Research Article - Der Pharma Chemica ( 2018) Volume 10, Issue 8
Green Synthesis, Structural Characterization and Pharmacological Evaluation for Analgesic and Anti-Inflammatory Activities of Salicylic Acid Based Triazolothiadiazole Derivatives
Parminder Kaur1* and Anshul Chawla2
1University Institute of Pharmaceutical Sciences, Chandigarh University, Gharuan, Mohali, Punjab, India
2CT Institute of Pharmaceutical Sciences, CT group of institutions, Shahpur, Punjab, India
- *Corresponding Author:
- Parminder Kaur
University Institute of Pharmaceutical Sciences
Chandigarh University
Gharuan, Mohali, Punjab, India
Abstract
Drug discovery and development process aims to make available medications that are safe and effective in improving the length and quality of life and relieving suffering. It comprises of lead discovery and lead optimization. Lead investigational compounds that survive initially are optimized or altered to make them more effective and safer by giving it different properties. In the present work, the carboxylic group of salicylic acid has been derivatized in order to improve its safety profile while maintaining anti-inflammatory and analgesic activity as their most common side effects are the occurrence of gastrointestinal damage with gastric upset and irritation, ulceration, bleeding and renal toxicity. Gastrointestinal damage is attributed to two factors-local irritation by carboxylic acid moiety and decreased cytoprotective PG production. In normal practice, chemotherapeutic, analgesic and anti-inflammatory are prescribed simultaneously which enhances the risk for developing NSAIDs related complications. It is known that bacterial infections often produce pain and inflammation. Hence there is a pressing need of the drugs having both anti- microbial and analgesic, anti- inflammatory activities with minimum side effects. 1,2,4-triazoles and 1,3,4-thiadiazoles both possess wide spectrum of therapeutic activities like antibacterial, antifungal, antiviral, analgesic, anti-inflammatory, anticonvulsant, antidepressant, antihypertensive, and hypoglycemic and diuretic properties. In view of aforementioned reports, Salicylic acid based triazolothiadiazole derivatives were synthesized through Microwave Assisted Organic Synthesis (MAOS) which is considered as an important approach towards green chemistry, because this technique is more environment-friendly and it accelerates the rate of reaction, increase the yield and quality of the product.
Keywords
1,2,4-triazole, 1,3,4-thiadiazole, Microwave synthesis, Analgesic, Anti-inflammatory activities.
Introduction
NSAIDS available in market cause serious side effects such as a range of gastrointestinal (GI) problems. When NSAIDs are used for pain management after surgery they cause increased risk of kidney problems. An estimated 10%-20% of NSAID patients experience dyspepsia. In the 1990s high doses of prescription NSAIDs were associated with serious upper gastrointestinal adverse events, including bleeding. Over the past decade, deaths associated with gastric bleeding have declined the use NSAIDs, like all drugs, mayinteract with other medications [1]. The chance of hospitalization or death from a gastrointestinal adverse event is 1.3% to 1.6% per year in patients with rheumatoid arthritis. Endoscopic studies indicate that 20%-30% of regular NSAID users develop ulcers [2]. Inflammation is a critical health problem and need attention. Thus, researchers have to paid attention to find out newer drug molecule with minimum side effects, with lower dosing frequency and with better patient compliance for inflammation.
One of the most important concepts of drug design is the covalent conjugation of biologically active moieties, acting by different mechanisms that would lead a compound with improved activity and reduced toxicity. Based on this concept and taking in consideration the pharmacological activities shown by various 1,2,4-triazole (1) and 1,3,4-thiadiazole derivatives (2), the aim of our project is to combine the structural features of salicylic acid and fused triazolothiadiazole derivatives (3) through “Microwave synthesis”. The demand for more efficient and more environmentally friendly method of synthesis for heterocyclic compounds compels the use of microwave irradiation. As, in traditional synthetic strategy longer reaction time period, expensive solvent types and lower yield are the basic problems.
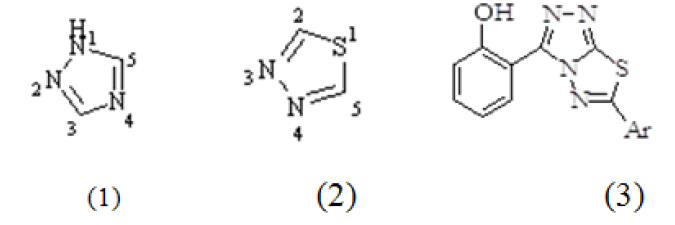
Materials and Methods
The structure of the synthesized compounds has been determined by using spectral analysis like Mass, 1H and 13C-NMR, IR spectroscopy and elemental analysis. The series of these novel compounds then evaluated for their in vivo pharmacological activities like anti-inflammatory and analgesic activities.
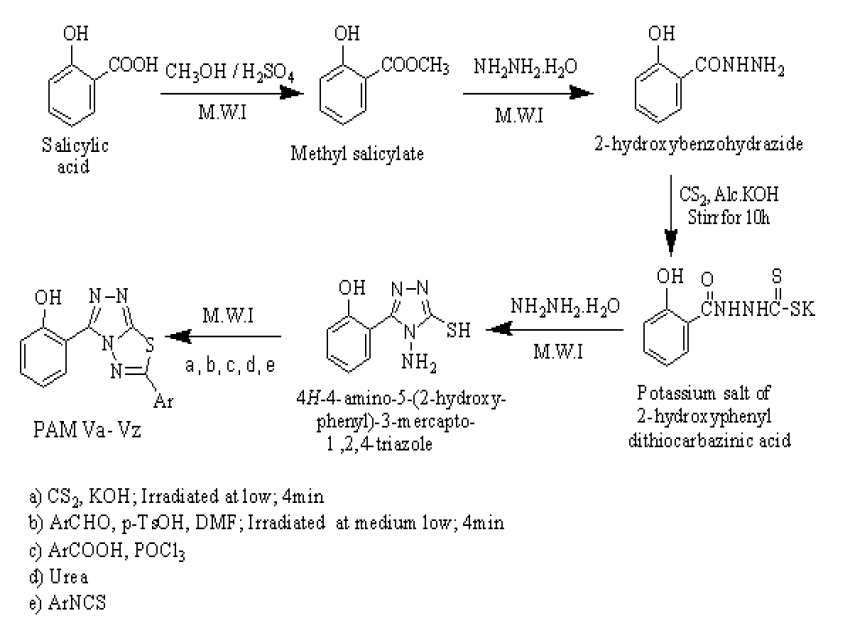
Initially, salicylic acid was esterified to methyl salicylate (PAM I) by treating it with dry methanol in the presence of concentrated sulphuric acid in the microwave. The 4-amino-3-substitutedaryl-5-mercapto-[1,2,4]-triazole was synthesized by the conversion of substituted aryl ester (PAM I) into hydrazide (PAM II) by using hydrazine hydrate and the subsequent reactivity of the hydrazide with carbon disulphide in presence of methanolic KOH to obtain the potassium salts of thiocarbohydrazide (PAM III), followed by addition of the hydrazine hydrate gives the cyclized compound (PAM IV). The titled compounds (PAM Va to PAM Vz) were synthesized by using simple, efficient and one pot condensation of PAM IV with different benzaldehyde, benzoic acid, phenyl isothiocyanate derivatives and with urea and acetyl chloride in the microwave.
