Research Article - Der Pharma Chemica ( 2018) Volume 10, Issue 6
In Vitro Evaluation of Monohalogenated Semicarbazones and Thiosemicarbazones as Potential Cytotoxic Agents Induction of Apoptosis and Genotoxicity
Luis P Morera1*, Rodrigo Novoa1, María S Di Genaro2 and Diego A Cifuente1
1Area of Organic Chemistry, INTEQUI-CONICET, Faculty of Chemistry, Biochemistry and Pharmacy, National University of San Luis, Chacabuco 917, 5700, San Luis, Argentina
2Area of Microbiology and Immunology, Faculty of Chemistry, Biochemistry and Pharmacy, National University of San Luis, Chacabuco 917, 5700, San Luis, Argentina
- *Corresponding Author:
- Luis P Morera
Area of Organic Chemistry
INTEQUI-CONICET
Faculty of Chemistry, Biochemistry and Pharmacy
National University of San Luis
Chacabuco 917, 5700, San Luis, Argentina
Abstract
A series of halogenated Semicarbazones (SCs) and Thiosemicarbazones (TSCs) (11-30) were synthesized from mono fluorinated-, bromine- and chlorinated acetophenones (1-10). Structures were confirmed by Nuclear Magnetic Resonance (NMR) spectral data. Both effects, the halogenated substituent and the position of the substitution on the antiproliferative activity, were systematically investigated for the first time. Cytotoxic activity was evaluated, using tetrazolium salt method (MTT), in two murine cell lines: CT26 (colon cancer) and B16 (melanoma). Only, o-, m- and p-fluorinated SCs and TSCs showed significant cytotoxic activity. Among them, compounds with fluorine at m-position in the phenyl ring showed the superior antiproliferative activity. The most actives derivatives were: m-Fluoroacetophenone semicarbazone (13) (μM; IC50 =7.2 ± 0.5, IC50=8.1 ± 0.2) and m-Fluoroacetophenone Thiosemicarbazone (23) (μM; IC50 = 3.1 ± 0.4, IC50=4.9 ± 0.5) in CT26 and B16, respectively. In addition, studying the genes Bcl-2 and Bax, compound 23 showed apoptosis induction and non-genotoxic properties.
Keywords
m-Fluorosemicarbazones, m-Fluorothiosemicarbazones, Cytotoxic Activity, Apoptosis induction
Introduction
Semicarbazones (SCs) and Thiosemicarbazones (TSCs) are a class of compounds that have shown great interests by their pharmacological properties and for their uses as antibacterial (Khana and Asiri, 2017), antiviral [1], anticonvulsants [2] and anti-protozoa agents [3]. It is known that the pharmacological activity of SCs and TSCs depends on the chemical moieties linked to the functional group. Antiproliferative and cytotoxic activities have benn described for SCs, TSCs and their derivatives analogues [4-6].
Particularly, fluorinated compounds have shown an important role as anticancer agents [7]. Fluorine-containing anticancer agents can certainly serve as potent warheads for the guided molecular missiles [8].
In the present paper, we study the antiproliferative effects of a series of SCs and TSCs synthesized from o-, p- and m-monohalogenated acetophenones. The cytotoxic activity was evaluated in cell lines CT26 (murine colon cancer) and B16 (murine melanoma). Only, the SCs and TSCs containing fluorine at o-, m- and p-position, showed a promising broad-spectrum cytotoxic activity. In addition, induction of apoptosis, for the most active compound, was evaluated studying the genes Bcl-2 and Bax by reverse transcription polymerase chain reaction (RT PCR). Finally genotoxicity was tested in agarose gel electrophoresis.
Material and Methods
General procedure for SCS and TSCs preparations
Acetophenones (1-10) and reagents were purchased from Sigma-Aldrich. SCs and TSCs were prepared from compounds 1-10 using semicarbazide hydrochloride and sodium acetate in ethanol or thiosemicarbazide and acetic acid (1%) in methanol (Figure 1). The reaction was refluxed for 10 h and monitored by Thin Layer Chromatography (TLC) analysis (TLC aluminum sheets, Merck, Silica gel 60, F254) with detection by iodine vapor. The products obtained (11-30) were filtered and recrystallized from 95% ethanol; their structure were confirmed by Nuclear Magnetic Resonance (NMR) spectral data. 1H and 13C-NMR spectra were obtained in a Bruker Spectra AC-200 (200 MHz, 50 MHz) in DMSO-d6.
