemical studies
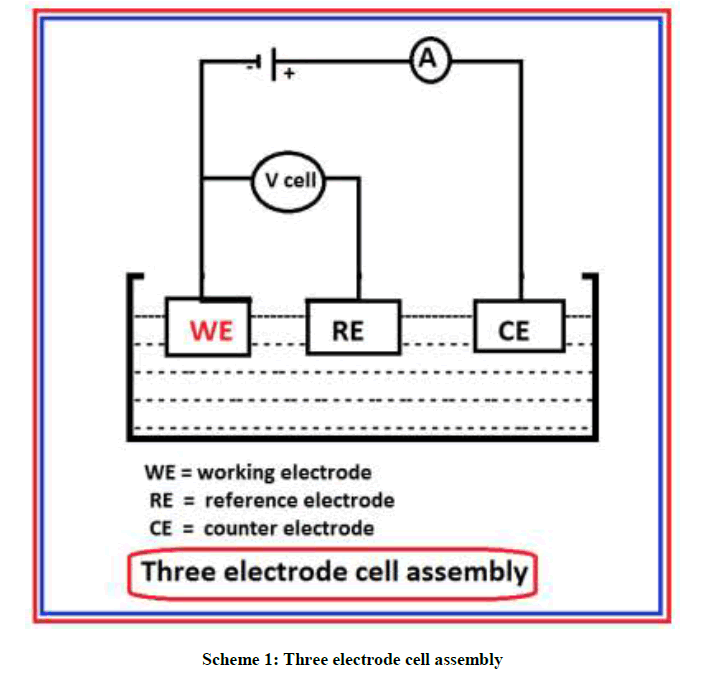
Polarization study and AC impedance spectra were recorded in a potentiostat. The experiments were recorded in a three electrode cell assembly. It is shown in Scheme 1.
Mild steel was used as working electrode. SCE was used as reference electrode. A platinum foil was used as counter electrode. The working electrode used was a mild steel rod of the same composition embedded in araldite and with an exposed area of 1 cm2.
From potentiodynamic polarization study corrosion current, corrosion potential and the Tafel slopes were derived. From AC impedance spectra, charge transfer resistance and double layer capacitance were calculated. The area of the counter electrode is much larger compared to the area of the working electrode. This will exert a uniform potential field on the working electrode. A solution quantity of 100 ml of the test solution was taken in a polarization cell. The working electrode was polished with 0/0, 1/0, 2/0, 3/0, and 4/0 emery papers successively and degreased with acetone. The working electrode, reference and auxiliary platinum electrodes were assembled and connections were made. Stirring was provided to the test solutions to avoid for the system the concentrations polarization before start of experiment. A time interval of about 15 minutes was given for the system to attain state and open circuit potential was recorded.
Results and Discussion
Weight loss study
The inhibition efficiency of leaves extract in controlling corrosion of mild steel in 0.5 M H2SO4 has been evaluated by weight loss method. Table 2 gives the inhibition efficiency of different concentrations of P. murex leaves extract in controlling corrosion of mild steel in 0.5 M H2SO4. It is observed that as the concentration of inhibitor increases, inhibition efficiency also increases. 500 ppm of inhibitor offers 83.54% inhibition efficiency.
| Inhibitor concentration (ppm) | Rate of corrosion g cm-2 hr-1 |
Inhibition efficiency (%) |
|---|---|---|
| Blank | - | - |
| 100 | 0.00163 | 53.33 |
| 200 | 0.00123 | 64.76 |
| 300 | 0.001 | 71.42 |
| 400 | 0.0006 | 81.9 |
| 500 | 0.00173 | 83.54 |
| 600 | 0.0017 | 83.86 |
| 700 | 0.00036 | 89.52 |
| 800 | 0.00033 | 90.47 |
Table 2: Inhibition effect on corrosion of mild steel in 0.5 M H2SO4 by Pedalium murex leaves extract
Adsorption isotherm
Adsorption isotherms are very important to determine the mechanism of organo-electrochemical reaction. It is important to know the mode of adsorption to understand this part of the study. Tables 3 and 4 give the various adsorption parameters.