| Code | Ar |
|---|---|
| PAM-Va | SH |
| PAM-Vb | CH3 |
| PAM-Vc | C6H5 |
| PAM-Vd | 2-Cl-C6H4 |
| PAM-Ve | 3-Cl-C6H4 |
| PAM-Vf | 4-Cl-C6H4 |
| PAM-Vg | 4-OH-C6H4 |
| PAM-Vh | 4-F-C6H4 |
| PAM-Vi | 4-OCH3-C6H4 |
| PAM-Vj | 3,4,5-triOCH3-C6H2 |
| PAM-Vk | 4-N,N- (CH3)2-C6H4 |
| PAM-Vl | 2,5-diOH-C6H3 |
| PAM-Vm | 3-NH2-C6H4 |
| PAM-Vn | 4-NH2-C6H4 |
| PAM-Vo | 3,5-diNH2-C6H3 |
| PAM-Vp | 2,4-diOH-C6H3 |
| PAM-Vq | 4-NO2-C6H4 |
| PAM-Vr | 2-Br-C6H4 |
| PAM-Vs | 4-Br-C6H4 |
| PAM-Vt | C5H4N |
| PAM-Vu | 2-NH2-C5H3N |
| PAM-Vv | O |
| PAM-Vw | NH-C6H5 |
| PAM-Vx | NH-C6H5 (4-Cl) |
| PAM-Vy | NH-C6H5 (2-OCH3) |
| PAM-Vz | NH-C6H5 (4-CH3) |
Synthesis of methyl salicylate (PAM I)
The salicylic acid (2 mmol, 1.04 g) was taken in an Erlenmayer flask (100 ml) and dry methanol (20 ml) was added followed by the addition of the catalyst, concentrated H2SO4 (4 drops). The flask was subjected to microwave irradiation at 300 W for 3 min, stirred after every minute and the completion of the reaction was monitored continuously by Thin Layer Chromatography (TLC). The contents were transferred into aqueous sodium bicarbonate solution. The organic product was extracted with ether (50 ml) and the ethereal layer was dried over anhydrous sodium sulfate. Then, the solvent was evaporated to get the product.
Synthesis of 2-hydroxybenzohydrazide (PAM II)
A mixture of methyl salicylate (10 mmo, l.3 ml) and hydrazine hydrate 80% (20 mmol, 1.2 ml) were thoroughly mixed to form a thick paste. The paste was air-dried and the reaction mixture was covered with funnel and subjected to microwave irradiation at 300 W for 3.5 min while stirring every 1 min. The progress of the reaction was monitored by TLC (Ethyl acetate: hexane; 5: 3). The solid that formed was washed with water, and purified by recrystallization from ethanol.
Synthesis of Potassium salt of dithiocarbazinic acid (PAM III)
2-hydroxybenzohydrazide (0.01 mol, 1.5 g) was added to absolute alcohol (15 ml) containing KOH (1.6 g) at ambient temperature. Carbon disulphide (0.015 mol, 1.14 ml) was added and the mixture was stirred at ambient temperature for 10 h. Then, the mixture was diluted with ether (10 ml) and stirred for further 1 h. The potassium salt separated out was filtered and washed with ether (5 ml). The potassium salt was used for the next stage without further purification.
Synthesis of 5-aryl-4-amino-3-mercapto-1,2,4-triazole (PAM IV)
Hydrazine hydrate (99%) (0.02 mol, 1.00 ml) was gradually added to the above potassium salt (0.01 mol, 2.49 g) dissolved in water (12 ml) with stirring and the mixture was irradiated in microwave at 300 W for 3 min during which hydrogen sulphide evolved and the colour of the reaction mixture changed to dark green colour. It was then cooled and acidified with conc. HCl to pH 1.00. A yellow solid separated out was filtered, washed with water and recrystallized from ethanol to obtain pure triazole.
Synthesis of mercapto substituted triazolothia diazole phenol (PAM Va)
A solution of KOH (0.004 mol, 0.44 g) in water (10 ml) was added drop-wise to a solution of substituted mercaptotriazole (0.002 mol, 0.82 g) and CS2 (0.004 mol, 0.74 ml) in ethanol (10 ml) in a microwave vial. The mixture was irradiated in microwave at medium low for 2.5 min while stirring every 1 min. The progress of the reaction was monitored by TLC. After the mixture was cooled to room temperature, the solution was poured into cold water (50 ml) and acidified to pH 1 with 37% HCl. The precipitated solid was filtered off and recrystallized from ethanol.
Synthesis of methyl substituted triazolothiadiazole phenol (PAM Vb)
To a solution of mercaptotriazole (0.01 mol, 1.04) in dry pyridine (25 ml), acetyl chloride (0.01 mol, 0.34 ml) added drop-wise and stirred at room temperature for 30 min. The reaction mixture, then, irradiated in microwave at low (136 W) for 2 min and poured into crushed ice. The solid product filtered and purified by recrystallization from ethanol.
Synthesis of 2-(6-substitutedaryl-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazol-3-yl)phenol (PAM Vc- PAM Vl)
A mixture of mercaptotriazole (0.01 mol, 1.04 g), substituted aromatic aldehyde (0.01 mol), p-TsOH (50 mg) and DMF (10 ml) taken in a round bottom flask (100 ml) was mixed properly with the help of a glass rod. The mixture then irradiated in microwave at 300 W for 3-4 min (monitored by TLC). After cooling to room temperature, poured into crushed ice and reaction mixture stirred thoroughly. The precipitates were filtered and washed with water, dried and recrystallized from ethanol.