Acetophenone Semicarbazone (a-SCs) (11)
Colorless crystals in 97% yield; NMR δH (ppm): 9.30 (s, 1H, -NH-), 7.80-7.58 (m, 5H, Ar-H), 6.40 (brs, 2H, -NH2, D2O exchangeable), 2.25 (s, 3H, -CH3); NMR δC (ppm): 157.8 (C=O), 147.9 (C=N), 140.7, 138.2, 126.1, 126.1, 125.8, 125.8, 13.2. EIMS (m/z): 177 (M+.).
o-Fluoroacetophenone Semicarbazone (2-F-a-SCs) (12)
Colorless crystals in 99% yield; NMR δH (ppm): 9.51 (s, 1H, -NH-), 7.96-7.87 (m, 4H, Ar-H), 7.79 (brs, 2H, -NH2, D2O exchangeable), 2.38 (s, 3H, -CH3); NMR δC (ppm): 162.6 (s), 157.3 (C=O), 142.8 (C=N), 133.2, 129.9, 126.5, 125.2, 116.1, 13.1. EIMS (m/z): 195 (M+.).
m-Fluoroacetophenone Semicarbazone (3-F-a-SCs) (13)
Colorless crystals in 99% yield; NMR δH (ppm): 9.52 (s, 1H, -NH-), 8.09-7.95 (m, 4H, Ar-H), 7.91 (brs, 2H, -NH2, D2O exchangeable), 2.31 (s, 3H, -CH3); NMR δC (ppm): 162.8, 157.1 (C=O), 142.6 (C=N), 131.2, 130.1, 126.1, 124.8, 115.8, 13.2. EIMS (m/z): 195 (M+.).
p-Fluoroacetophenone Semicarbazone (4-F-a-SCs) (14)
Colorless crystals in 98% yield; NMR δH (ppm): 9.56 (s, 1H, -NH-), 8.11 (dd, J=7.0 Hz, J=1.9 Hz, 2H, Ar-H), 7.92 (dd, J=6.9 Hz, J=2.0 Hz, 2H, Ar-H), 7.88 (brs, 2H, -NH2, D2O exchangeable), 2.32 (s, 3H, -CH3); NMR δC (ppm): 162.0, 156.9 (C=O), 142.8 (C=N), 131.3, 125.0, 116.5, 13.3. EIMS (m/z): 195 (M+.).
o-Bromoacetophenone Semicarbazone (2-Br-a-SCs) (15)
Colorless crystals in 95% yield; NMR δH (ppm): 9.42 (s, 1H, -NH-), 8.05-7.90 (m, 4H, Ar-H), 7.71 (brs, 2H, -NH2, D2O exchangeable), 2.28 (s, 3H, -CH3); NMR δC (ppm): 157.1 (C=O), 146.8, 143.4, 141.8 (C=N), 131.1, 130.1, 127.9, 124.3, 14.6. EIMS (m/z): 255 (M+.).
m-Bromoacetophenone Semicarbazone (3-Br-a-SCs) (16)
Colorless crystals in 95% yield; NMR δH (ppm): 9.47 (s, 1H, -NH-), 8.10-7.82 (m, 4H, Ar-H), 7.73 (brs, 2H, -NH2, D2O exchangeable), 2.44 (s, 3H, -CH3); NMR δC (ppm): 157.3 (C=O), 146.7, 143.9, 141.8 (C=N), 131.6, 131.3, 128.0, 124.6, 14.4. EIMS (m/z): 255 (M+.).
