Review Article - Der Pharma Chemica ( 2021) Volume 13, Issue 11
Hydrochemistry of Surface Water bodies of the Himalayan Region (Impact of Anthropogenic activities on water quality) : A Review
Kanchan Deoli Bahukhandi*Kanchan Deoli Bahukhandi, Department of Engineering, Sustainability Cluster, School of Engineering, University of Petroleum and Energy Studies, India, Email: kanchan@ddn.upes.ac.in
Abstract
The Himalayan ecosystem is one of the most fragile ecosystems in the world. Amid the growing changes in land use pattern due to deforestation, construction of multiple dams, mining, increased urbanization, and industrialization together with emission of greenhouses gases i.e CO2, NOx, CH4 and CFCs contributing to Global Warming has come to be seen as a risk affecting drastically the water chemistry of the region. The frequent cloud burst, avalanche, landslide along with flash flood are the most common human induced disaster which recurrently occurred in past few decades in the Himalayan region. This paper discusses about the impact of land use pattern on water chemistry of the region and spatial and temporal variation in water quality of the region.
Among anions, bicarbonate is most dominant anions which constitute around 60 % to 70 % anion followed by SO4 constituting around ~ 15% of anion. The high equivalent ratio of (Ca2+ + Mg2+)/ HCO3 ratio ~ (1.2) and relatively high contribution of (Ca+Mg) ) to total cations (TZ+) indicates carbonate weathering as the primary source of major ions. The carbonate weathering contributes around 80 % to 90 % of HCO3 while silicate contributes 5% -12 % HCO3 in the Himalayan catchment which implies the dominant of carbonate weathering. The ionic ratios and relative concentration of ions suggests that the major source of ions in water in the study areas remains rock weathering, although anthropogenic activities have influenced the ionic composition. Overall cation and anions concentration shows following trend in the study area HCO3>SO4>NO3>Ca>Cl>Mg>Na >Si > K >PO4>F. The surface water of study area belongs to Ca-Mg-HCO3 and Ca-Mg-SO4 Hydrochemical facies.
Keywords
Land use pattern; Himalayan River; Water quality; Hydrochemistry; Temporal and spatial variation
Introduction
Water makes up more than three-fourth of our earth surface. It is estimated that 70 % of our earth surface is water-covered of which 97% of water represents ocean. However, the ocean water cannot be used for drinking, agricultural and industrial purposes because of high salt content [1]. Therefore, only 3% water is available in the form of fresh water out of which approximately 78% remains in the form of ice caps and glacier [2] and thereby, only 1 % water is accessible by human being which is present in the form of rivers, lakes, stream, groundwater etc. The quality and quantity of the surface and ground water is severely affected due to land use pattern because of industrialization, urbanization, and intensive agricultural practices and, also climate change attribution to increase in temperature [3-7]. It is imperative to protect water quality as well as its quantity. Freshwater resources are vulnerable to climate change due to large climatic variability associated with the monsoon system and rising water demand. It has been estimated that climate changes can affect surface water quality across East Asia [8]. The changes in land use pattern i.e industrialization, urbanization, construction of Dam reservoir has led to over exploitation of water resources. The water quality has been deteriorating in past few decades due to discharge of physical, chemical and biological pollutants in the Himalayan regions. Improper disposal of solid waste, discharge of waste water in water bodies or in land ecosystem without proper treatment has changed the ionic composition of water in different places of Himalayan region [9-21]. In order to understand suitability of water for various needs and for understanding chemical weathering process the knowledge of hydrochemistry is essential which will help in determining the ionic sources and factors controlling the water chemistry of the region. The water quality is described as a function of physical and chemical parameters which is greatly influenced by geological formations, climatic conditions and anthropogenic activities [22-24].
Globally, weathering of rocks is the major source of ions in the water bodies. Physical, chemical and biological processes are involved in rock weathering. The chemical reactions between rock minerals and soil water produces cations, anions and heavy metals in surface and groundwater bodies. The chemical composition of the world river has been reviewed by [25-28]. This compilation provides a general idea of chemical composition of rivers flowing through different geological terrains and climatic regimes. From the data compilation by Livingstone and Meybeck it can be observed that ionic ratios in the water of Major River are fairly constant. The 90% of World Rivers are rock dominant type of the water [29]. Gibbs plotted TDS verses the Na/Na+Ca, the two-end member of sea water and fresh water to show the mechanism controlling water chemistry in river and found that that the chemistry of river water is influenced by precipitation, rock interaction and evaporation. The rivers with Na/Na+Ca between 0.2 to 0.6 and TDS concentration approximately between 90mg/l to 450 mg/l owe most of the chemical composition due to rock weathering. Among cations, Ca were found to be most abundant cation while among anions, HCO3 were found to be most abundant anions in water in these rock dominant river. The remaining 10% rivers are Na, Cl, SO4 ion dominant type where water is either rain dominant type or the evaporation-crystallization types [30,31]. The rock – dominated type of water reflects the weathering of minerals found in surface rocks, particularly the calcium carbonate minerals in sedimentary rock.
Weathering of both silicate and non-silicate rock contribute ~70% of river alkalinity while remaining 30% is caused by river based biological processes due to decomposition of organic matter [32]. The relative weathering contribution in the beginning of monsoon season is likely to be high due to large discharge of various river systems flushing out from weathered top soil in the initial stages of rain water when biochemical reactivity is inhibited by rapid flow of water. The faecal pollution contamination in water was found due to urban runoff and birds from the Bagnallas beach [33]. The presence of fluoride in groundwater in the rift valley region of Ethiopia, reported dental and skeletal health problems due to drinking of high fluoride water (up to 33 mg/L fluoride) [34]. However, no studies have been done in the past on the analysis for understanding the changes in water quality and its relationship between land use patterns. The present focus is to understand the impact of land use pattern on Himalayan river quality of Uttarakhand regions. And accordingly, the secondary data of past fifteen years have been taken from Central pollution Control Board (Uttarakhand Pollution Control Board) and other researcher’s water quality data of the region to understand the impact of land use pattern. With an aim to improve the Ganga river water quality, Central government’s ambitious project to clean River Ganga under its Namami Gange Project (NGP) has borne some fruit with river water from Rudraprayag in Uttarakhand to Uluberia in West Bengal improving between 2014 and 2019. The Central Government has approved the projects for ‘World Bank’ assistance to National Ganga River Basin Authority(NGRBA) for abatement of pollution of river Ganga’ at an estimated cost of Rs.7000crore.
Himalayan River
The whole Garhwal Himalaya region except western part of Uttarkashi and Dehradun district and small tract lying in eastern margin of Garhwal district is drained by Ganga system. The Alaknanda and Bhagirithi are the two major rivers which meet at confluence point at Devprayag [35]. The Bhagirithi River originates from Gangotri glacier which is about 30 km long and 2 km wide. The whole Ganga system is divided into three main sub system i.e Sub system of Alaknanda, Sub system of Bhagirithi and the Ramganga system. The Alaknanda originates from Saptopanth glacier. The Dhauliganga originates from Kailing in Niti Pass and joins the Alaknanda at Vishnupryag. The river Mandakini originates from Kedar Himal and it joins Alaknanda at Rudraprayag. The Bhilangana River originates from Bharti Kantha the part of Tehri district and it joins the Bhagirithi river at Ganeshprayag in Tehri. The river Bhagirathi is the source stream of Ganga. It emanates from Gangotri Glacier at Gaumukh at an elevation of 3, 892 m (12,770 feet). Many small streams comprise the headwaters of Ganga. The important among these are Alaknanda, Dhauliganga, Pindar, Mandakini and Bhilangana. At Devprayag, where Alaknanda joins Bhagirathi, the river acquires the name Ganga. The Tehri dam has been built on Bhagirithi River. The western part of Uttarakhand drainage by the Yamuna system. Yamuna originate from Yamnotri Glacier which lies on the south-western slope of Bandar Punch Peak. Kosi is the one of the major rivers of Kumaon Garhwali, it originates from Kausani region and it is not a glacial river.
Land use Pattern in Himalayan
The land use refers to the human employment of land and is the planned hiring and management strategy laced on the land defined by humans [36]. It includes distribution of forest, water, desert, ice and the immediate sub surface including biota, soil, topography, surface, and groundwater. It is a dynamic process that changes by human induced disaster, anthropogenic activities causing negative impact on natural ecosystem [37]. These changes in land use due to anthropogenic activities, in turn also changes the physical, chemical and biological characteristic of water [38-45]. The Hindu Kush-Himalayan (HKH) region is the highest mountain range in the world, and it extends about 3500 km from Afghanistan to China in east-west, and 250 to 300 km in north-south extension [46]. Geologically, this Himalayan region is one of the youngest mountains in the world. It was dated to late Tertiary/early Quaternary (about 1.7-1.5 million) years before the present [47]. The Himalayan Rivers are ranked amongst the top rivers in terms of suspended sediment load [48]. There anthropogenic activities i.e deforestation, construction of Dams, river valley projects, all weather road projects, urbanization and industrialization has drastically changed land use pattern of this region [49-61].