Synthesis of 2-(6-substitutedaryl-[1,2,4]triazolo [3,4-b][1,3,4]thiadiazol-3-yl)phenol (PAM Vm- PAM Vu)
A mixture of mercaptotriazole (0.01 mol, 1.04 g), substituted aromatic acids (0.01 mol) and phosphorus oxychloride (10 ml) was irradiated in microwave at 160 W for 2-2.5 min. Excess of phosphorus oxychloride was removed under reduced pressure. The resulting reaction mass was cooled and poured into cold water with vigorous stirring. The solid thus obtained was filtered, washed with dilute sodium bicarbonate solution followed by water, dried and recrystallized from ethanol.
Synthesis of 3-(2-hydroxyphenyl)-[1,2,4]triazolo [3,4-b][1,3,4]thiadiazol-6(5H)-one (PAM Vv)
A mixture of mercaptotriazole (0.1 mol, 1.04 g) and urea (0.13 mol, 0.39 g) was irradiated in microwave at 180°C-190°C for 3 min. The reaction mixture was cooled and added to a solution of sodium hydroxide (5%, 20 ml), then filtered and the filterate acidified with dilute HCl. The solid product was purified by recrystallization from ethanol.
Synthesis of 2-(6-(substitutedphenylamino)-[1,2,4]triazolo[3,4-b][1,3,4]thiadia-zol-3-yl) phenol (PAM Vw-PAM Vz)
An equimolar mixture (0.01 mol) of mercaptotriazole (0.01 mol, 1.04 g) and substituted aryl isothiocyanate (0.01 mol) was taken in DMF (15 ml) containing powdered NaOH (0.4 g) and irradiated in microwave at 300 W for 1-2 min while stirring after every minute until the complete evolution of H2S. The completion of the reaction was monitored by TLC. The reaction mixture was poured onto ice cold water. The obtained solid was filtered, washed with water, dried and recrystallized from ethanol.
Pharmacological evaluation
The synthesized compounds were subjected to anti-inflammatory and analgesic evaluation for possible preliminary pharmacological screening. Wistar rats of either sex weighing 150-200 g were used. The animals were housed in groups of five at room temperature of 25°C ± 2°C under 12 h light/12 h dark cycle with free access to food and water ad libitum. The studies were undertaken with prior approval from the Institutional Animal Ethics Committee (IAEC) numbered as IAEC-CTIPS/2017/VIII/0058(PCH-M) and utmost care was taken to insure that the animals were treated in the most humane and acceptable manner. Animal activity was performed in CT institute’s animal house having registration number 1704/PO/Re/S/13 /CPCSEA
Anti-inflammatory activity (in vivo)
Anti-inflammatory activity was performed by using Carrageenan Induced Rat Hind Paw Edema Method. Synthesized compounds (PAM Vd, PAM Vf, PAM Vi, PAM Vk) were evaluated at 20 mg/kg. Aspirin was used as standard drug.
Carrageenan induced rat hind paw Edema Method
The animals were deprived of food for 24 h before the commencement of the experiment but allowed free access to water. Safety and toxicity dose of drugs for LD50 determined according to OECD guidelines. The animals were divided into six groups and each group consisting of five animals. The one control group received vehicle 0.05 ml of 1.0% w/v tween80 in normal saline. One group served as standard and received aspirin at the dose of 100 mg/kg as suspension in tween80 orally and rest of groups were received test compounds dissolved or suspended in the same volume of vehicle at the dose of 20 mg/kg. After half an hour 0.05 ml of 1% w/v suspension of carrageenan in normal saline was injected subcutaneously to the sub plantar region of right hind paw of each rat. The paw was marked with ink at the level of the lateral malleolus and immersed in mercury up to this mark. The paw volume was measured plethysmographically immediately after injection at an interval of 0 h, 0.5 h, 1 h, 2 h, 3 h and eventually 4 h. The difference between the paw volume at 4th and 0th h was calculated and taken as edema volume. Percentage inhibition in the paw edema was calculated by using the formula,
Percentage inhibition=100 (1-Vt/Vc),
where Vt=mean increase in paw volume of test, and Vc=mean increase in paw volume of control. Percentage inhibition shown by tested compounds was recorded. Various data related to anti-inflammatory activity have been presented [3,4].
Analgesic activity (in vivo)
Analgesic activity was performed by using Tail immersion method method. Synthesized compounds (PAM Vd, PAM Vf, PAM Vi, PAM Vk) were evaluated at 20 mg/kg. Aspirin was used as standard drug. The Wistar rats (150-200 g body weight) were screened for sensitivity by immersing their tails gently in hot water maintained at 55°C-55.5°C. The rats that lifted their tails from the hot water within 5 s were selected for the study. The selected rats were then divided into six groups (n=5). One group of animals received vehicle 0.05 ml of 1.0% w/v tween80 in normal saline orally and served as control. One group served as standard and received Aspirin at the dose of 100 mg/kg as suspension in tween80 orally and rest of groups were received test compounds dissolved or suspended in the same volume of vehicle at the dose of 20 mg/kg. The lower 5 cm portion of the tail was marked. This part of the tail was immersed in a cup of freshly filled water of exactly 55°C. The rats reacted by withdrawing their tails within a few seconds. The reaction time was determined before and periodically after oral administration of the test compounds, e.g., after 0.5, 1, 2, 3 and 4 h. The cut off time of the immersion is 15 s [5,6].
Result and Discussion
The yield of different synthesized compounds was found to be in the range of 45%-85%. Purity of all the compounds was checked by Thin Layer Chromatography (TLC) by using silica gel Gas stationary phase and the suitable solvent system and Rf values were calculated. The melting points of all the synthesized compounds were determined by open capillary tubes and were expressed in °C. Their solubility was also checked (Table 1).