p-Bromoacetophenone Semicarbazone (4-Br-a-SCs) (17)
Colorless crystals in 99% yield; NMR δH (ppm): 9.45 (s, 1H, -NH-), 8.04 (dd, J=7.0 Hz, J=2.0 Hz, 2H, Ar-H), 7.89 (dd, J=7.0 Hz, J=2.0 Hz, 2H, Ar-H), 7.65 (brs, 2H, -NH2, D2O exchangeable), 2.35 (s, 3H, -CH3); NMR δC (ppm): 156.9 (C=O), 146.8, 144.4, 141.8 (C=N), 126.9, 123.3, 13.1. EIMS (m/z): 255 (M+.).
o-Chloroacetophenone Semicarbazone (2-Cl-a-SCs) (18)
Colorless crystals in 97% yield; NMR δH (ppm): 9.48 (s, 1H, -NH-), 8.26 (brs, 1H, -NH2, D2O exchangeable), 7.56 (brs, 1H, -NH2, D2O exchangeable), 7.48-7.05 (m, 4H, Ar-H), 2.43 (s, 3H, -CH3); NMR δC (ppm): 157.1 (C=O), 147.7 (C=N), 136.4, 96.6, 137.7, 137.7, 130.1, 130.0, 17.9. EIMS (m/z): 211(M+.).
m-Chloroacetophenone Semicarbazone (3-Cl-a-SCs) (19)
Colorless crystals in 93% yield; NMR δH (ppm): 9.45 (s, 1H, -NH-), 8.33 (brs, 1H, -NH2, D2O exchangeable), 7.60 (brs, 1H, -NH2, D2O exchangeable), 7.47-7-15 (m, 4H, Ar-H), 2.4 (s, 3H, -CH3); NMR δC (ppm): 157.1 (C=O), 147.7 (C=N), 136.4, 96.6, 137.7, 137.7, 131.1, 129.8, 17.6. EIMS (m/z): 211(M+.).
p-Chloroacetophenone Semicarbazone (4-Cl-a-SCs) (20)
Colorless crystals in 97% yield; NMR δH (ppm): 9.47 (s, 1H, -NH-), 8.12 (brs, 1H, -NH2, D2O exchangeable), 7.63 (brs, 1H, -NH2, D2O exchangeable), 7.55 (dd, J=7 Hz, J=2 Hz, 2H, Ar-H), 7.27 (dd, J=6.9 Hz, J=1.9 Hz, 2H, Ar-H), 2.43 (s, 3H, -CH3); NMR δC (ppm): 157.1 (C=O), 147.7 (C=N), 137.7, 136.4, 129.6, 127.1, 16.8. EIMS (m/z): 211(M+.).
Acetophenone Thiosemicarbazone (a-TSCs) (21)
Colorless crystals in 94% yield; NMR δH (ppm): 10.13 (s, 1H, -NH-), 8.00 (brs, 1H, -NH2, D2O exchangeable), 7.90 (brs, 1H, -NH2, D2O exchangeable), 7.89-7.41 (m, 5H, Ar-H), 2.18 (s, 3H, -CH3). NMR δC (ppm): 177.6 (C=S), 146.8 (C=N), 137.6, 129.2, 128.2, 128.2, 126.6, 126.6, 12.9. EIMS (m/z): 196 (M+) (100 %).
o-Fluoroacetophenone Thiosemicarbazone (2-F-a-TSCs) (22)
Colorless crystals in 96% yield; NMR δH (ppm): 10.27 (s, 1H, -NH-), 8.29 (brs, 1H, -NH2, D2O exchangeable), 7.92 (brs, 1H, -NH2, D2O exchangeable), 7.88-7.56 (m, 4H, Ar-H), 2.29 (s, 3H, -CH3); NMR δC (ppm): 179.1 (C=S), 162.6, 157.8, 146.0 (C=N), 130.8, 126.7, 124.4, 116.3, 17.5. EIMS (m/z): 211 (M+.).
m-Fluoroacetophenone Thiosemicarbazone (3-F-a-TSCs) (23)
Colorless crystals in 98% yield; NMR δH (ppm): 10.29 (s, 1H, -NH-), 8.31 (brs, 1H, -NH2, D2O exchangeable), 7.95 (brs, 1H, -NH2, D2O exchangeable), 7.90-7.53 (m, 4H, Ar-H), 2.23 (s, 3H, -CH3); NMR δC (ppm): 178.9 (C=S), 164.7, 159.9, 145.3 (C=N), 140.0, 129.7, 122.7, 116.1, 13.9. EIMS (m/z): 211 (M+.).