Development of Hydel Power Plant
The land use patterns are changing drastically in Himalayan region of Uttarakhand. The Himalayan being the first youngest mountain range, its ecosystem is fragile and sensitive ecosystem [62] and thus it is prone to landslide, cloudburst and soil erosion. The regions fall in a very high seismic zone (Seismic Zone V) (Down to earth 2021). The Himalayan belt has over 2,300 glacial lakes that are melting due to global warming and, therefore, it is significantly changing land use by human induced natural disaster i.e avalanche, landslide, flash flood and soil erosion. The Garhwal Himalaya valley is drained by Alaknanda and Bhagirihti River. These rivers emerge from the Snow –clad Chaukhamba range which consists of enormous glaciers. There are around 20 operational dams in the Alaknanda and Bhagirithi valley amongst which 30 percent of them are large projects (Down to earth 2021, March). The continuous development of these hydel power projects are changing the environmental flow of these rivers hence changing hydrochemistry of these river bodies. The huge construction of these dams led to human induced disasters i.e .landslide, flashflood in this region. The impact of land slide caused by avalanche like in Chamoli disaster which took place in Dhaulignaga on February 7, 2021, one of the tributaries of Alaknanda is more severe as compare to other landslides. This disaster took place at Reini village, the birthplace of Chipko movement. The muddy deluge gushed down damaging the 13.2 MW Rishiganga hydropower project and washed away under construction 520 MW Tapovab- Vishugad project downstream. The rising temperature due to Global warming and climate changes possibly caused reiterating glaciers which led to such avalanche, landslide and flash flood in Himalayan region.
Extreme landslides have become very frequent and intense in Himalayas in past few decades. The study jointly conducted by Wadia Institute of Himalayan Geology and the National Geotechnical Facility shows that Uttarakhand has faced 27 major landslide events between 1180 and 2015 and one third of which have occurred in just 15 years between 2001 to 2015 (Down to Earh 2021). The land use changes have caused adverse environmental problem both at regional and global level changes in climate and biogeochemical cycles, loss of biodiversity [63,64].
The water quality of Himalayan ecosystem is deteriorating day by day due to anthropogenic activities. Many studies have been carried to analyse the water quality of the Himalayan region. The river water is used for drinking, agricultural, industrial and to full fill other basic needs of human being [65]. The water quality in all around world is significantly deteriorating at a very fast rate due to change in land use pattern i.e industrialization, urbanization, construction of Dam reservoir [66]. The glacial-fed streams have more current velocity (0.35-1.7 m/s) and coarse bottom of boulders, rocks or pebbles [67]. The hydroelectrically project has caused the water bodies to stagnant. The agricultural practices also added chemical fertilizers and pesticides residue - Endosulfan, Dieldrin and DDT in Ganga River and its tributaries. Mass bathing in sacred water bodies, open defecation on river bank and other religious activities are adding huge amount of pollution in these river. The construction of reservoir is also affecting the river flow of the region. The physical, chemical and biological qualities of river water are significantly altering due to obstruction of natural flow of a river a dam or a barrage [68].
The impact of HEPs (Hydroelectric Project) can be estimated in three stages of the project i.e pre project construction, project construction and project operation. These stages can cause number of negative impact i.e displacement, loss of lands, homes, and livelihoods, soil erosion and landslides, loss of flora and fauna, changes in micro-climate), disposal of debris and earth (loss of trees, river water pollution), drying of springs, disposal of muck into the river, disruption of river flows (biotic changes, disruption of natural functions e.g. sediments disposal, land shaping, nutrient cycling), sedimentation (effect on river water quality) and other secondary effects (release of greenhouse gases, warming of valleys, increased earthquake risks, floods, downstream urban and industrial development. All these impacts also deteriorate water quality directly and indirectly due various sources.
Hydrochemistry of the Himalayan River
The chemistry of some of the large rivers of the Asian region such as the Ganges, Brahmaputra, Changjiang and Huanghe has been studied extensively by a number of researchers. The major ion and trace element geochemistry in the Upper Ganga River of Himalayan water categorized as HCO3-, SO42-, Ca2+ dominant which constitute >60% of the total water composition. It was observed that only 5-12% of bicarbonate is derived from silicate lithology indicating strong influence of carbonate lithology on water chemistry in the head waters of Ganga River. More than 80% Na + and K+ are derived from silicate lithology. The ion chemistry of the river is influenced by natural as well as anthropogenic sources. The following formula can be used for calculating alkalinity derived from silicate weathering where all values are in mill equivalents unit [69].
(Following method of Alkalinity carbonate weathering = 0.74 Ca total + 0.4 Mg total
Alkalinity silicate = Alka total – Ala. Carb.weathering
It is estimated that on an average 90 %, if bicarbonate comes from carbonate weathering while taking the account of Ca and Mg and ~ 9% bicarbonate comes from silicate weathering. 5-12% of HCO3 is derived from silicate lithology implying carbonate weathering account for major amount of HCO3 in the upper Ganga catchment of the Himalaya. This is also evident from the good Ca+ Mg and HCO3 correlations (r2 = 0.6) in present area of study. The (Ca +Mg) and HCO3 accounts for 80 % of cations and anions in the water bodies of Himalayan river [70].
Thus, the weathering of rock forming minerals, with minor contribution from cyclic sea salt and pollution, are the major sources of ions in river. The chemical composition of glacial melt water also demonstrates that the chemical weathering take place beneath the glacier. The major source of HCO3– in river water is from carbonate and silicate weathering which together accounts for 95% of global water composition. Weathering of both silicate and non- silicate rocks contribute 70% of the river alkalinity while remaining 30% is accounted by river-based biological process due to decomposition of organic matter.
The south Asians rivers reveals strong seasonal variation in their solute transport reflecting variable export of silica, Ca, Mg and alkalinity in response to seasonal changes i.e. precipitation and temperature. The value for alkalinity, Ca and Mg in winter season are generally higher than summer season in downstream region of river Indus in Pakistan. Similar variations were also observed for the river Indus from Ladakh Himalaya as well as for Ganga and Brahmaputra. The ratio of Ca/Na and Mg/Na observed by Quade et al. (2003) result in seasonal variable fractions of CO2 consumed by silicate weathering and observed silicate weathering contribution of ~ 15 -19% and 36 % during the rainy and non-rainy season, respectively [71,72]. These estimates are slightly higher than that reported by Galy and France- Lanord. The silicate weathering to be much more intense in huge Ganga basin as compared to higher Himalayan basin.
The rivers draining the Himalaya contribute significantly to the global sediment and water discharge. This has got attentions of many researchers in the past because of the possible connection between chemical weathering in the Himalaya and global climate. It is estimated that silicate weathering is possibly a global sink for CO2 on geologic time scales.
The major ion chemistry and chemical weathering in Ganga basin has been carried out by several workers, notably among them are Trevedi et al, Krishnaswami et al, Sarin et al 1992, Abbas and Subramanian etc. Sarin and Sarin et al carried out major ion and isotope chemistry of high land and low land river of Ganga and Brahamputra basin and observed that the chemistry of high land river was dominated by weathering of carbonate rocks present in the basin and silicate weathering in these drainage basins is of minor importance.
The seasonal variation in these rivers indicates that during lean flow (premonsoon season), the contribution from alkaline and saline salt affected soil is more pronounced and during peak flow (post monsoon), the chemical composition of these rivers closely follow that of “rock dominant” type of water. Study on major ion chemistry and weathering control in a high-altitude basin at Alaknanda River Garhwal Himalaya was carried out by. They observed that among anions, bicarbonate is the most dominating anion (78%) with minor contribution of sulphate (19%), and chloride (3%). Dalai et al., (2002a, b, c and 2003), carried out a part of detailed geochemical and isotopic investigation of the Yamuna River System (YRS) in the Himalaya (i) to characterize chemical weathering in the YRS basin and (ii) to assess relative mobility of elements during weathering and transport [73-75]. He found that carbonate weathering is contributing to major ion composition of Yamuna water. However, geochemical studies of sediments in the headwaters of rivers in the Himalaya are limited. Variable geochemical studies of river sediments in the Indian Himalaya include reconnaissance survey of the Ganga and the Yamuna and the Indus.
The sediment loads of Asian rivers are reported to be the highest in the world, delivering approximately 80% of the global sediment input to the oceans. A total of 1.8 Gt year-1 of suspended sediments or ~ 9% of the total annual load carried from the continents to the oceans (worldwide) are transported in three river systems, the Ganga, Indus and Brahmaputra, in a combined runoff of 1.19 x 103 km2. These large sediment loads are due to the exposure of the geologically young rocks forming the Himalayan mountain chain, which has the world's greatest range of relief and extremes of climate.