| Code | M.P.ºC | %Yield |
|---|---|---|
| PAM-I | 223 (B. P.) | 85.18 |
| PAM-II | 146-152 | 83.43 |
| PAM-III | 240-244 | 68.54 |
| PAM-IV | 204-206 | 80.28 |
| PAM-Va | 144-148 | 63.26 |
| PAM-Vb | 218-220 | 46.5 |
| PAM-Vc | 148-152 | 75.67 |
| PAM-Vd | 134-136 | 70.72 |
| PAM-Ve | 124-126 | 45.45 |
| PAM-Vf | 146-150 | 81.81 |
| PAM-Vg | 176-180 | 76.66 |
| PAM-Vh | 188-192 | 81.82 |
| PAM-Vi | 168-172 | 80 |
| PAM-Vj | 160-164 | 68.75 |
| PAM-Vk | 194-196 | 60.71 |
| PAM-Vl | 200-202 | 54 |
| PAM-Vm | 162-166 | 50.64 |
| PAM-Vn | 176-180 | 62.98 |
| PAM-Vo | 218-222 | 49.68 |
| PAM-Vp | 174-176 | 79.01 |
| PAM-Vq | 158-162 | 57.98 |
| PAM-Vr | 166-168 | 64.51 |
| PAM-Vs | 182-186 | 76.34 |
| PAM-Vt | 160-164 | 67.34 |
| PAM-Vu | 168-172 | 67.53 |
| PAM-Vv | 160-164 | 88.37 |
| PAM-Vw | 134-136 | 81 |
| PAM-Vx | 138-142 | 62.05 |
| PAM-Vy | 168-172 | 63.12 |
| PAM-Vz | 136-140 | 67.08 |
Table 1: Melting point and percentage yield of synthesized compounds
Compounds PAM Vd and PAM Vf shown potent anti-inflammatory and analgesic activities at the dose of 20 mg/Kg than standard drug Aspirin (100 mg/Kg). Compound PAM Vi showed moderate activity as compared to the standard while compounds PAM Vk showed weak activity. The major findings and SAR studies indicated that the derivatives having electron withdrawing groups like chloro substitution were observed more potent activity.
PAM I
IR (KBr, cm-1): 3188.33(O-H), 2956.24 (CH str.; aro), 2853.61 (CH; CH3), (1679.70 (C=O), 1585.21 and 1485.11 (C=C; aro), 1090 (C-O), 757 (ortho substitution); 1H-NMR (δ, 400 MHz, DMSO-d6): 7.71-6.82 (m, 4H, 4-Ar), 10.68 (s, 1H, -OH), 3.84 (s, 3H, CH3); 13C-NMR (δ, 100 MHz, DMSO-d6): 160.66, 112.21 (2C, Ar), 135.36, 129.59, 118.89, 117.06 (4CH, Ar), 169.64 (1C, C=O), 51.90 (1C, CH3); MS (m/z, M+): 152.48 CHNSO Analysis (%): C (63.15), H (5.30), O (31.55).
PAM II
IR (KBr, cm-1): 3271.36 (O-H), 3318.43 (NH str.), 3055.48 (CH str.), 1485.30 (C=C), 1136.53 (C-N), 1644.39 (C=O); 1H-NMR (δ, 400 MHz, DMSO-d6): 7.82-6.83 (m, 4H, 4-Ar), 8.00 (s, 1H, OH), 2.54 (s, 2H, NH2), 7.98 (s, 1H, NH); 13C-NMR (δ, 100 MHz, DMSO-d6): 159.57, 117.58 (2C, Ar), 127.11, 118.58, 133.31, 114.39 (4CH, Ar), 167.97 (1C, C=O); MS (m/z, M+): 152.50; CHNSO Analysis (%): C (55.26), H (5.30), N (18.41), O (21.03).
PAM III
IR (KBr, cm-1): 3243.49 (O-H), 3415.42 (NH str.), 1485.27 (C=C), 1144.45 (C-N), 1640.44 (C=O), 1947.92 (N=C=S); 1H-NMR (δ, 400 MHz, DMSO-d6): 7.65-6.96 (m, 4H, 4-Ar), 10.40 (s, 1H, -OH), 3.43 (s, 1H, NH), 7.99 (s, 1H, CONH); 13C-NMR (δ, 100 MHz, DMSO-d6): 156.26, 119.41 (2C, Ar), 117.0, 119.41, 133.43, 109.34 (4CH, Ar), 159.79 (1C, C=O), 177.02 (1C, C=S); MS (m/z, M+): 194.46; CHNSO Analysis (%): C (36.07), H (2.65), N (10.52), S (24.07), O (12.01), K (14.68).
PAM IV
IR (KBr, cm-1): 3073.63 (OH), 3350.66 (NH str.), 2593.97 (SH), 1613.59 (C=N), 1574.70 (C=C), 2946.62 (C-H; Aro), 1515.48 (C-N), 1309.61 (C=S), 743.73 (C-S); 1H-NMR (δ, 400 MHz, DMSO-d6): 7.60-6.49 (m, 4H, 4-Ar), 10.40 (s, 1H, -OH), 3.86 (s, 2H, NH2), 1.64 (s, 1H, SH); 13C-NMR (δ, 100 MHz, DMSO-d6): 155.64, 116.44 (2C, Ar), 125.28, 119.70, 131.27, 109.53 (4CH, Ar), 161.06, 179.06 (2C, 1,2,4-triazole); MS (m/z, M+): 208.36; CHNSO Analysis (%): C (46.14), H (3.87), N (26.90), S (15.40), O (7.68).
PAM Va
IR (KBr, cm-1): 3338.45 (OH), 2593.52 (SH), 1615.41 (C=N), 1516.40 (C=C), 3077.50 (C-H; Aro), 1351.42(C=S), 765.58 (C-S); 1H-NMR (δ, 400 MHz, DMSO-d6): 7.63-6.93 (m, 4H, 4-Ar), 7.9 (s, 1H, -OH), 2.87(s, 1H, SH); 13C-NMR (δ, 100 MHz, DMSO-d6): 156.25, 116.99 (2C, Ar), 128.98, 119.38, 133.37, 109.33 (4CH, Ar), 159.80, 162.25, 177.04 (3C, Triazolothiadiazole); MS (m/z, M+): 250.45; CHNSO Analysis (%): C (43.19), H (2.42), N (22.38), S (25.62), O (6.39).
PAM IV
IR (KBr, cm-1): 3256 (OH), 2912 (CH str.), 1610 (C=N), 1621 (C=C), 3172 (C-H; aro), 1530 (C-N), 711(C-S); 1H-NMR (δ, 400 MHz, DMSO-d6): 7.63-6.93 (m, 4H, 4-Ar), 7.9 (s, 1H, -OH), 2.35(s, 3H, CH3); 13C-NMR (δ, 100 MHz, DMSO-d6): 155.34, 118.52 (2C, Ar), 128.98, 121.94, 130.20, 116.41 (4CH, Ar), 159.80, 162.25, 158.74 (3C, Triazolothiadiazole), 16.9 (1C, CH3); MS (m/z, M+): 232.26; CHNSO Analysis (%): C (51.71), H (3.47), N (24.12), S (13.81), O (6.89).