p-Fluoroacetophenone Thiosemicarbazone (4-F-a-TSCs) (24)
Colorless crystals in 97% yield; NMR δH (ppm): 10.31 (s, 1H, -NH-), 8.40 (brs, 1H, -NH2, D2O exchangeable), 8.15 (brs, 1H, -NH2, D2O exchangeable), 7.95 (dd, J=7 Hz, J=2 Hz, 2H, Ar-H), 7.92 (dd, J=7.0 Hz, J=1.9 Hz, 2H, Ar-H), 2.21 (s, 3H, -CH3); NMR δC (ppm): 179.3 (C=S), 164.4, 162.0, 147.3 (C=N), 138.3, 129.3, 14.3. EIMS (m/z): 211 (M+.).
o-Bromoacetophenone Thiosemicarbazone (2-Br-a-TSCs) (25)
Colorless crystals in 96% yield; NMR δH (ppm): 10.43 (s, 1H, -NH-), 8.52 (brs, 1H, -NH2, D2O exchangeable), 8.29-7.85 (m, 5H), 2.31 (s, 3H, - CH3); NMR δC (ppm): 179.3 (C=S), 147.4 (C=N), 145.3, 143.9, 129.8, 127.8, 125.3, 123.3, 13.9. EIMS (m/z): 270 (M+.).
m-Bromoacetophenone Thiosemicarbazone (3-Br-a-TSCs) (26)
Colorless crystals in 94% yield; NMR δH (ppm): 10.33 (s, 1H, -NH-), 8.62 (brs, 1H, -NH2, D2O exchangeable), 8.34-7.96 (m, 5H), 2.47 (s, 3H, - CH3); NMR δC (ppm): 179.2 (C=S), 147.6 (C=N), 146.0, 144.1, 130.0, 129.1, 125.9, 124.7, 13.8. EIMS (m/z): 270 (M+.).
p-Bromoacetophenone Thiosemicarbazone (4-Br-a-TSCs) (27)
Colorless crystals in 93% yield; NMR δH (ppm): 10.41 (s, 1H, -NH-), 8.55 (brs, 1H, -NH2, D2O exchangeable), 8.23 (brs, 1H, -NH2, D2O exchangeable), 8.33 (dd, J=6.9 Hz, J=1.9 Hz, 2H, Ar-H), 8.14 (dd, J=6.9 Hz, J=1.9 Hz, 2H, Ar-H), 2.48 (s, 3H, -CH3); NMR δC (ppm): 179.3 (C=S), 147.4 (C=N), 145.3, 143.9, 127.8, 123.3, 13.9. EIMS (m/z): 270 (M+.).
o-Chloroacetophenone Thiosemicarbazone (2-Cl-a-TSCs) (28)
Colorless crystals in 92% yield; NMR δH (ppm): 10.55 (s, 1H, -NH-), 8.47 (brs, 1H, -NH2, D2O exchangeable), 8.32 (brs, 1H, -NH2, D2O exchangeable), 8.29- 8.06 (m, 4H, Ar-H), 2.49 (s, 3H, -CH3); NMR δC (ppm): 178.7 (C=S), 147.4 (C=N), 145.6, 144.1, 127.9, 127.7, 122.9, 123.1, 13.8. EIMS (m/z): 227 (M+.).
m-Chloroacetophenone Thiosemicarbazone (3-Cl-a-SCs) (29)
Colorless crystals in 95% yield; NMR δH (ppm): 10.29 (s, 1H, -NH-), 8.61 (brs, 1H, -NH2, D2O exchangeable), 8.01 (brs, 1H, -NH2, D2O exchangeable), 8.30-8.15 (m, 4H, Ar-H), 2.53 (s, 3H, -CH3); NMR δC (ppm): 179.1 (C=S), 147.4 (C=N), 145.3, 143.9, 127.8, 127.8, 123.3, 123.3, 13.9. EIMS (m/z): 227 (M.+).