High seismicity, steep valleys with frequent avalanching, intense monsoonal rainfall and glacial activities support high erosion rates in the Himalayan catchments. Himalayan glaciers produce a large amount of rock debris and have large lateral moraines compared with glaciers in many other areas. Further, high velocity erosive glaciers driven by high gradients, accumulation, and ablation rates result in high concentrations of sediments in the melt water. Such glaciers are thought to be important active agents of erosion and sediment transport in regional denudation systems.
Silicon oxide is the most abundant component of the Earth’s crust. It occurs as a silicate minerals associated with igneous, metamorphic and sedimentary rocks. Weathering of silicate rock is also a measure of sources of alkalinity in river water. South Asian river basins are important in transferring silica and alkalinity to rivers and oceans because of geological and anthropogenic factors. Alkalinity load and dissolved silica is shown to be related with land use pattern and therefore prone to the anthropogenic alteration. Lesser Himalayan studies indicate that steep relief, resulting in abundant supply of eroded material, is not a limiting factor in case of Himalayan Rivers. The chemistry of' the sediments, in particular shales and other finer sediments such as loess, have become a very important tool for estimating the composition of the upper crust in a particular area, or on global scale [76]. This tool is being used in conjunction with other studies to understand crustal evolution. In the Himalayas, this approach has not previously been adopted, although preliminary information are now available on some of' the major Indian rivers including the Ganges and the Brahmaputra.
The Himalayan lake in Kumaun region namely: Nainital, Bhimtal, Sattal and Naukchiatal and found that water chemistry is dominated by Ca, Mg and HCO3 indicating carbonate lithology the major source of ions Chakrapani. He also studied major element geochemistry in upper Ganga River and found large seasonal variation. He observed that the water of the Ganga HCO3- SO42- and Ca2+ are the dominant ions which constitute > 60% of total water composition and about 5 -12 % of HCO3 and more than 80% Na+ and K+ were derived from silicate lithology indicating the predominance of carbonate lithology on the ionic composition of Ganga water [77-79].
The water quality of Himalayan region from Gangotri to Roorkee has been determined by using WQI index in four seasons i.e winter (41.6), summer (39.4), autumn (35.6) and monsoon season and found that water quality was in good condition in all the seasons except monsoon season (65.3). In order to study metal contamination he used metal index (MI) and pollution index (PI) for the metals Fe, Pb, Zn, and Cu and found MI: > 6 and PI: > 5) which indicated water quality has been severely deteriorated which possibly attributed changes in due to anthropogenic sources i.e industrialization, urbanization, mining, discharge of municipal solid waste and sewage waste into water bodies and construction of hydel power plants increased over the past few decades in Himalayan ecosystem.
Chemical Weathering Process
The control on the abundances of dissolved major cations (Na, K, Mg and Ca) and anions (HCO3, SO4 and Cl) in the water can be explained in terms of weathering of various rocks in the drainage basin. The dissolved concentrations in river water are derived from various sources including rock weathering, rainfall, glacial melt water, wind-blown dust but among all these sources, rock weathering dominate the resultant water composition. Plotting of the data as per the Gibb’s schemes (1970) also shows that the water chemistry of the study area is controlled by rock weathering. Rock weathering involves congruent and incongruent dissolution as per the following reactions.
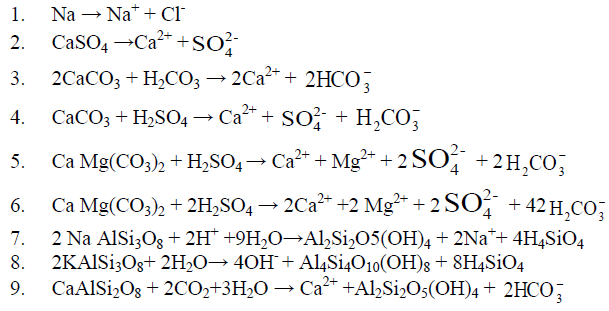
A source of proton is necessary for the rapid weathering of these carbonate minerals and the most common source of protons is carbonic acid (H2CO3, reaction 3). The relative proportion of ions in water dependent on their relative abundances in the host rock. The two major anions HCO3 and SO4 in surface water are mainly derived from dissolution of atmospheric CO2 in water and the oxidation of sulphides. These two reactions provide the bulk of protons which chemically weather carbonate, and silicates in the drainage basin. The dissolution of carbonates rocks proceeds more rapidly than silicate rocks. Some of the weathering reactions for carbonate minerals such as calcite (CaCO3), dolomite CaMg(CO3)2 and gypsum or anhydrite (CaSO4.2H2O, CaSO4) associated with sedimentary rocks are as follows:
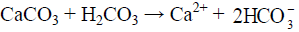
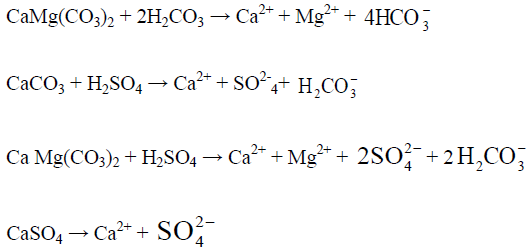
The equivalent ratio of Ca: HCO3 in entire study area (surface and groundwater) is 1.2 and that of surface water of Haridwar and Dehradun district is 1.09 and 0.95 respectively, suggesting calcite weathering as their source. The equivalent ratio of Ca: HCO3 of Ganga River at Haridwar and Risikesh is 1.09 and in Dehradun surface water it is 0.95.
The sulphuric acid is mainly derived from the oxidation of sulphate minerals such as gypsum and pyrites and from anthropogenic sources (Holland, 1978: Jickells etal, 1982). Pyrite which oxidized in a carbonate environment yields H2SO4 which is neutralized largely by Ca2+, Mg2, Na + and K+ in the solution. However, the solution products of silicate weathering are not well defined because the degradation of silicates generate a variety of solid phases (mostly clays) along with the dissolve species. Thus, the weathering of silicate rocks with carbonic acid is written as (Sarin1983)
(Na, K, Mg, Ca+CO2 +H2O) Silicate → H4SiO4 + HCO3- +Na+ +K++Mg2+ +Ca2++ Solid products
Since the abundance of cations and anions released to the solution are determined by the bulk composition of the parent rock and the availability of protons for rock weathering, the relative proportion of Ca2+ and HCO3, released during the weathering of calcium silicate (Ca feldspar) can be same as that resulting from calcite dissolution. Therefore, it is difficult to find out the origin of Ca in the water based on water chemistry alone. Additional information on geology of the terrains is required to distinguish the relative inputs of Ca and Mg from weathering of carbonate and silicates. In addition to the inputs of Ca, Mg and HCO3, there are other reaction product such as SiO2, Na and K which are released to the solution during silicate weathering.
In case of silicate weathering, all the alkalinity produced is of atmospheric origin whereas in case of carbonate weathering it is only half of it. Therefore, silicate weathering on land represents an important sink for atmospheric CO2 and is of special interest within the context of controlling the concentration of CO2 in the atmosphere and ocean over geological time scales Silicate weathering is dependent on temperature and precipitation and possibly would be enhanced through global warming. The ratio of (Ca+Mg):(Na+K) is 6 in Indus river which is more than twice the world average (2.2) implying that weathering of carbonate lithology is major factor in water composition in the basin.
Another factor affecting weathering is the erosion rate, i.e., the delivery of potentially weatherable material. In Outer and Lesser Himalaya abundant supply of and resulting eroded material is not a limiting factor in case of Himalayan Rivers due to steep relief.
The extent of silicate weathering borne out from (Na*+K): TZ+ ratio (0.10) indicates contribution of silicate weathering is limited to ~ 10 %. It is difficult to estimate contribution of cations through silicate weathering. A rough estimate of the contribution from silicates obtained using the (Na*+K):TZ ratios (Na signifies sodium corrected for chloride) as an index of silicate weathering indicates that the sodium and potassium derived from silicate weathering can account about 9 % in all three season viz. summer, winter and post monsoon season (Bartarya 2012, Bahukhandi 2017). The low abundance of SiO2, 73 μmole/l to 136 μmole/l with mean value of 110 μmole/l and low SiO2: HCO3 molar ratios (0.044 to 0.09 with mean value of 0.06) also indicate the contribution of ions from silicate weathering is very less in Ganga basin (Bartarya 2012, Bahukhandi 2017, Divya 2011). Na+ and Cl- when derived from rainfall or halite deposit, show a molar ratio of 1. The molar ratio of Na/Cl of Ganga water at Haridwar vary from 0.3 to 1.4 with mean value of 1.05 indicating either rainfall or halite dissolution as their source.