PAM Vc
IR (KBr, cm-1): 3256 (OH), 1614 (C=N), 1585 (C=C), 3125 (C-H; aro), 1530 (C-N), 711(C-S); 1H-NMR (δ, 400 MHz, DMSO-d6): 7.31-6.79 (m, 4H, 4-Ar), 7.8 (s, 1H, -OH), 7.48-7.22 (s, 5H, Ar), 7.8 (s, 1H, -OH); 13C-NMR (δ, 100 MHz, DMSO-d6): 155.32, 118.54 (2C, Ar), 128.88, 121.74, 130.30, 116.48 (4CH, Ar), 159.80, 162.25, 175.45 (3C, Triazolothiadiazole), 133.56, 127.54, 129.38, 128.86, 129.34, 127.58 (6C, Ar); MS (m/z, M+): Mass spectra of PAM Vc showed molecular ion peak at 294.33; CHNSO Analysis (%): C (61.21), H (3.42), N (19.04), S (10.89), O (5.44).
PAM Vd
IR (KBr, cm-1): 3256 (OH), 1614 (C=N), 1585 (C=C), 3125 (C-H; aro), 1530 (C-N), 711 (C-S); 1H-NMR (δ, 400 MHz, DMSO-d6): 7.79 (s, 1H, -OH), 7.81-7.42 (m, 4H, Ar), 7.8-6.79 (m, 4H, Ar); 13C-NMR (δ, 100 MHz, DMSO-d6): 155.34, 118.57(2C, Ar), 128.58, 121.64, 130.36, 116.49 (4CH, Ar), 159.86, 162.27, 175.56 (3C, Triazolothiadiazole), 132.56, 136.96, 129.45, 130.24, 127.56, 128.92 (6C, o-Cl-Ar); MS (m/z, M+): 328.78; CHNSO Analysis (%): C (54.80), H (2.76), N (17.04), S (9.75), O (4.87), Cl (10.78).
PAM Ve
IR (KBr, cm-1): 3256 (OH), 1614 (C=N), 1585 (C=C), 3125 (C-H; aro), 1530 (C-N), 711(C-S); 1H-NMR (δ, 400 MHz, DMSO-d6): 7.64-6.92 (m, 4H, Ar), 7.49-7.23 (m, 4H, Ar), 7.75 (s, 1H, -OH); 13C-NMR (δ, 100 MHz, DMSO-d6): 155.34, 118.57 (2C, Ar), 128.58, 121.64, 130.36, 116.49 (4CH, Ar), 159.86, 162.27, 175.56 (3C, Triazolothiadiazole), 134.95, 127.48, 134.85, 128.35, 130.74, 125.67 (6C, m-Cl-Ar); MS (m/z, M+): 328.78; CHNSO Analysis (%): C (54.80), H (2.76), N (17.04), S (9.75), O (4.87), Cl (10.78).
PAM Vf
IR (KBr, cm-1): 3256 (OH), 1614 (C=N), 1585 (C=C), 3125 (C-H; aro), 1530 (C-N), 711(C-S); 1H-NMR (δ, 400 MHz, DMSO-d6): 7.64-6.92 (m, 4H, Ar), 7.75 (s, 1H, -OH), 7.42-7.32 (m, 4H, Ar); 13C-NMR (δ, 100 MHz, DMSO-d+): 155.34, 118.57 (2C, Ar), 128.58, 121.64, 130.36, 116.49 (4CH, Ar), 159.86, 162.27, 175.56 (3C, Triazolothiadiazole), 131.64, 128.92, 129.34, 134.38, 129.46, 128.97 (6C, p-Cl-Ar); MS (m/z, M+): 328.78; CHNSO Analysis (%): C (54.80), H (2.76), N (17.04), S (9.75), O (4.87), Cl (10.78).
PAM Vg
IR (KBr, cm-1): 3190.69 (OH), 1622.69 (C=N), 1484.52 (C=C), 2918.71 (C-H; Aro), 1536.57 (C-N), 751.71 (C-S), 1397.73 (N=C‒S), 1230.52 (N‒N=C); 1H-NMR (δ, 400 MHz, DMSO-d6): 7.62-6.90 (m, 4H, Ar), 7.77 (s, 1H, -OH), 7.31-6.79 (m, 4H, Ar); 13C-NMR (δ, 100 MHz, DMSO-d6): 155.34, 118.57 (2C, Ar), 128.58, 121.64, 130.36, 116.49 (4CH, Ar), 159.86, 162.27, 175.56 (3C, Triazolothiadiazole), 126.14, 128.94, 116.56, 158.57, 116.56, 128.94 (6C, p-OH-Ar); MS (m/z, M+): 310.33; CHNSO Analysis (%): C (58.05), H (3.25), N (18.05), S (10.33), O (10.31).
PAM Vh
IR (KBr, cm-1): 3095.55 (OH), 1488.52 (C=C), 2936.56 (C-H; aro), 1592.44 (C-N), 767.69 (C-S), 1170.52 (C-F); 1H-NMR (δ, 400 MHz, DMSO-d6): 7.65-6.93 (m, 4H, Ar), 7.76 (s, 1H, -OH), 7.46-7.03 (m, 4H, Ar); 13C-NMR (δ, 100 MHz, DMSO-d6): 155.34, 118.57 (2C, Ar), 128.58, 121.64, 130.36, 116.49 (4CH, Ar), 159.86, 162.27, 175.56 (3C, Triazolothiadiazole), 129.14, 129.14, 116.48, 116.48, 162.92, 129.56 (6C, p-F-Ar); MS (m/z, M+): 312.32; CHNSO Analysis (%): C (57.68), H (2.90), N (17.94), S (10.27), O (5.12), F (6.08).
PAM Vi
IR (KBr, cm-1): 3325 (OH), 1609 (C=N), 1586 (C=C), 3113 (C-H; aro), 1530 (C-N), 711(C-S), 996(C-O), 2980 (C-H; CH3), 1374 (N=C‒S), 1237 (N‒N=C); 1H-NMR (δ, 400 MHz, DMSO-d6): 7.65-6.93 (m, 4H, Ar), 7.76 (s, 1H, -OH), 7.46-7.03 (m, 4H, Ar); 13C-NMR (δ, 100 MHz, DMSO-d6): 155.34, 118.57 (2C, Ar), 128.58, 121.64, 130.36, 116.49 (4CH, Ar), 159.86, 162.27, 175.56 (3C, Triazolothiadiazole), 125.82, 128.54, 128.54, 114.82, 114.82, 160.75 (6C, p-OCH3-Ar), 59.97 (1C, OCH3); MS (m/z, M+): 324.36; CHNSO Analysis (%): C (59.25), H (3.73), N (17.27), S (9.89), O (9.87).