| Inhibitor Concentration (ppm) | ϴ/(1-ϴ) | 3+logϴ/(1-ϴ) | 3+log C |
|---|---|---|---|
| Blank | - | - | - |
| 100 | 1.1427 | 3.0579 | 5.000 |
| 200 | 1.8376 | 3.2642 | 5.301 |
| 300 | 2.4989 | 3.3977 | 5.4771 |
| 400 | 4.5248 | 3.6555 | 5.602 |
| 500 | 5.0753 | 3.7054 | 5.6989 |
| 600 | 5.1957 | 3.7156 | 5.7781 |
| 700 | 8.5152 | 3.9301 | 5.8450 |
| 800 | 8.4612 | 3.9274 | 5.9030 |
Table 3: Adsorption parameters obtained when mild steel is immersed in 0.5 M H2SO4 in presence of Pedalium murex leaves extract
| System | Langmuir isotherm | |||
|---|---|---|---|---|
| Kads x 10-3 M-1 | Slope | R2 | ΔG0ads KJ mol-1 | |
| Pedalium murex leaves extract | 0.010391 | 0.9292 | 0.9603 | -1386.8667 |
Table 4: Adsorption coefficients deduced from Langmuir isotherm for Pedalium murex leaves extract
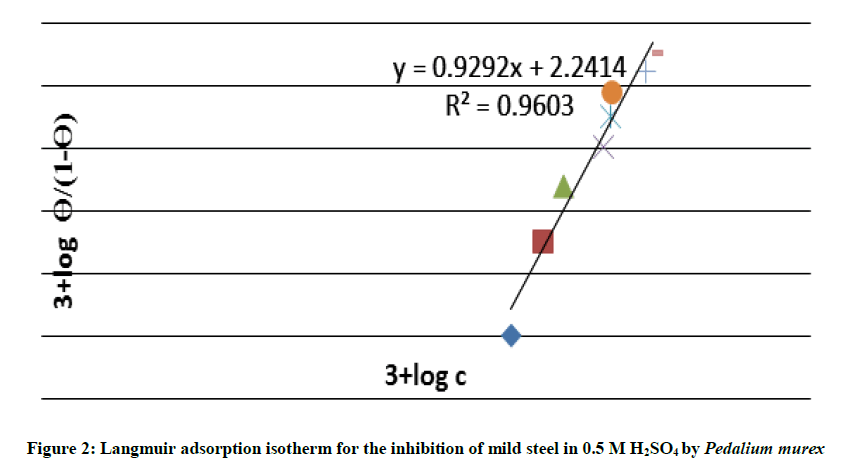
The obtained values were fitted to Langmuir isotherm and the best fit to the experimental data was obtained. Langmuir adsorption isotherm is
Ө=Kads.C/1+ Kads.C
Ө(1+kads. C)=Kads.C
Ө=Kads.C-Kads.CӨ
Ө=(1-Ө) Kads.C
Ө/1-Ө=Kads.C
The plot of log (Ө/1-Ө) vs. log C is a straight line as shown in Figure 2. Thus the Langmuir isotherm is valid for the inhibitor adsorption on the metal surface.
Free energy change
The free energy change adsorption of adsorption (ΔG0ads) of inhibitor on mild steel surfaces related to the constant of the adsorption according to equation.
From the results, values of ΔG0ads were found to be negative and were below the threshold value of -40 kJ/mol indicates the spontaneous adsorption of the ingredients of P. murex on the surface of mild steel and the mechanism of physical adsorption is applicable.