Seasonal variation in river water composition due to chemical weathering has been documented by Raymond and Cole (2003) for Mississippi river, Stallard and Edmond for Amazon River and by Subramanian (2000) for several rivers in the Indian subcontinent. The weathering of silicate rocks and its product such as clay are the main causes of alkalinity variation in river water and the process of weathering depends upon temperature, precipitation and human activities. Considerate excess of Na over Cl and higher Na/Cl molar ratio ranging from 1.4 to 7.3 (mean 3.6) suggest that a much of the Na and K has a source other than precipitation possibly through rock weathering. Since Himalayan rivers are far away from the sea, the cyclic contribution through precipitation is not of significant importance. This suggests that Na could be derived from either evaporitic dissolution or silicate weathering.
Temporal Variation in Water Quality
The spatial variation in water quality due to land use pattern have been carried out by. In order to understand the temporal variation the water quality data of past then years were collected by State Pollution Control Board (SPCB) (Table 1).
| Location | pH | Temperature | Range EC µ Simes/cm |
TDS (mg/l) | BOD (mg/l) | COD (mg/l) | Total Coliform/100 ml | Alkalinity (mg/l) | Cl (mg/l) | Ca (mg/l) | Mg (mg/l) | Total Hardness (mg/l) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ganga Haridwar | 192 - 195 | 97.0 - 167 | 0.5-3 | 4.0- 6.0 | 38-2419 | 60 - 120 | 1.2 - 10 | 17.32 -34 | 2.12- 12.7 | 38.4- 138 | ||
| Ganga River Laxamjhula | 7.3- 8.6 | 7.7 -14 | 148 - 173 | 70 - 114 | 0.8-1.63 | 4.0 -4.2 | 2 - 108.09 | 129 -130 | 2.8 - 8 | 16.7 - 25.2 | 1.53 -8.64 | 30.5 - 170 |
| Yamuna (Dakhpather) | 7.7- 7.9 | 6.6 - 16.72 | 173 - 228 | 93 - 200 | 0.8-2.2 | 4 .0 - 6.0 | 4.0 - 230 | 70 - 90 | 3.17 - 10 | 18 - 30 | 2 - 9.36 | 30 - 115 |
| Alaknanda | 6.62- 8.2 | 15 -23 | 463.0 - 463.2 | 63.2 - 301 | 0 -2 | 4 | 2.0 - 40.0 | 50- 100 | 5.25 - 12.5 | 16.0 - 32 | 14 -14.1 | 64 - 87 |
| Bhagirithi (Gangotri) | 7.71 -8.2 | 5 - 15.75 | ND | 55.8 - 56 | 0-1 | 4 | 0 | 58 - 62 | 5- 5.5 | 17.5 -17.6 | 5.04 - 9.12 | 64 - 110 |
| Bhagirithi | 6.7-8.4 | 12.0- 16.0 | 116.0 - 116.1 | 84.0 - 84.1 | 0.25-1.2 | 4 | 0 | 44 - 65 | 4.0 - 8.0 | 30 - 30.1 | 6.0 - 6.24 | 67 - 73 |
| Mandakini | 7 - 7.1 | 12.0 - 16.0 | 156.9 - 157 | ND | 0.47 -1.2 | 4 | 0 | 78 - 80 | 5.0 - 8.0 | 19.6- 46 | ND | 105 -106 |
| Nainital Lake | 7.6 -7.9 | 12.0 -19.0 | 390 - 477.5 | 287 - 290 | 1.4-2 | 4.0 - 6.8 | ND | 194 - 260 | 5.0 - 17 | 42 - 66.4 | 5.9 - 33.12 | 67 - 230 |
| Bheemtal Lake | 7.46 -7.8 | 14.0 - 20.0 | 170 - 225.1 | 121 - 130 | 1.2-1.8 | 5.08 - 7.36 | ND | 121 -136 | 11 - 25.0 | 33.2- 49.2 | 2.8 - 18.24 | 174 - 312 |
| Gola river Haldwani | 7.35- 7.7 | 14 .0 -12.0 | 180 - 230 | 160 - 166 | 1.3 -3.6 | 10.0 - 24.0 | ND | 125 - 148 | 11.0 - 22.58 | 33.2 - 49.2 | 14.8 - 26.7 | 121 - 206 |
| Kosi River (Kasipur) | 7.21-7.41 | 12 -24.89 | 334 - 750 | 503.1 - 503.4 | 3.87 - 6.8 | ND | ND | ND | 19.0 - 21.66 | 33.2 -39.2 | ND | 147 - 242 |
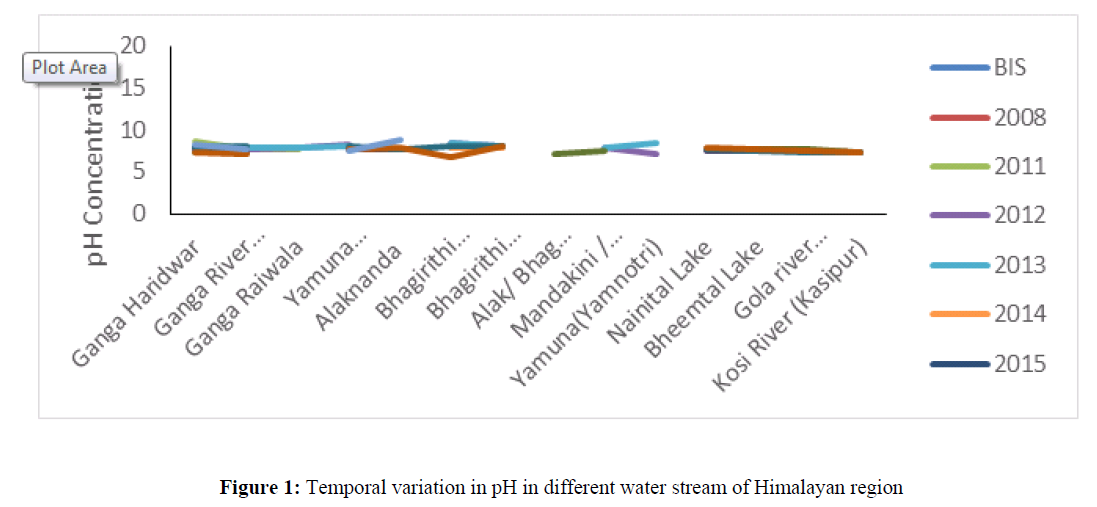
The pH concentration in all sampling location was ranged from 6.7 to 8.38 (Figure 1) which was found under permissible limit (6.5 to 8.5) of BIS standard of drinking water quality. The pH is an important variable in water quality assessment as it influences many biological and chemical processes within a water body and all processes associated with water supply and treatment. When measuring the effects of an effluent discharge, it can be used to help determine the extent of the effluent plume in the water body.