PAM Vj
IR (KBr, cm-1): 3325 (OH), 1609 (C=N), 1586 (C=C), 3113 (C-H; aro), 1530 (C-N), 711(C-S), 996(C-O), 2980 (C-H; CH3), 1374 (N=C‒S), 1237 (N‒N=C); 1H-NMR (δ, 400 MHz, DMSO-d6): 7.63-6.91 (m, 4H, Ar), 7.71 (s, 1H, -OH), 6.44 (s, 2H, Ar), 3.73(s, 9H, CH3); 13C-NMR (δ, 100 MHz, DMSO-d6): 155.34, 118.57 (2C, Ar), 128.58, 121.64, 130.36, 116.49 (4CH, Ar), 159.86, 162.27, 175.56 (3C, Triazolothiadiazole), 128.24, 104.95, 151.78, 140.82, 147.28 (6C, tri-OCH3-Ar), 56.24, 56.58, 56.24 (3C, OCH3); MS (m/z, M+): 384.41; CHNSO Analysis (%): C (56.24), H (4.20), N (14.57), S (8.34), O (16.65).
PAM Vk
IR (KBr, cm-1): IR spectrum of PAM Vk showed various absorption peaks at 3292.64 (OH), 1609.42 (C=N), 1485.46 (C=C), 3084.48 (C-H; Aro), 695.65 (C-S), 2941.52 (C-H; CH3), 1374.47 (N=C‒S), 1233.55 (N‒N=C); 1H-NMR (δ, 400 MHz, DMSO-d6): 7.65-6.94 (m, 4H, Ar), 7.74 (s, 1H, -OH), 7.30-6.65 (m, 4H, Ar), 2.85 (s, 6H, CH3); 13C-NMR (δ, 100 MHz, DMSO-d6): 158.75, 118.92 (2C, Ar), 133.09, 118.66, 133.76, 116.98 (4CH, Ar), 159.69, 165.88, 176.98 (3C, Triazolothiadiazole), 128.58, 130.26, 130.26, 114.58, 114.58, 156.21 (6C, p-N-(CH3)2-Ar), 39.61 (2C, CH3); MS (m/z, M+): 338.52; CHNSO Analysis (%): C (56.24), H (4.20), N (14.57), S (8.34), O (16.65).
PAM Vl
IR (KBr, cm-1): 3256 (OH), 1627 (C=N), 1587 (C=C), 3120 (C-H; aro), 1530 (C-N), 711(C-S), 1345 (N=C‒S), 1250 (N‒N=C); 1H-NMR (δ, 400 MHz, DMSO-d6): 7.65-6.94 (m, 4H, Ar), 7.74 (s, 1H, -OH), 5.62-5.56 (s, 2H, -OH), 6.78-6.52 (m, 3H, Ar); 13C-NMR (δ, 100 MHz, DMSO-d6): 155.34, 118.57 (2C, Ar), 128.58, 121.64, 130.36, 116.49 (4CH, Ar), 159.86, 162.27, 175.56 (3C, Triazolothiadiazole), 125.15, 147.94, 117.84, 117.35, 151.64, 114.86 (6C, o-OH-Ar); MS (m/z, M+): Mass spectra of PAM Vl showed molecular ion peak at 326.33; CHNSO Analysis (%): C (55.21), H (3.09), N (17.17), S (9.83), O (14.71).
PAM Vm
IR (KBr, cm-1): 3256 (OH), 1627 (C=N), 1587 (C=C), 3120 (C-H; aro), 1530 (C-N), 711(C-S), 1345 (N=C‒S), 1250 (N‒N=C), 3150 (NH), 698 (meta- substitution); 1H-NMR (δ, 400MHz, DMSO-d6): 7.62-6.89 (m, 4H, Ar), 7.73 (s, 1H, -OH), 7.07-6.42 (m, 4H, Ar), 4.2 (s, 2H, NH2); 13C-NMR (δ, 100MHz, DMSO-d6): 155.34, 118.57 (2C, Ar), 128.58, 121.64, 130.36, 116.49 (4CH, Ar), 159.86, 162.27, 175.56 (3C, Triazolothiadiazole), 134.34, 114.24, 148.95, 116.38, 130.14, 117.56 (6C, m-NH2-Ar); MS (m/z, M+): 309.35; CHNSO Analysis (%): C (58.24), H (3.58), N (22.64), S (10.37), O (5.17).
PAM Vn
IR (KBr, cm-1): 3256 (OH), 1627 (C=N), 1587 (C=C), 3120 (C-H; aro), 1530 (C-N), 711(C-S), 1345 (N=C‒S), 1250 (N‒N=C), 3150 (NH), 825 (para- substitution); 1H-NMR (δ, 400 MHz, DMSO-d6): 7.67-6.93 (m, 4H, Ar), 7.70 (s, 1H, -OH), 7.23-6.52 (m, 4H, Ar), 4.13 (s, 2H, NH2); 13C-NMR (δ, 100 MHz, DMSO-d6): 155.34, 118.57 (2C, Ar), 128.58, 121.64, 130.36, 116.49 (4CH, Ar), 159.86, 162.27, 175.56 (3C, Triazolothiadiazole), 123.56, 128.36, 116.84, 148.47, 116.84, 128.36 (6C, p-NH2-Ar); MS (m/z, M+): 309.35; CHNSO Analysis (%): C (58.24), H (3.58), N (22.64), S (10.37), O (5.17).
PAM Vo
IR (KBr, cm-1): 7.67-6.93 (m, 4H, Ar), 7.70 (s, 1H, -OH), 6.52-6.04 (m, 4H, Ar), 3.56 (s, 4H, NH2); 1H-NMR (δ, 400 MHz, DMSO-d6): 7.67-6.93 (m, 4H, Ar), 7.70 (s, 1H, -OH), 7.23-6.52 (m, 4H, Ar), 4.13 (s, 2H, NH2); 13C-NMR (δ, 100 MHz, DMSO-d6): 155.34, 118.57 (2C, Ar), 128.58, 121.64, 130.36, 116.49 (4CH, Ar), 159.86, 162.27, 175.56 (3C, Triazolothiadiazole), 135.17, 104.28, 104.28, 149.72, 149.72, 102.74 (6C, 3,5-di-NH2-Ar); MS (m/z, M+): 324.36; CHNSO Analysis (%): C (55.54), H (3.73), N (25.91), S (9.89), O (4.93).