Potentiodynamic polarization studies
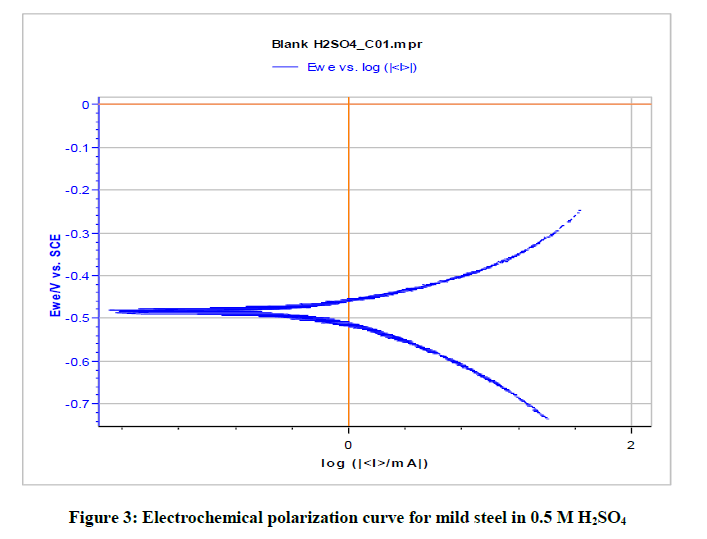
Polarization study has been used to investigate the formation of protective film during the process of corrosion inhibition study [33-37]. When there is corrosion inhibition, corrosion current value decreases.
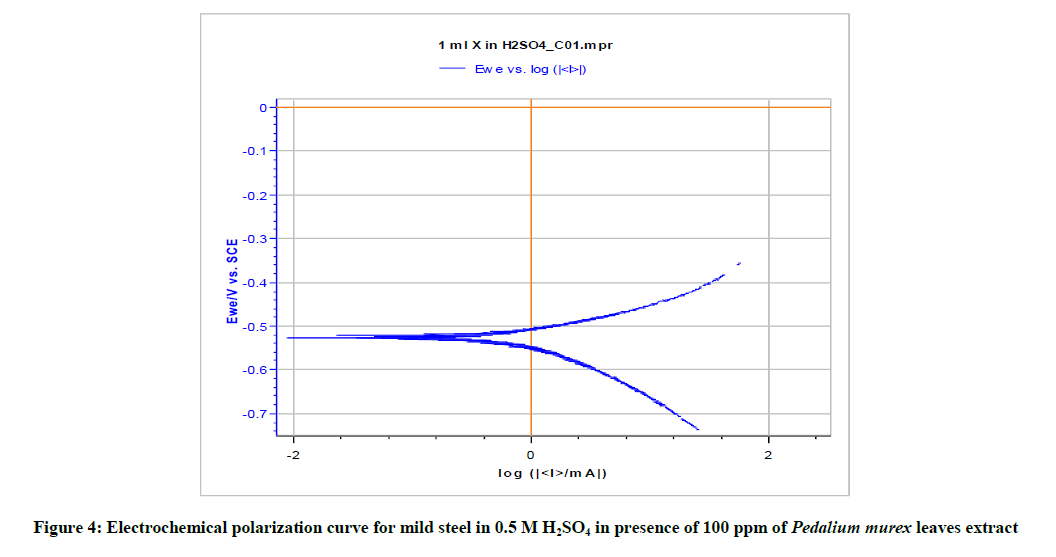
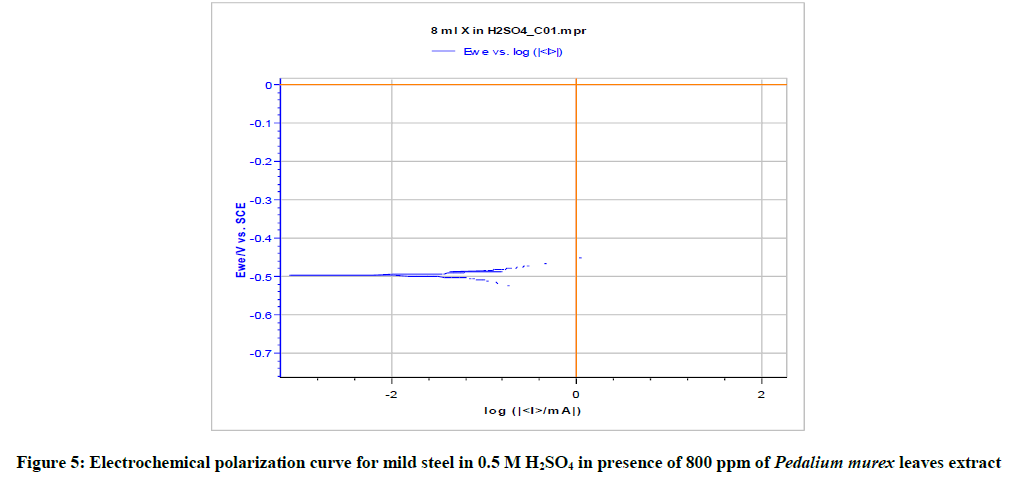
Table 5 and Figures 3-5 give the values of potentiodynamic parameter such as corrosion current (Icorr), corrosion potential (Ecorr) and the cathodic Tafel slopes (bc and ba) for the different concentrations of the green inhibitor under study. It can be seen that there is absorption of the inhibitor molecules on metal surface which is revealed by the decrease in corrosion current in presence of inhibitor.