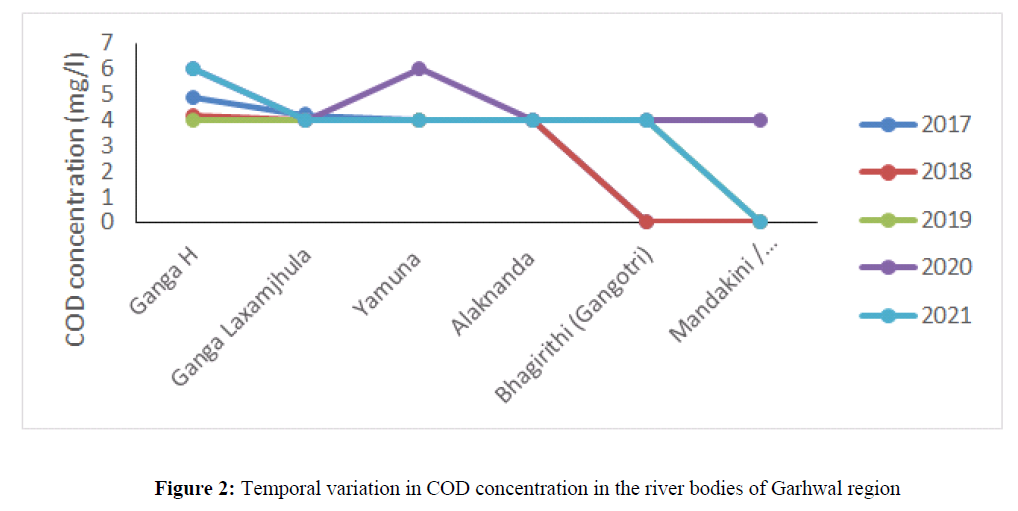
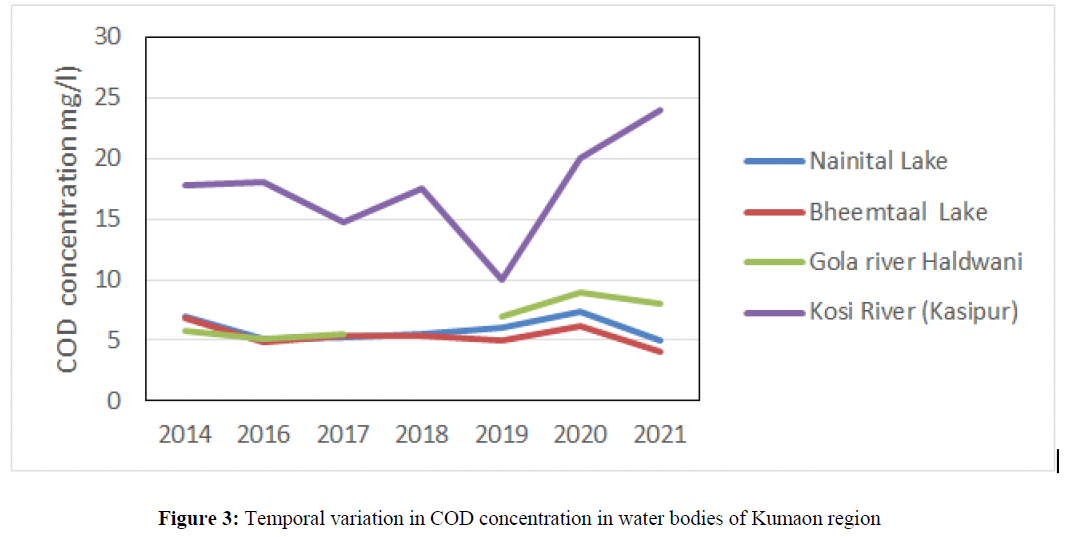
The COD concentration in water bodies indicate presence of organic pollution in water bodies. The COD concentration was 4.8 mg/l in 2017 at Ganga River Haridwar which has increased up to 6 mg/l in the year 2020 and 2021. The high concentration of COD was attributed due to anthropogenic sources i.e. discharge of industrial effluent and sewage waste into the water bodies (Matta 2021). The COD concentration did not show any temporal variation in and found ~ 4 mg/l in river Alaknanda, Bhagirithi, Mandakani in the year 2017, 2018, 2019, 2020 and 2021 (Figure 2). The COD concentration in Nainital Lake was ranged from 5 mg/l to 7 mg/l, in Bheemtal it varied from 4 mg/l to 6.8 mg/l and in Gola River it varied from 5.08 mg/l to 9 mg/l in the year 2014, 2016, 2017, 2018, 2019, 2020 and 2021 (Figure 3). The decrease trend of COD was observed in the year 2021 as compared to the year 2020 which may attribute due to lockdown imposed in 2020 and 2021 leading the industries to shut down, closing of tourism industries which possibly reduced sewage waste and solid waste into the water bodies. The concentration of COD has shown temporal variation in Kosi River and it was 17.8 mg/l in 2014, 18 mg/l in 2016, 20 mg/l in 2020 and found highest 24 mg/l in 2021.
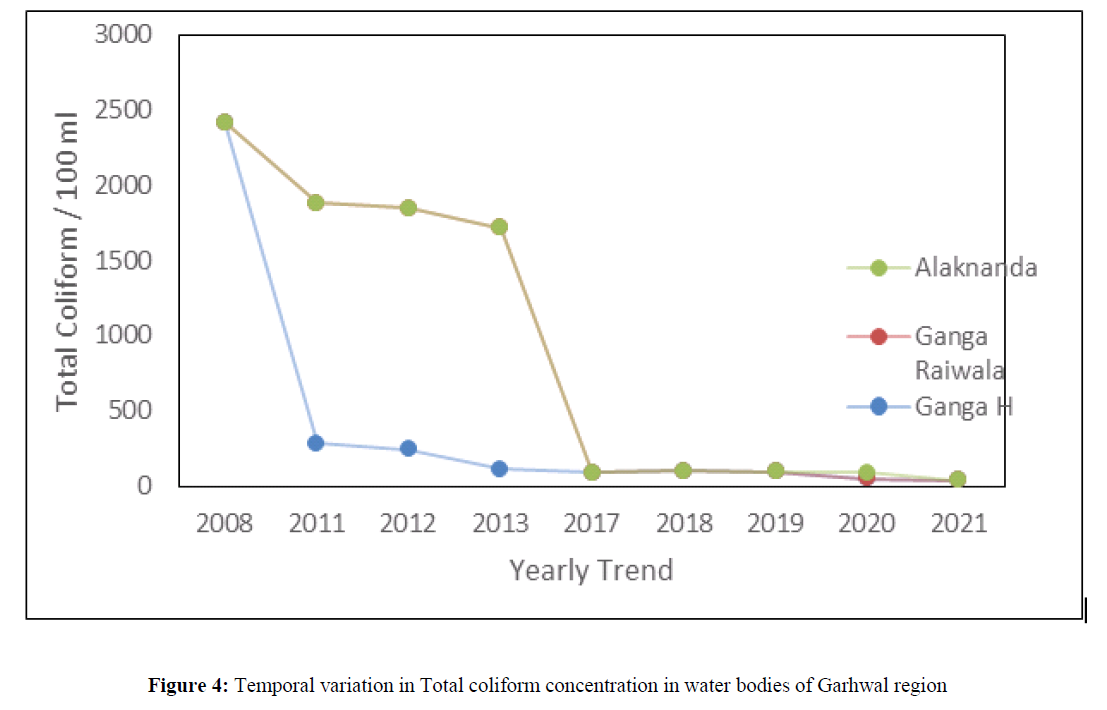
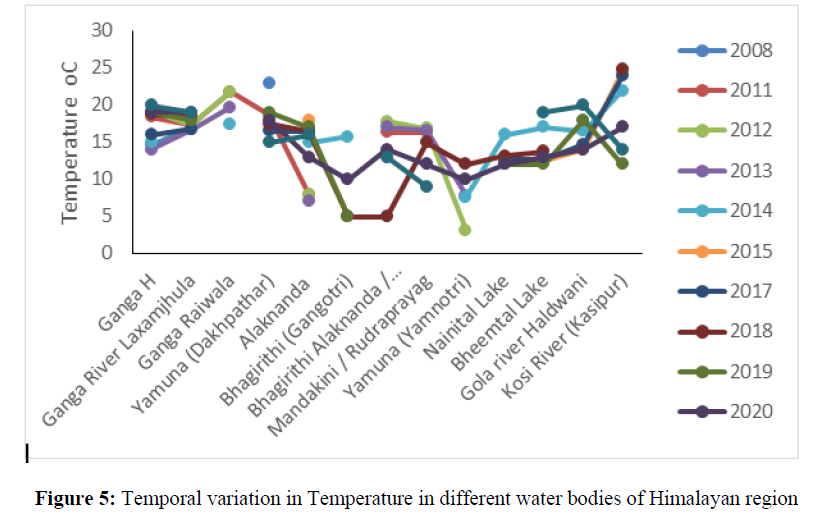
The total coliform number was ranged from 2 to 2419 during 2008, 2011, 2012, 2013, 2017, 2018, 2019, 2020 and 2021 in the river Ganga, Yamuna, Alaknanda and Bhagirithi and crossed the maximum permissible limit of (total count 0 in per 100 ml sample) BIS Standard of drinking water quality (IS 10500:2012) (Figure 4). The presence of total coliform bacteria in water indicates presence of pathogenic organisms such as Clostridium pafringens, Salmonella, and Protozoa. These pathogens cause various water borne disease such as diarrhea, giardiasis, dysentery, and gastroenteritis. The temperature ranged from 7ºC to 24.6ºC between the years 2008 to 2021 in different water bodies i.e Ganga, Yamuna, Alaknanda, Mandakini, Nainital, Bheemtal, Kosi and Gola river of Himalayan region (Figure 5). The temperature is one of the important physical parameter for aquatic communities. The different plankton groups flourish under different temperatures for example diatoms dominate at 20ºC - 25ºC, green algae dominate at 30ºC – 35ºC, and cyano-bacteria dominate above 35ºC.