PAM Vp
IR (KBr, cm-1): 3256 (OH), 1627 (C=N), 1587 (C=C), 3120 (C-H; aro), 1530 (C-N), 711(C-S), 1345 (N=C‒S), 1250 (N‒N=C); 1H-NMR (δ, 400 MHz, DMSO-d6): 7.65-6.94 (m, 4H, Ar), 7.74 (s, 1H, -OH), 5.62-5.56 (s, 2H, -OH), 7.14-6.26 (m, 3H, Ar); 13C-NMR (δ, 100 MHz, DMSO-d6): 155.34, 118.57 (2C, Ar), 128.58, 121.64, 130.36, 116.49 (4CH, Ar), 159.86, 162.27, 175.56 (3C, Triazolothiadiazole), 116.35, 156.74, 104.17, 159.94, 109.56, 130.38 (6C, 2,4-di-OH-Ar); MS (m/z, M+): 326.33; CHNSO Analysis (%): C (55.54), H (3.73), N (25.91), S (9.89), O (4.93).
PAM Vq
IR (KBr, cm-1): 3256 (OH), 1610 (C=N), 1581 (C=C), 3132 (C-H; aro), 1530 (C-N), 711(C-S), 1209 (C-O), 1348 & 1529 (NO2); 1H-NMR (δ, 400 MHz, DMSO-d6): 7.67-6.93 (m, 4H, Ar), 7.70 (s, 1H, -OH), 8.25-7.74 (m, 4H, Ar); 13C-NMR (δ, 100 MHz, DMSO-d6): 155.34, 118.57 (2C, Ar), 128.58, 121.64, 130.36, 116.49 (4CH, Ar), 159.86, 162.27, 175.56 (3C, Triazolothiadiazole), 139.65, 128.47, 128.47, 121.64, 121.64, 148.46 (6C, p-NO2-Ar); MS (m/z, M+): Mass spectra of PAM Vq showed molecular ion peak at 373.23; CHNSO Analysis (%): C (53.09), H (2.67), N (20.64), S (9.45), O (14.15).
PAM Vr
IR (KBr, cm-1): 3256 (OH), 1627 (C=N), 1587 (C=C), 3120 (C-H; aro), 1530 (C-N), 711(C-S), 1345 (N=C‒S), 1250 (N‒N=C), 1050 (C-Br), 740 (ortho- substitution); 1H-NMR (δ, 400 MHz, DMSO-d6): 7.66-6.94 (m, 4H, Ar), 7.75 (s, 1H, -OH), 7.49-7.11 (m, 4H, Ar); 13C-NMR (δ, 100 MHz, DMSO-d6): 155.34, 118.57 (2C, Ar), 128.58, 121.64, 130.36, 116.49 (4CH, Ar), 159.86, 162.27, 175.56 (3C, Triazolothiadiazole), 139.82, 120.38, 132.24, 131.45, 128.37, 129.74 (6C, o-Br-Ar); MS (m/z, M+): 373.23, CHNSO Analysis (%): C (48.27), H (2.43), N (15.01), S (8.59), O (4.29), Br (21.41).
PAM Vs
IR (KBr, cm-1): 3256 (OH), 1627 (C=N), 1587 (C=C), 3120 (C-H; aro), 1530 (C-N), 711(C-S), 1345 (N=C‒S), 1250 (N‒N=C); 1H-NMR (δ, 400 MHz, DMSO-d6): 7.62-6.88 (m, 4H, Ar), 7.70 (s, 1H, -OH), 7.49-7.37 (m, 4H, Ar); 13C-NMR (δ, 100 MHz, DMSO-d6): 155.34, 118.57 (2C, Ar), 128.58, 121.64, 130.36, 116.49 (4CH, Ar), 159.86, 162.27, 175.56 (3C, Triazolothiadiazole), 132.54, 129.75, 129.75, 132.27, 132.27, 129.71 (6C, p-Br-Ar); MS (m/z, M+): 373.23; CHNSO Analysis (%): C (48.27), H (2.43), N (15.01), S (8.59), O (4.29), Br (21.41).
PAM Vt
IR (KBr, cm-1): 3256 (OH), 1627 (C=N), 1587 (C=C), 3120 (C-H; aro), 1530 (C-N), 711(C-S), 1345 (N=C‒S), 1250 (N‒N=C); 1H-NMR (δ, 400 MHz, DMSO-d6): 7.65-6.91 (m, 4H, Ar), 7.78 (s, 1H, -OH), 8.81-7.44(m, 4H, Ar); 13C-NMR (δ, 100 MHz, DMSO-d6): 155.34, 118.57 (2C, Ar), 128.58, 121.64, 130.36, 116.49 (4CH, Ar), 159.86, 162.27, 175.56 (3C, Triazolothiadiazole), 133.48, 134.15, 124.74, 148.56, 149.18 (5C, 3-pyridyl); MS (m/z, M+): 295.32; CHNSO Analysis (%): C (56.94), H (3.07), N (23.71), S (10.86), O (5.42).
PAM Vu
IR (KBr, cm-1): 3256 (OH), 1627 (C=N), 1587 (C=C), 3120 (C-H; aro), 1530 (C-N), 711(C-S), 1345 (N=C‒S), 1250 (N‒N=C), 3150 (NH), 738 (ortho- substitution); 1H-NMR (δ, 400 MHz, DMSO-d6): 7.68-6.96 (m, 4H, Ar), 7.75 (s, 1H, -OH), 8.07-6.66 (m, 3H, Ar), 4.2 (s, 1H, NH); 13C-NMR (δ, 100 MHz, DMSO-d6): 155.34, 118.57 (2C, Ar), 128.58, 121.64, 130.36, 116.49 (4CH, Ar), 159.86, 162.27, 175.56 (3C, Triazolothiadiazole), 118.73, 135.56, 113.57, 146.51, 156.74 (5C, 2-NO2-3-pyridyl); MS (m/z, M+): Mass spectra of PAM Vu showed molecular ion peak at 310.33; CHNSO Analysis (%): C (54.18), H (3.25), N (27.08), S (10.33), O (5.16).