| System | Inhibitor, ppm | Icorr, μA | Ecorr, mV vs SCE | ba, mV dec-1 | bc, mVdec-1 | Inhibition Efficiency (%) |
|---|---|---|---|---|---|---|
| Blank | - | 1563.682 | -510.743 | 164.6 | 167.9 | - |
| Pedalium murex leaves extract | 100 | 762.219 | -545.51 | 123.9 | 165.1 | 51.25 |
| 800 | 195.168 | -509.879 | 74.70 | 163.7 | 87.51 |
Table 5: Corrosion parameters obtained from polarization curves for mild steel in 0.5 M H2SO4 in the presence and absence of inhibitor
AC impedance spectra
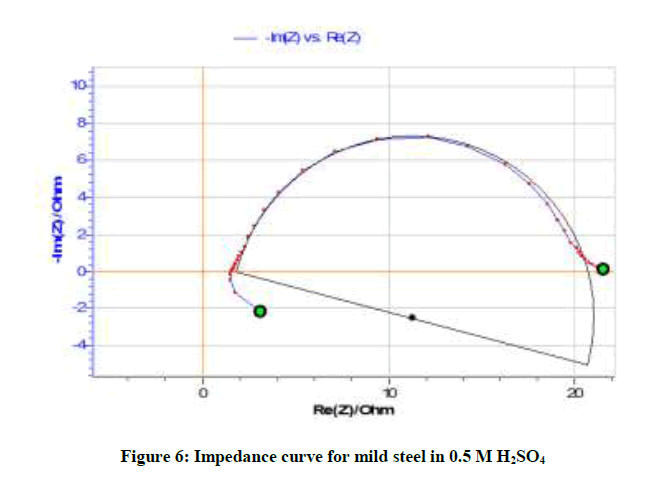
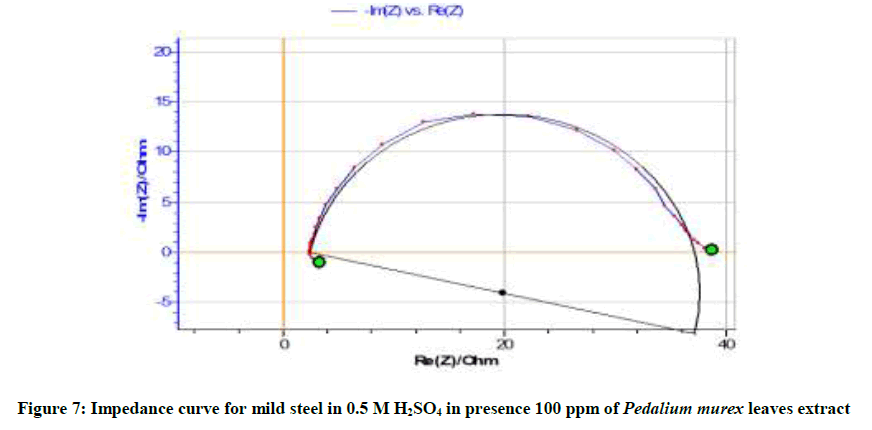
AC Impedance spectra have been used to investigate the formation of protective film on the metal surface during corrosion inhibition process. When there is corrosion inhibition, charge transfer resistance value increases and double layer capacitance decreases [38-43].