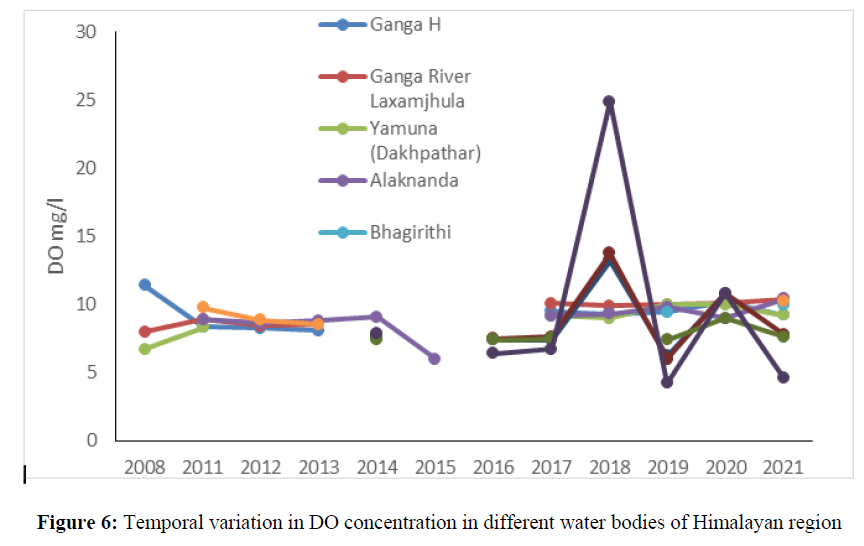
The DO concentration in water bodies are affected by various physical, chemical and biological processes and also indicate temporal, spatial and seasonal changes which can be attributed due to anthropogenic activities. The DO concentration was 11.4 mg/l in 2008 at Ganga River which decreased up to 9.2 mg/l in 2021. In Yamuna, Alaknandam Bhagirithi and Mandakini river, the DO concentration ranged from 6 mg/l to 10.4 mg/l from the year 2008 to 2021 (Figure 6). The DO concentration ranged from 4.2 mg/l (at Kosi River) to 13.75 mg/l (Bheemtal Lake) from the year 2014 to 2021. The DO concentration less than 2 mg/l is considered lethal for aquatic biodiversity. A low DO (less than 2mg/l) would indicate poor water quality and thus would have difficulty in sustaining many sensitive aquatic life.
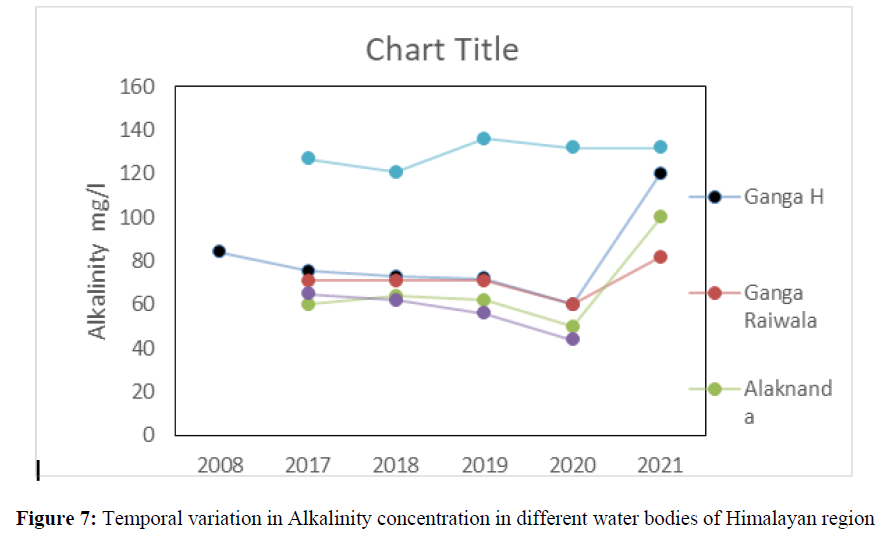
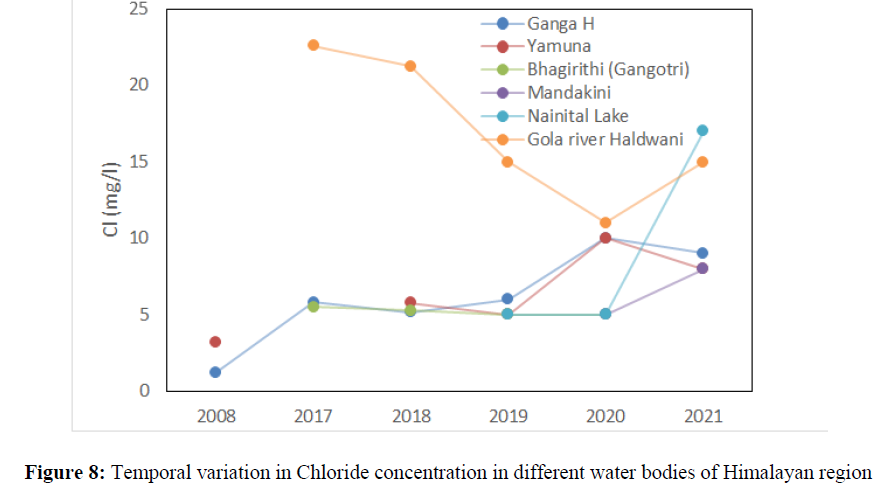
The alkalinity concentration was 84 mg/l in 2008, 76 mg/l in 2017 and 120 mg/l in 2021 in Ganga River at Haridwar. In Alaknanda the alkalinity ranged from 50 mg/l to 64 mg/l in the year 2017, 2018, 2019, and 2020 while it increased up to 100 mg/l in in the year 2021. The highest concentration of Alkalinity was found in Nainital lake 126.83 mg/l in 2017, 214 mg/l 2018, 260 mg/l in 2019, 256 mg/l in 2020 which further reduced to 194 mg/l in 2021 (Figure 7). The alkalinity has crossed the acceptable limit (200 mg/l) at Nainital lake in the year 2018 (214 mg/l), 2019 (260 mg/l) and in the year 200 (256 mg/L) but found under permissible limit (600 mg/l) of BIS standard of drinking water quality. The chemical weathering is one of the major causes of high concentration of alkalinity in Himalayan River. The chloride concentration was ranged from 1.2 mg/l to 12.5 mg/l in Ganga river, Yamuna river, Alaknanda, Bhagirithi, and Mandakini river between the year 2008 to 2021 (Figure 8). In 2008 the chloride concentration was 1.2 mg/l and in 2021, it increased up to 9 mg/l, in Yamuna its concentration was 2.8 mg/l and increased up to 8 mg/l while in Bhagirithi River the chloride concentration was 5.5 mg/l in 2017 and increased up to 12.5 mg/l in 2021. The higher concertation in the year 2020, 2021 possibly attributed due to changes in land use pattern and increased anthropogenic activities in Himalayan region [80-85].
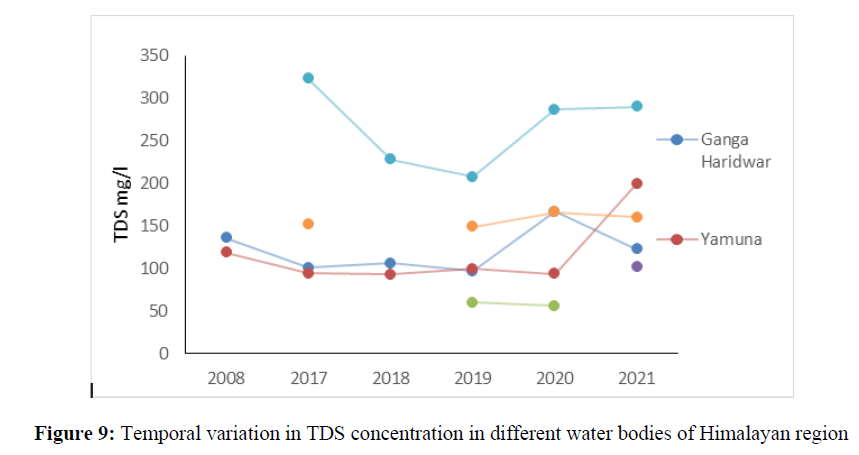
The TDS concentration which was 63 mg/l in 2017 increased up to 301 mg/l in 2021 indicating temporal variation caused by changes in the land use pattern over the years in Himalayan ecosystem. The high TDS concertation was found in Nainital Lake in the year 2017 (323 mg/l), 2020 (287 mg/l) and 2021 (290 mg/l) and found under permissible limit of BIS 2012 standard of drinking water quality.
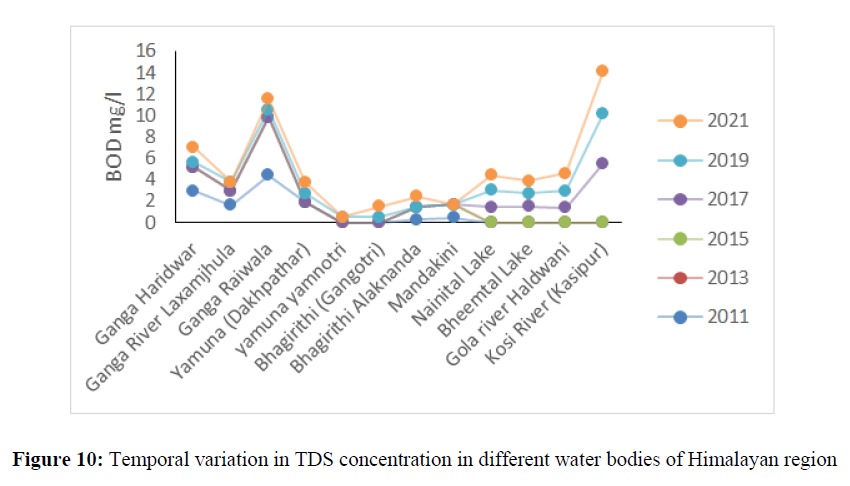
The BOD (Biological Oxygen Demand) indicates the organic pollution concentration in the water bodies is largely affected by sewage waste, industrial effluent and dumping of waste into water bodies. The BOD concentration ranged from 0.25 mg/l to 6.28 mg/l in Ganga River, Yamuna, Alaknanda, Bhagirhithi and Mandakini river between 2011 to 2021 (Figure 9). The BOD concentration in Nainital, Bheemtal Lake and in Gola River varied from 1.2 mg/l to 1.52 mg/l between the year 2014 to 2021. The higher concentration of BOD was found in Kosi river and ranged 1.3 mg/l to 5.7 mg/l from the year 2014 to 2021 (Figure 10).