PAM Vv
IR (KBr, cm-1): 3348.63 (OH), 1613.53 (C=N), 1587 (C=C), 3120 (C-H; aro), 1530 (C-N), 711(C-S), 1345 (N=C‒S), 1250 (N‒N=C), 1675 (C=O); 1H-NMR (δ, 400 MHz, DMSO-d6): 7.66-6.92 (m, 4H, Ar), 7.76 (s, 1H, -OH), 8.24 (s, 1H, NH); 13C-NMR (δ, 100 MHz, DMSO-d6): 133.06, 118.57 (2C, Ar), 128.54, 116.97, 117.13, 109.16 (4CH, Ar), 156.21, 159.67, 176.97 (3C, Triazolothiadiazole); MS (m/z, M+): 234.25; CHNSO Analysis (%): C (54.18), H (3.25), N (27.08), S (10.33), O (5.16).
PAM Vw
IR (KBr, cm-1): 3209.49 (OH), 1627 (C=N), 1587 (C=C), 2918.75 (C-H; Aro), 1555.51 (C-N), 697.45 (C-S), 1344.49 (N=C‒S), 1243.61 (N‒N=C), 3036.56 (NH); 1H-NMR (δ, 400 MHz, DMSO-d6): 7.51-7.09 (m, 4H, Ar), 9.72 (s, 1H, OH), 7.01-6.46 (m, 4H, Ar), 3.92 (s, 1H, NH); 13C-NMR (δ, 100 MHz, DMSO-d6): 155.34, 118.57 (2C, Ar), 128.58, 121.64, 130.36, 116.49 (4CH, Ar), 159.86, 162.27, 152.74 (3C, Triazolothiadiazole), 143.17, 116.35, 116.35, 129.61, 129.61, 116.38 (6C, NH-Ar); MS (m/z, M+): 309.26; CHNSO Analysis (%): C (46.15), H (2.58), N (23.92), S (13.69), O (309.35).
PAM Vx
IR (KBr, cm-1): 3256 (OH), 1627 (C=N), 1587 (C=C), 3120 (C-H; aro), 1530 (C-N), 711(C-S), 1345 (N=C‒S), 1250 (N‒N=C), 3050 (NH), 1040 (Cl), 805 (para- substitution); 1H-NMR (δ, 400 MHz, DMSO-d6): 7.63-6.92 (m, 4H, Ar), 7.75 (s, 1H, -OH), 7.02-6.40 (m, 4H, Ar), 3.85 (s, 1H, NH); 13C-NMR (δ, 100 MHz, DMSO-d6): 155.34, 118.57 (2C, Ar), 128.58, 121.64, 130.36, 116.49 (4CH, Ar), 159.86, 162.27, 152.74 (3C, Triazolothiadiazole), 141.24, 117.75, 129.74, 124.35, 129.74, 117.75 (6C, NH-p-Cl-Ar); MS (m/z, M+): 343.79; CHNSO Analysis (%): C (58.24), H (3.58), N (22.64), S (10.37), O (5.17), Cl (10.31).
PAM Vy
IR (KBr, cm-1): 3256 (OH), 2925 (CH str), 1627 (C=N), 1587 (C=C), 3120 (C-H; aro), 1530 (C-N), 711(C-S), 1345 (N=C‒S), 1250 (N‒N=C), 3050 (NH), 1209 (C-O), 745 (ortho- substitution); 1H-NMR (δ, 400 MHz, DMSO-d6): 7.65-6.94 (m, 4H, Ar), 7.74 (s, 1H, -OH), 6.57-6.35 (m, 4H, Ar), 3.85 (s, 1H, NH), 3.73 (s, 3H, CH3); 13C-NMR (δ, 100 MHz, DMSO-d6): 155.34, 118.57 (2C, Ar), 128.58, 121.64, 130.36, 116.49 (4CH, Ar), 159.86, 162.27, 152.78 (3C, Triazolothiadiazole), 132.65, 117.38, 121.94, 119.87, 115.12, 147.56 (6C, NH-o-OCH3-Ar), 55.94 (1C, CH3); MS (m/z, M+): 339.37;.
PAM Vz
IR (KBr, cm-1): spectrum of PAM Vz showed various absorption peaks at 3256 (OH), 2925 (CH str), 1627 (C=N), 1587 (C=C), 3120 (C-H; Aro), 1530 (C-N), 711(C-S), 1345 (N=C‒S), 1250 (N‒N=C), 3050 (NH), 815 (para- substitution); 1H-NMR (δ, 400 MHz, DMSO-d6): 7.63-6.90 (m, 4H, Ar), 7.71 (s, 1H, -OH), 6.81-6.34 (m, 4H, Ar), 3.85 (s, 1H, NH), 2.35 (s, 3H, CH3); 13C-NMR (δ, 100 MHz, DMSO-d6): 155.34, 118.57 (2C, Ar), 128.58, 121.64, 130.36, 116.49 (4CH, Ar), 159.86, 162.27, 152.78 (3C, Triazolothiadiazole), 140.17, 116.25, 116.25, 129.94, 128.47 (6C, NH-p-CH3-Ar), 24.35(1C, CH3); MS (m/z, M+): 323.37; CHNSO Analysis (%): C (59.43), H (4.05), N (21.66), S (9.92), O (4.95).
Acknowledgement
The authors are thankful to Punjab University, Chandigarh for spectral assistance and sophisticated analytical instrumentation facility.
References
- C. Montiel-Duarte, E. Ansorena, M.J. Lopez-Zabalza, E. Cenarruzabeitia, M.J. Iraburu, Biochem. Pharmacol., 2004, 67, 6, 1025-1033.
- A. Al-Saeed, Oman Medical Journal, 2011, 26, 6, 385-391.
- A.M. Vittalrao, T. Shanbhag, K. Kumari, K.L. Bairy, S. Shenoy, Indian J. Physiol. Pharmacol., 2011, 55, 1, 13-24.
- A. Almasirad, Z. Mousavi, M. Tajik, M.J. Assarzadeh, A. Shafiee, DARU J. Pharmaceut. Sci., 2014, 22, 1-8.
- S.K. Reddy, S.A. Kumar, V.D. Kumar, S. Ganapaty, Tropic. J. Pharmaceut. Res., 2012 11, 6, 971-976.
- K. Gnananath, A.S. Kumar, N. Srinivas, P. Gomathi, K.K. Kumar, Int. J. Pharma Bio Sci., 2012, 3, 2, 407-414.