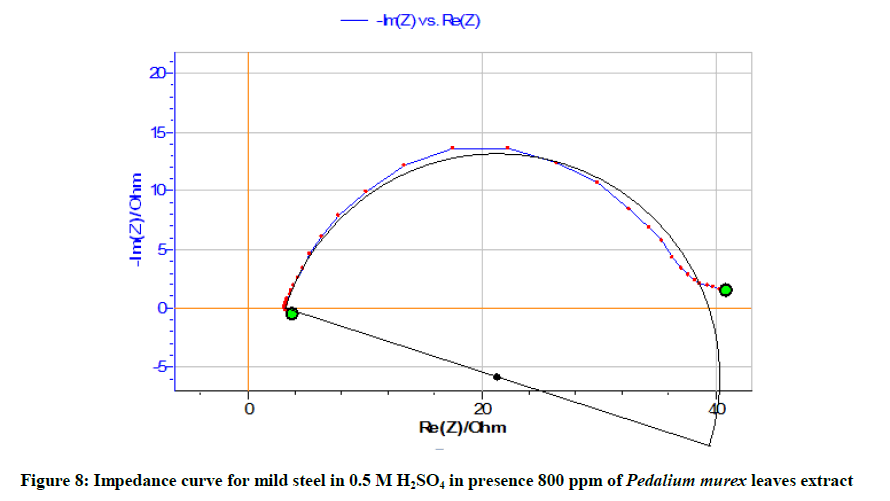
Table 6 and Figures 6-8 show the AC impedance curves of mild steel immersed in acid medium in presence of different inhibitor concentrations. The charge transfer resistance (Rt) value increases and double layer capacitance (Cdl) value decreases in presence of inhibitor concentrations. This implies the formation of protective film on the metal surface. It can be also seen from Table 7 that there is a close agreement between the values of inhibition efficiencies obtained from weight loss measurements, polarization and impedance studies.
| EA (ppm) | Rt Ohm, cm2 | Cdl, F/cm2 | Inhibition efficiency (%) |
|---|---|---|---|
| Blank | 18.98 | 0.2251 | - |
| 100 | 34.93 | 0.1010 | 55.13 |
| 800 | 36.35 | 0.0240 | 89.33 |
Table 6: Corrosion parameters obtained from impedance study for mild steel in 0.5 M H2SO4
| [Inhibitor], (ppm) | Inhibition efficiency (%) | ||
|---|---|---|---|
| Weight loss studies | Polarization studies | Impedance studies | |
| 100 | 53.33 | 51.25 | 55.13 |
| 800 | 90.47 | 87.51 | 89.33 |
Table 7: Comparison of inhibition efficiency measured by Weight loss, Polarization and Impedance method
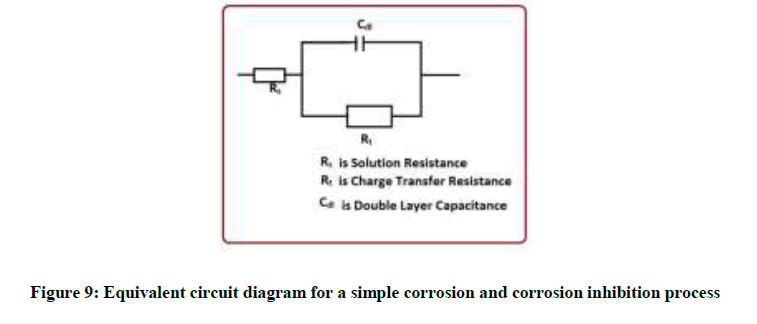
It is inferred from the Nyquisit plots that the process represent simple corrosion and corrosion resistance one. Semi circles are obtained. The equivalent circuit diagram for such a system is shown in Figure 9.