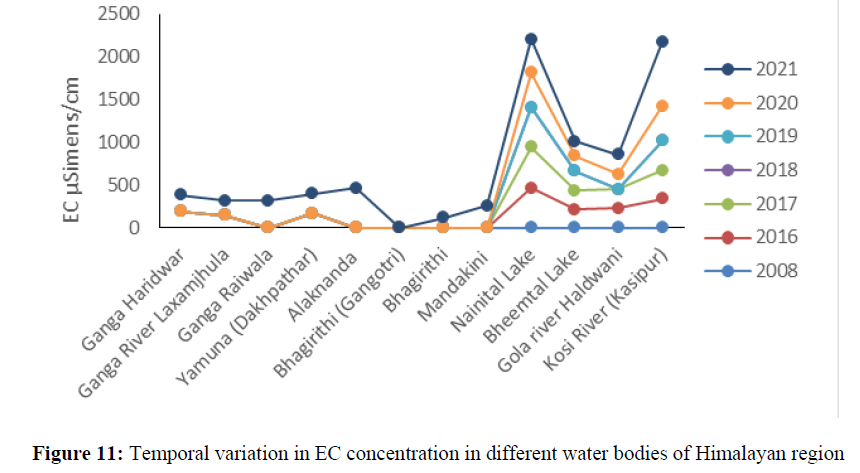
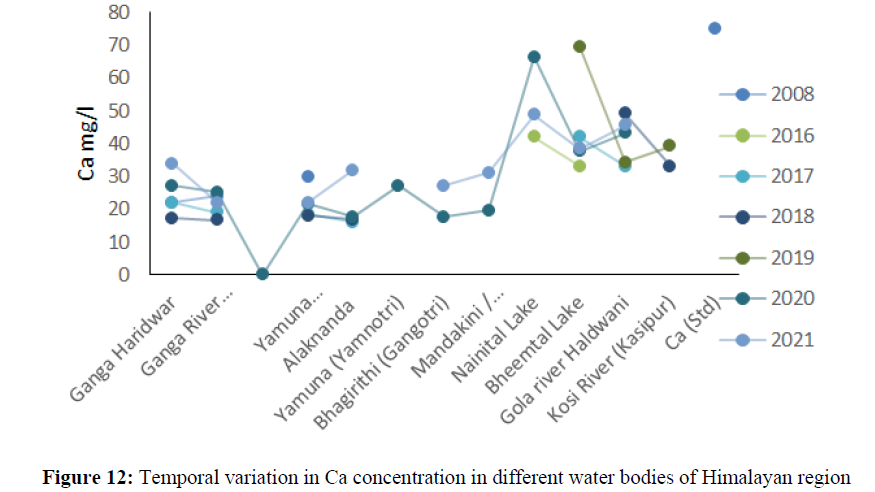
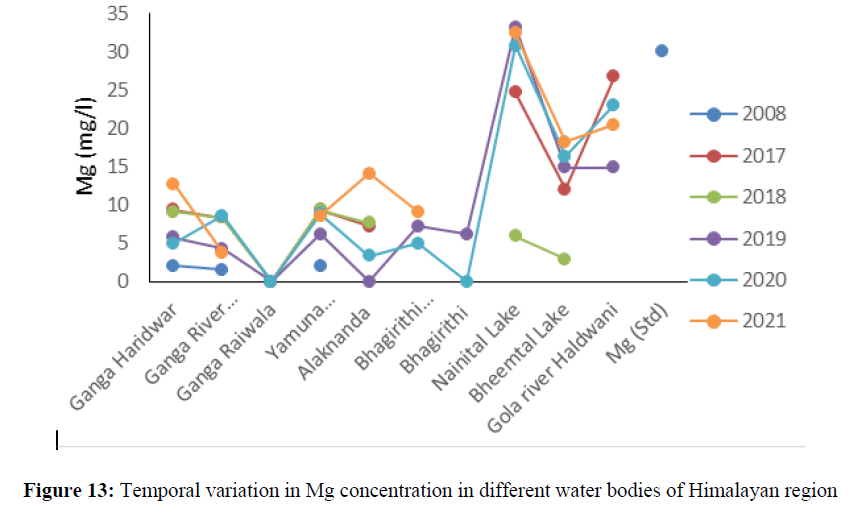
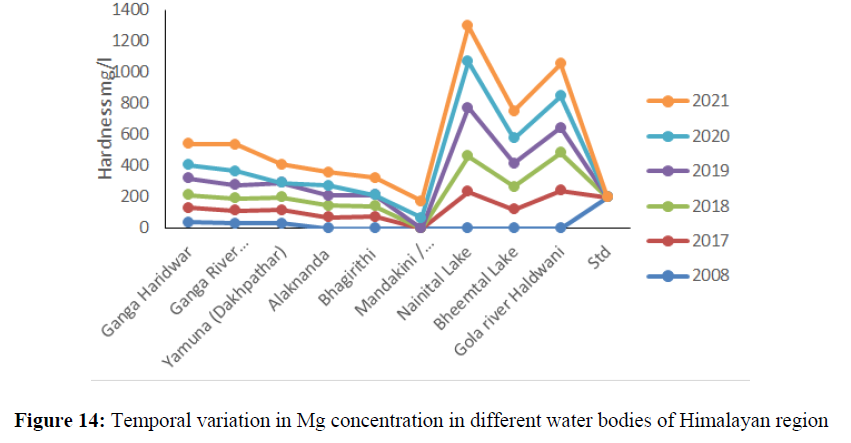
The higher concentration of EC was found in Nainital lake 390 mg/l to 466.6 mg/l between the year 2016 to 2021 (Figure 11). The calcium concentration ranged from 22 mg/l to 69.6 mg/l between the years 2008 to 2021 in all the sites and found under permissible limit of BIS Standard (75 mg/l) of drinking water quality (Figure 12). The magnesium concentration ranged from 1.53 mg/l to 8.64 mg/l between the years 2008 to 2021 in all water bodies and found under permissible limit of BIS Standard (30 mg/l) of drinking water quality. The hardness concertation has shown increasing trend over the years and in 2008 it was recorded 38.4 mg/l and increased up to 138 mg/l in 2021. The chloride concentration in Yamuna river was 30 mg/l in 2008 and increased up to 115 mg/l and in Mandakini and Bhagirithi river its concentration was 71 mg/l, 74 mg/l in the year 2008 which was increased 110 mg/l and 106 mg/l in the year 2021and found under permissible limit (200 mg/l) of BIS standard of drinking water quality (Figure 13) and (Figure 14). The hardness concentration in Nainital Lake ranged from 230 mg/l to 312 mg/l between the years 2017 to 2021 and crossed the required acceptable limit (200 mg/l) but found under permissible limit (600 mg/l) of drinking water quality standard.
Conclusion
The rock weathering is one of the important factors for controlling the hydrochemistry of the region. However, due to changes in land use pattern over the last few decades because of increase in dominance of anthropogenic factors has greatly influenced the water chemistry of the region. The bicarbonate weathering is the major source of ions followed by silicate weathering. Among the anions, the HCO3 was the most dominant ion followed by SO4, NO3, PO4 and F and among the cations, Ca is the dominant source of ions followed by Mg, Na and K. The factor analysis indicated two factors influencing the water chemistry- one naturally occurring lithological process cause dominance of HCO3, Ca and Mg ions and the other factor is due to changes in land use pattern which caused the increased concentration of SO4, NO3, Cl and K in the water bodies. The concentration of i.e. chloride, hardness, magnesium, calcium, electric conductivity and alkalinity has drastically increased in past one and half decades and shown temporal variation in water bodies of Himalayan region in past decade. The higher concentration over the years may be attributed with changes in land use pattern which drastically changes the hydrochemistry of the region. The parameter i.e. alkalinity, TDS, hardness has crossed the required acceptable of BIS 2012 standard of drinking water quality but found under permissible limit of BIS 2012 standard of drinking water quality. The total coliform crossed the under permissible limit of BIS 2012 standard of drinking water quality. The parameter pH, alkalinity, Ca and Mg was found under permissible limit of BIS 2012 standards.
References
- Thliza A, Khan AU, Dangora DB et al., Curr Sci Int. 2015, 14: p. 91–97.