p-Chloroacetophenone Thiosemicarbazone (4-Cl-a-TSCs) (30)
Colorless crystals in 93% yield; NMR δH (ppm): 10.41 (s, 1H, -NH-), 8.52 (brs, 1H, -NH2, D2O exchangeable), 8.21 (brs, 1H, -NH2, D2O exchangeable), 8.17 (dd, J=7.0 Hz, J=2.0 Hz, 2H, Ar-H), 8.07 (dd, J=7.0 Hz, J=2.0 Hz, 2H, Ar-H), 2.51 (s, 3H, -CH3); NMR δC (ppm): 179.2 (C=S), 147.4 (C=N), 144.9, 143.9, 126.1, 122.0, 14.1. EIMS (m/z): 227 (M.+).
Cell lines
Cell lines CT26 (murine colon cancer) and B16 (murine melanoma) were provided by Dra. Maccioni UNC-CIBICI (Universidad Nacional de Córdoba-Centro de Investigaciones en Bioquímica Clínica e Inmunología). Cell was grown at 37°C in a humidified atmosphere containing 5% CO2 in DMEM Hyclone medium, supplemented with 2 mM de L-glutamine (Invitrogen), 10% de heat inactivated calf serum (Natocor), 100 U/ml de penicillin y 100 U/ml de streptomycin (Invitrogen), HEPES, 1 mM sodium pyruvate, penicillin (100 IU/ml), and streptomycin (100 μg/ ml).
Cell viability (MTT)
Antiproliferative activity was evaluated using MTT cell proliferation assay seeded in 96-well microculture plates (1 x 104 cells). Compounds 1- 30, in five different concentrations (1, 12.5, 25, 50, and 110 μg/ml), were dissolved in DMSO/ethanol (4:1). After 24 h, MTT reagent (10 μl) was added to each well (final volume 100 μl) and the samples incubated for a further 4 h at 37°C. Cell viability was determined based on mitochondrial conversion of MTT to formazan in samples and untreated cells (cell culture medium containing 1% Dimethyl sulfoxide (DMSO). Absorbance was measured at 550 nm on a ThermoMax Microplate Reader (Molecular Devices, Sunnyvale, USA). The cell inhibitory rate was calculated as follows: inhibitory rate (%) = [(Abscontrol cells - Abstreated cells) / Abscontrol cells] x 100. All assays were done with six parallel samples. The IC50 value was defined as the drug concentration required inhibiting cell growth by 50% relative to controls. Data were analyzed using the statistical analysis programs of ANOVA test and Graph Pad Instat. Meaningful differences were considerate for a p<0.05.
RT-PCR
PCR was performed in 25 μl reactions using Taq DNA polymerase (Life Technologies, UK). cDNA equivalent to 0.1 μg RNA was used. Forward (F) and reverse (R) primers for quantitation of mRNA, designed to span at least one intron, were as follows: GAPDH F: AAC TTT GGC ATT GTT GTG GAA GG. GAPDH R: ACA CAT TGG GGG TAG GAA CA. Bcl-2 F: ATG GCG CAA GCC GGG AA Bcl-2 R: CTT GTG GCC CAG GTA TGC. Bax F: ATG GAC GGG TCC GGG GAG C. Bax R: TCG CCC ATC TTC TTC CAG AT. PCR annealing temperatures of 57°C, 59°C and 59°C were used for amplification using GAPDH, Bcl-2 and Bax primers.