Conclusion
The inhibitive influence of P. murex L. extract on the corrosion of mild steel in 0.5 M H2SO4 was studied by weight loss method, polarization measurements and AC impedance spectra. The inhibition efficiency values determined by these techniques showed close agreement. The corrosion rate decreased with addition of P. murex L. extract. This is probably due to the progressing adsorption of the inhibitor molecules present in the extract on the metal surface. The maximum inhibition efficiency was found to be 90.47%.
1. The corrosion of mild steel in 0.5M H2SO4 solution is inhibited by the addition of P. murex L. extract.
2. The percentage of inhibition efficiency increases with increases of the inhibitor concentration.
3. The corrosion inhibition P. murex L. extract is attributed to the adsorption of the any of the components on to the mild steel surface. The adsorption is assumed to arise from the π-bond of the components on the mild steel surface.
4. The values obtained from the weight loss technique for the studied inhibitor fit into the Langmuir adsorption isotherm and the kinetic thermodynamic model. The values free energy for the adsorption process indicates that both physisorption and chemisorptions (comprehensive adsorption) of the P. murex L.extract studied on mild steel surface.
5. Polarization studies reveal that this formulation controls the cathodic reaction predominantly.
6. AC impedance studies reveal that a protective film is formed on the metal surface. These results suggested P. murex L. leaves extract is a best eco-friendly inhibitor.
Acknowledgement
The authors are thankful to their managements for their help and encouragement.
References
- S.O. Ajeigbe, N. Basar, M.A. Hassan, M. Aziz, ARPN J Engineering and Applied Sci., 2017,12(9), 2763-2771.
- Z. Sanaei, T. Shahrabi, B. Ramezanzadeh, Dyes and Pigments., 2017, 139, 218-232.
- M. Shyamala, A. Arulanantham, Malaysian J Analytical Sci., 2017, 21(2), 346-355.
- R. Kusumastuti, R.I. Pramana, J.W. Soedarsono, AIP Conference Proceedings., 2017, 1823, 020012.
- A. Ehsani, M.G. Mahjani, M. Hosseini, R. Moshrefi, H. Mohammad Shiri, J. Colloid. Interface. Sci., 2017, 490, 444-451.
- Noreen Anthony, E. Malarvizhi, P. Maheshwari, Susai Rajendran, N. Palaniswamy, Indian J.Chem. Tech., 2004, 11, 346-350.
- S. Rajendran, A.J. Amalraj, M.J. Joice, N. Anthony, D.C. Trivedi, M. Sundaravadivelu, Corrosion Reviews., 2004, 22(3), 233-248.
- S. Rajendran, V. Ganga Sri, J. Arockiaselvi, A. J. Amalraj, Bulletin of Electrochemistry., 2005, 21(8), 367-377.
- S. Rajendran, S. Muthulakshmi, R. Rajeswari, A. Vijitha, J. Electrochem. Soc., 2005, 54(2), 50-52.
- S. L. Priya, A. Chitra, S. Rajendran, K. Anuradha, Surface Engineering, 2005, 21(3), 229-231.
- S. Rajendran, S. Shanmugapriya, T. Rajalakshmi, A. J. Corrosion., 2005, 61(7), 685-692.
- K. Anuradha, R. vimala, B. Narayanasamy, J. Arockiaselvi, S. Rajendran, Chem. Engg. Commun., 2008, 195(3), 352-366.
- J. Arockia Selvi, V. Gangasree, S. Rajendran, Portugaliae Electrochimica Acta., 2008, 27(1), 1-11.
- S. Rajendran, J. Jeyasundari, J. Arockia Selvi, B. Narayanasamy, A.P.P. Regis, P. Rengan, Portugaliae Electrochimica. Acta., 2009, 27(2), 153-164.
- S. Rajendran, J. Paulraj, P. Rengan, J. Jeyasunbdari, M. Manivannan, J. Dent. Oral. Hyg., 2009, 1(1), 1-8.
- S. Rajendran, M. Manivannan, Zastita Materijala., 2009, 50, 131-140.
- S. Rajendran, M. Agasta, R. Bama Devi, B. nShyamala Devi, K. Rajam, J. Jayasundari, Zastita Materijala., 2009, 50(2), 77-84.