- Abbas and Subramanain. Hydrol. 1984, 69: p. 3-182.
- Abok Elisha Onyango, Michael Wandayi Okoth, Catherine Nkirote Kunyanga et al., J Environ Public Health. 2018, p.10.
- Addiscott TM and Thomas D. Soil Till Res 273cation. 2000.
- Ali SA and Ali U. Appl Water Sci. 2018, 8: p. 39.
- Altaf F, Meraj G and Romshoo SA. Geogr J. 2013.
- Amman AA, Michalke B and Schramel P. Anal Biochem. 2002, 372: p. 448–452.
- American Public Health Association, 1996
- Arora S, Bahukhandi KD and Mishra PK. Sci Total Environ. 2020, 74: p. 140573.
- Bahukhandi KD, Saini R, Ahuja NA et al., Himalayan Geology. 2021, 42(1): p. 175-188.
- Bahukhandi KD, Kumar S, Siddiqui NA et al., J chem pharm res. 2018, 10(7): p. 145-150.
- Bahukhandi KD and Siddiqui NA. Environ Pollut. 2008, 12(1).
- Bahukhandi KD and Bartarya SK. 2012, 3(74): p. 1109-1119.
- Bahukhandi KD and Bartarya SK. J Environmental Research. 2014, 2(2): p. 168-177.
- Bahukhandi KD, Mondal P and Singh S. Int J Sci Res. 2015, 5(5): p. 1-8.
- Bahukhandi KD and Aaron A. Ind J Global Ecology and Environment, 2017, 5(3): p. 133-143.
- Bahukhandi KD, Bartarya SK and Siddiqui NA. Int J Chemtech Res. 2017, 10(10): p. 95-118.
- Bahukhandi K, Siddiqui NA and Arora S. Advances in Water Pollution Monitoring and Control. 2018.
- Kanchan Bahukhandi, Shilpi Agarwal & Shailey Singhal. Int J Environ Anal Chem. 2020.
- Bartarya SK. J Hydrol. 1993, 146: p. 149-174.
- Bartarya SK and Bahukhandi KD. GJEDT. 2012, 1(1): p. 11-22.
- Bhat SA, Meraj G and Pandit AK. Arab J Geosci. 2016, 9: p. 50.
- Bhat SA, Meraj G, Yaseen S et al., Int J Environ Sci. 2013, 3(5): p. 1625–1640.
- Bhat SA, Meraj G, Yaseen S et al., J Ecosyst. 2014.
- Bhatt KP and Saklani S. Arab J Geosci. 1996, 48: p.171-182.
- BIS (Bureau of Indian Standards). Specification for drinking water IS 10500: New Delhi, 2012.
- Biswas S, Sudhakar S and Desai VR. J Indian Soc Remote Sens. 1999, 27(3): p.155–166.
- Chakrapani GJ, Subramanian, Gibbs RJ. Et al., Environ Geol. 1995, 25: p. 192-196.
- Chakrapani GJ. Environ Geol. 2005, 48: p. 189-201.
- Chakrapani G. India. Env Geol. 2002, 43: p. 99–107.
- Deborah Chapman. Water Quality Assessments - A Guide to Use of Biota, Sediments and Water in Environmental, 1996.
- Deoli Kanchan Bahukhandi and Bartarya SK. International Conference on Chemical, Biological and Environmental Engineering 2012, 3: p. 1109- 1119.
- Durum WH and Haffty J. Geochim Cosmochim Acta. 1963, 21: p. 1
- SHARMA E, BHUCHAR S, XING MA et al., International Society for Tropical Ecology, 2007.
- Sharma E, Bhuchari S and Maxing G. Tropical Ecology. 2007, 48(2): p. 151-161.
- Faith Ngwenya. J Biosci. 2016, 5(9): p. 4556-4567.
- Gibbs RJ. Science, 1970, 170: p. 1088.
- Hooijer A, Klijn F, Pedroli GBM et al., River Res Appl. 2004, 20(3): p. 343–357.
- Hu M, Stallared RF and Edmond JM. Nature. 1963, 298: p. 550-553.
- Ramesh K and Bhuvana Jagadeeswari P. Res J Environment Sci. 2012, 1(1): p. 19-27.
- Kim J, Li W, Philips BL et al., J Biol Sci. 2011, 4(10): p. 4298–4305.
- King JM, Scheepers ACT, Fisher RC et al., Report to the Water Research Commission, 2003.
- Kumar A, Matta G and Bhatnagar S. Environ Sci Pollut Res. 2021.
- Kumar M, Chauhan R and Ramanathan. Environmental Earth Sciences. 2007, 57(4): p. 873-884.
- Livingstone DA. Geochim Cosmochim Acta. 1963, 27: p. 1055.
- Sarin MM, Krishnaswami S, Dilli K et al., Cosmochimica Acta, 1989, 5 (5): p. 997-1009.
- Malarkodi M and Krishnaswamy. International Ground Water Conference. 2007.
- Matta G, Kumar A, Nayak et al., Arab J Geosci. 2020, 13: p. 1027.
- Meraj G, Romshoo SA, Ayoub S et al., Geocarto Int. 2018, 33(10): p. 1114–1138.
- Meraj G, Romshoo SA and Yousuf AR. Int J Curr Res Rev. 2012, 04(16): p. 47–61.
- Meraj G, Yousuf AR and Romshoo SA. Jammu and Kashmir Environmental Information System.
- Meybeck M and Ragu A. UNEP. 1995
- Meybeck M. (1976). Hydro Sci Bull. 1976, 21: p. 265.
- The Ministry of Environment and Forests Government of India. 2014
- Mumtazuddin S, Azad AK, Bharti Prabhat et al., Res J Environmen Sci. 2012, 1(1): p. 7-11.
- Anyamene NC and Ojiagu DK. Int J Agric Biol. 2014, 3: p. 120–122.
- Semwal N and Akolkar P. Source: Current Science. 2006.
- Nitin Kamboj and Vishal Kamboj. Water Science. 2019, 33: p. 65-74.
- Oludairo O and Aiyedun J. BJVM. 2016. 13: p. 73–81.
- Pande K, Sarin MM, Trivedi JR et al., Chem Geol. 1994, 116: p. 245-259.
- Parekh VP and Patel AS. Gujrat International Ground Water Conference. 2007.
- Parekh VP and Patel AS. Gujrat International Ground Water Conference. 2007.
- Paul D. Ann Agrarian Sci. 2017, 15: p. 278–286.
- Rajiv P, Hasna Abdul Salam, Kamaraj M et al., India I Res J Environmen Sci. 2012, 1(1): p. 2-6.
- Pradhan B. J Spat Hydrol. 2010, 9(2): p.1–18.
- Probst A, El Gh A, Mari B et al., Chemical Geology. 1998, 170: p. 153-177.
- Rai SC, Sharma E and Sundriyal RC. Environmental Conservation. 1994, 21: p. 30-34.
- Bhattacharya T, Chakraborty S and Tuck Neha. I Res J Environmen Sci. 2012, 1(1): p. 28-33.
- Ramesh K and Elango L. International Ground Water Conference. 2007.
- Ramkumar T, Venkarramanan S, Anithamary I et al., Arab J Geosci. 2012, 6: p. 101–108.
- Rashid I and Romshoo SA. Environ Monit Assess. 2013, 185: p. 4705–4719.
- Raymahasay BS. hydro geochemistry and biogeochemistry. 1970, 1: pp. 82-89.
- Sarin MM, Krishnaswami S, Sharma KK et al., Cuu Sci. 1992, 62(12): p. 801-805.
- Sarin MM, Krishnaswami S, Trivedi JR. Hydrological Sciences. 1992, 43(5): p.112-120.
- Sarin MM and Krishnaswami,S. Nature. 1994, 312: p. 538-541.
- Shivayogimath CB, Kalburgi PB, Deshannavar UB et al., I Res J Environment Science. 2012, 1(1): p. 12-18.
- Shukla AK, Ojha CSP, Garg RD et al., India J Hazard Toxic Radioact Waste. 2020. 24: p. 04020028.
- Singh JS, Pandey AN and Pathak PC. Environ Conserv. 1983, 10: p. 343-345.
- Skilodimou H, Livaditis G, Bathrellos G et al., Geogr Ann A. 2003, 85A(2): p. 197–204.
- Subramani T, Elango L, Damodarasamy SR. Environ Geol. 2005, 47: p. 1099–1110.
- Tiwari Seema, Int j eng. 2015, 9.
- Turekian KK. Jhon Wiley and Sons, Newyork, 1971, p. 9-73.
- Turekian KK. Handbook of Geochemistry Springer Verlag, Berlin, 1971, p.297-323.
- UCOST (Uttarakhand State Council for Science and Technology). 2012.
- Valdiya KS. Dynamic Himalaya. University Press, Hyderabad, India. 1998.