Genotoxicity
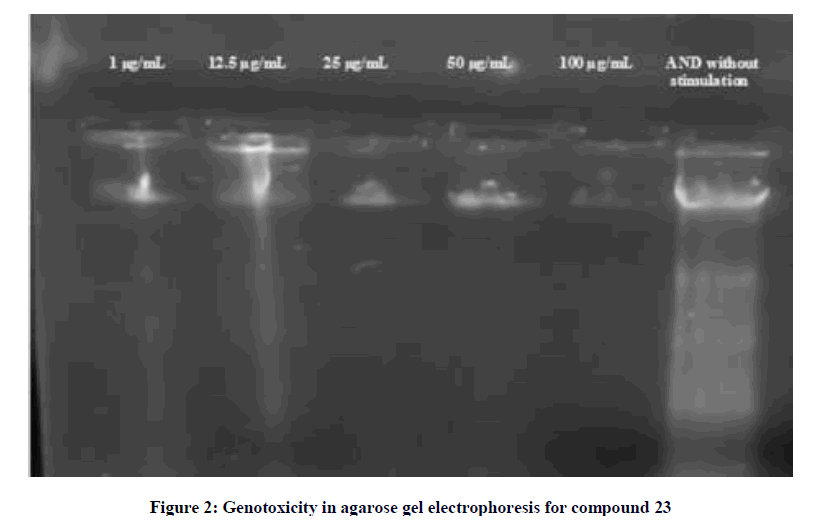
A solution of 1 mg of B16 cell line DNA was dissolved in 1 ml of sterile distilled water. Stock concentrations of the investigated compounds were prepared by dissolving 1 mg/ml DMSO. An equal volume of each compound and DNA were mixed thoroughly and kept at room temperature for 2-3 h. The effect of the chemicals on the DNA was analyzed by agarose gel electrophoresis. A 2 μl of loading dye was added to 15 μl of the DNA mixture before being loaded into the well of an agarose gel. The loaded mixtures were fractionated by electrophoresis, visualized by UV and photographed.
Results and Discussion
A series of SCs and TSCs were prepared by refluxing of an alcoholic solution of the desired ketone with semicarbazide hydrochloride or thiosemicarbazide using sodium acetate or acetic acid (1%) as catalyst (Pavi et al., 2014) (Scheme 1). The structure of SCs and TSCs 11-30 was confirmed by NMR and EIMS.
All compounds (1-30) were tested in vitro for their capacity to inhibit the growth of two cancer cell lines, CT26 (murine colon cancer) and B16 (murine melanoma), at five different concentrations (1.0, 12.5, 25, 50 and 100 μg/ml). The cytotoxic effects were investigated by means of MTT.
Results presented in Table 1 indicate that, in CT26 and B16 the MTT results showed that the new hydrazones derivatives (11-30), were more active than the acetophenones (1-10). This would be consistent with the fact that many nitrogenated functionalities are present in bioactive compounds with anticancer properties. Also changing oxygen to sulfur significantly increased the cytotoxic activity. TSCs (21-30) exhibited more population growth of the carcinoma cells than the semicarbazones (11-20). At the same time, the presence of fluorine atom at the aromatic ring in the hydrazone derivatives (12-14, 22-24), pointed out a structural requirement in this compounds with significant antiproliferative activity. In this sense, among the TSCs (21-30), a potent increase of antiproliferative capacity was observed for the fluorinate derivatives (22-24) with respect to chlorinated and /or brominated ones (25-30).