- S. Rajendran, P. Sumithira, B. Shyamala Devi, J. Jeyasundari, Zastita Materijala., 2009, 50, 223-226.
- S. Rajendran, V. Uma, A. Krishnaveni, J. Jeyasundari, B. Shyamaladevi, M. Manivannan, Arabian J Sci and Engineering., 2009, 34(2c), 47-158.
- Noreen Antony, H. Benita Sherina, S. Rajendran, Int. J. Engg. Sci. Technol., 2010, 2(7), 2774-2782.
- S. Rajendran, P. Sumithra, B. Shyamala Devi, J. Jeyasundari, Zastita Materijala., 2009, 5, 223-226.
- M. Sangeetha, S. Rajendran, T.S. Muthu Megala, K. Krishnaveni, Zastitam Materijala., 2011, 52, 35-19.
- B. Syamala Devi, S. Rajendran, Int.J. Chem. Sci. Technol., 2011, 1(1),79-87.
- V. Sirbharathy, Susai Rajendran, J. Sathyabama, Int. J. Chem. Sci. Technol., 2011, 1(3), 108-115.
- M. Sangeetha, S. Rajendran, J. Sathiya Bama, A. Krishnaveni, P. Santhy, N. Manimaran, B. Shyamaladevi, Portugaliae Electrochimica Acta., 2011, 29(6), 429-444.
- M. Sangeetha, J. Sathyabama, S. Rajendran, P. Prabhakar, Nat. Prod. Plant. Resour., 2012, 2(5),601-610.
- J. Felicita Florence, Susai Rajendran, K.V. Srinivasan, Electroplating & finising., 2012, 31(7),1-4.
- V. Sirbharathy, S. Rajendran, Chem. Sci. Rev. Lett., 2012, 1(1), 25-29.
- K. Rajam, S. Rajendran, N. Nazeera Banu, J. Chem., 2013, 2013(2013), 11.
- V. Johnsirani, J. Sathiyabama, Susai Rajendran, A. Suriya Prabha, ISRN Corrosion., 2012, 2012, 7.
- V. Sribharathy, Susai Rajendran, ISRN Corrosion., 2013, 2012, 7.
- M. Sermakkani, V. Thangapandian, 2010, 2(1), 110-114.
- S. Shanmugapriya, S. Rajendran, P. Prabakar, N. Karthika, Int. J. Nano Corr. Sci. Engg., 2016, 3(4), 144-156.
- S. Anusuya, S. Devi Meenakshi, Int. J. Nano Corr. Sci. Engg., 2016, 3(4), 204-215.
- M. Abinaya, S. Devi meenakshi, Int. J. Nano Corr. Sci. Engg., 2016, 3(4), 216-222.
- S. Rajendran, A. Krishnaveni, G. Muthukumar, Int. J. Nano Corr. Sci. Engg., 2016, 3(4), 254-266.
- J. AngelinThangakani, S. Rajendran, J. Sathiabama, Int. J. Nano Corr. Sci. and Engg., 2016, 3(4),280-301.
- M. Lavanyaa, M. Suganya, Int. J. Nano Corr. Sci. Engg., 2016, 3(4), 88-103.
- A. Josephine Vanitha, A. Sahaya Raja, Susai Rajendran, Int. J. Nano Corr. Sci. Engg.,2016, 3(4), 223-245.
- A. Anandan, Susai Rajendran, J. Sathiyabama, D. Sathiyaraj, Int. J. Nano Corr. Sci. Engg.,2016, 3(4), 9-27.
- K. Nithya, S. Devi Meenakshi, Int. J. Nano Corr. Sci. Engg., 2016, 3(4), 44-55.
- P. Nithya Devi, J. Sathiyabama, S. Rajendran, R. Joseph Rathis, S. Santhana Prabha, Int. J. Nano Corr. Sci. Engg., 2016, 3(4), 56-79.
- S. Madhumitha, V. Priyadharshini, A. Sheela, C. Aadhithya, M. Sangeetha, S. Rajendran, Int. J. Nano Corr. Sci. Engg., 2016, 3(4), 80-87.