| Compound | CT26 (µM)a | B16 (µM)a | Compound | CT26 (µM)a | B16 (µM)a |
|---|---|---|---|---|---|
| Acetophenone (1) | 72.1 ± 0.2b | 75.5 ± 0.7b | 4-Br-a-SCs (17) | 147.7 ± 0.5 | 144.1 ± 0.6 |
| 2-F-a (2) | 68.1 ± 0.2 | 65.5 ± 0.7 | 2-Cl-a-SCs (18) | 111.2 ± 0.3 | 115.5 ± 0.2 |
| 3-F-a (3) | 62.7 ± 0.1 | 66.9 ± 0.6 | 3-Cl-a-SCs (19) | 121.1 ± 0.9 | 126.6 ± 0.1 |
| 4-F-a (4) | 67.7 ± 0.3 | 64.0 ± 0.4 | 4-Cl-a-SCs (20) | 114.3 ± 0.7 | 115.9 ± 0.9 |
| 2-Br-a (5) | 148.7 ± 0.5 | 175.5 ± 0.3 | a-TSCs (21) | 52.1 ± 0.2 | 45.5 ± 0.7 |
| 3-Br-a (6) | 181.1 ± 0.9 | 185.2 ± 0.3 | 2-F-a-TSCs (22) | 16.6 ± 0.2 | 16.9 ± 0.4 |
| 4-Br-a (7) | 175.4 ± 0.1 | 185.5 ± 0.7 | 3-F-a-TSCs (23) | 3.1 ± 0.4 | 4.9 ± 0.5 |
| 2-Cl-a (8) | 111.2 ± 0.3 | 115.5 ± 0.2 | 4-F-a-TSCs (24) | 17.4 ± 0.4 | 17.1 ± 0.6 |
| 3-Cl-a (9) | 121.1 ± 0.9 | 126.6 ± 0.1 | 2-Br-a-TSCs (25) | 113.2 ± 0.3 | 175.5 ± 0.2 |
| 4-Cl-a (10) | 114.3 ± 0.7 | 115.9 ± 0.9 | 3-Br-a-TSCs (26) | 121.4 ± 0.2 | 185.2 ± 0.1 |
| a-SCS (11) | 61.1 ± 0.8 | 63.1 ± 0.4 | 4-Br-a-TSCs (27) | 115.7 ± 0.8 | 185.5 ± 0.5 |
| 2-F-a-SCs (12) | 22.0 ± 0.9 | 25.2 ± 0.7 | 2-Cl-a-TSCs (28) | 111.2 ± 0.3 | 110.5 ± 0.2 |
| 3-F-a-SCs (13) | 7.2 ± 0.5 | 8.1 ± 0.2 | 3-Cl-a-TSCs (29) | 121.1 ± 0.9 | 126.6 ± 0.1 |
| 4-F-a-SCs (14) | 24.08 ± 0.2 | 28.6 ± 0.3 | 4-Cl-a-TSCs (30) | 110.3 ± 0.7 | 111.9 ± 0.9 |
| 2-Br-a-SCs (15) | 137.5 ± 0.9 | 175.5 ± 0.6 | Cis-platinc | 0.9 ± 0.7 | 0.7 ± 0.2 |
| 3-Br-a-SCs (16) | 158.4 ± 0.8 | 182.3 ± 0.7 | Curcuminc | 8.2 ± 0.2 | 9.1 ± 0.3 |
aIC50 values were determined from the dose-response curves as the mean of two parallel experiments;
three wells per every concentration employing the MTT assay;
bData are presented as mean ± SEM;
cCis-platin and curcumina were used as a positive control;
aSD=standard deviation of quadruplicate of two independent experiments
Table 1: Cytotoxic activity (IC50 in μM ± SD) of compounds 1-30 against CT26 (murine colon cancer) and B16 (melanoma murine) cell lines
In our model, it was found that fluorinated substituents at o-, m- and p-position in the phenylethanol moiety significantly increased the antiproliferative activity.
At the same time, with respect to the substitution position of fluorine atom, the change of substitution pattern reduce the antiproliferative activity, as observed for compounds o- and p-fluorine substituted. We found, (Table 1), that compounds with fluorine functional group at the m-position of phenyl ring (3, 13, 23) showed better inhibitory activity than o-or p-substituted analogs (2, 4, 12, 14, 22, 24) and the IC50 values approximately followed the sequence of m-position < o-position and p-position. In particular, SCs (13) and TSCs (23) showed a potent growth inhibitory effect on both cell lines. Compounds 13 and 23 were able to inhibit cell growth in the same order as curcumin with IC50 (μM) values of 7.2, 3.1 and 8.1, 4.9. This was indicative of a possible selective effect. Thus the presence of a fluorine atom at m-position in the aromatic ring, as well as, the new functional group semi- or thiosemicarbazone, are structural requirements to enhance the cytotoxic activity.
On the other side, compounds 5-10, 15-21 and 25-30, carrying bromine and chlorine atoms at o-, m- and p-position of the aryl group, did not showed significant cytotoxic activity.
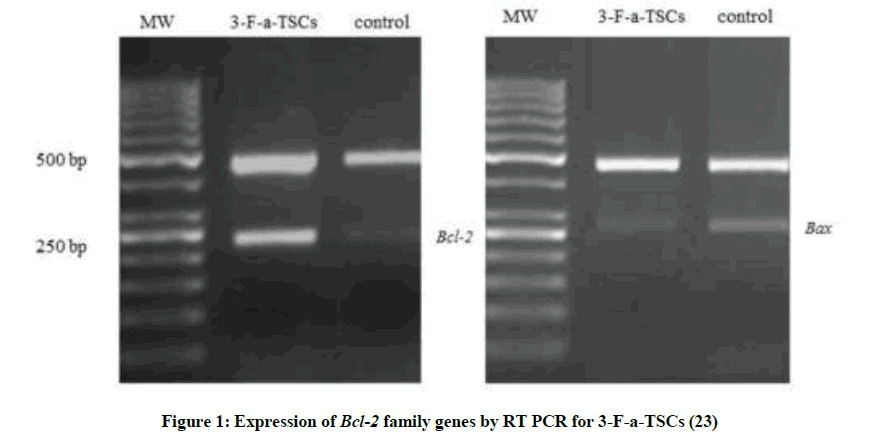
In addition we evaluated apoptosis induction and genotoxicity by the compound with the highest cytotoxic activity (23). Apoptosis was assessed by RT-PCR for Bcl-2 family (Figure 1).
The results also showed for compound 23 that the expression of Bcl-2 (anti-apoptotic gen) (p<0.05) decreased whereas that of Bax (pro-apoptotic gen) (p<0.05) increased compared to the control cells. The decrease of the Bcl-2/Bax ratio and DNA damage could be the critical mechanisms of apoptosis induced by compound 23 in both cell lines, CT26 and B26.
Also, compound 23 showed no genotoxicity at significant doses administered in agarose gel electrophoresis (Figure 2).
Conclusion
A series of new semi- and Thiosemicarbazones derived from monohalogenated aromatic ketones were obtained and evaluated for their antiproliferative properties in two cell lines CT26 (murine colon cancer) and B26 (murine melanoma). The results showed that only two hidrazones, m-fluorosubstituted in the aromatic ring, suppresses the proliferation and induces the cell cytotoxicity in the same order as the positive control curcumin. The most actives compounds were: m-Fluoroacetophenone SCs (13) and m-Fluoroacetophenone TSCs (23). In addition, compound 23 showed in vitro antiproliferative activity in a time- and dose-dependent manner through induction of cell apoptosis. Docking studies are in progress.
References
- C.C. Pacca, R.E. Marques, J.W.P. Espindola, G.B.O.O. Filho, A.C.L. Leite, M.M. Teixeira, M.L. Nogueira, Biomed. Pharmacother., 2017, 87, 381-387.
- A.E. Vieira, G.L. Araujo, C.M. Galassi, R.F. Rodrigues, G.D. Cassalli, M. Kaiser, T. Dalla Costa, H. Beraldo, C.A. Tagliati, Food Chem. Toxicol., 2013, 55, 434-43.
- A.C. da Silva, T.A.R. Dos Santos, I.V.B. da Silva, M.V.G. de Oliveira, D.R.M. Moreira, A.C.L. Leite, V.R.A. Pereira, Exp. Parasitol., 2017, 177, 57-65.
- A. Czubatka-Bienkowska, J. Sarnik, A. Macieja, G. Galita, Z.J. Witczak, T. Poplawski, Bioorg. Med. Chem. Lett., 2017, 27(12), 2713-2720.
- C. Gan, J. Cui, S. Su, Q. Lin, L. Jia, L. Fan, Y. Huang, Steroids., 2014, 87, 99-107.
- J. Qi, J. Deng, K. Qian, L. Tian, J. Li, K. He, X. Huang, Z. Cheng, Y. Zheng, Y. Wang, Eur. J. Med. Chem., 2017, 134, 34-42.
- G.S. Prakash, S. Chacko, Curr. Opin. Drug. Discov. Devel., 2008, 11(6), 793-802.
- I. Ojima, J. Fluor. Chem., 2017, 198, 10-23.