Review - Der Pharma Chemica ( 2023) Volume 15, Issue 1
LONGER-ACTING DRUG DELIVERY SYSTEMS FOR DIABETIC RETINOPATHY
Nisha Rajendra Gawali1, Prashant Lakshaman Pingale1*, Dattatray Manohar Shinkar1, Sahebrao Sampat Boraste1 and Sunil Vishvnath Amrutkar22Department of Pharmaceutical Chemistry, GES’s Sir Dr. M. S. Gosavi College of Pharmaceutical Education and Research, Nashik-422005, MS, India
Prashant Lakshaman Pingale, Department of Pharmaceutics, GES’s Sir Dr. M. S. Gosavi College of Pharmaceutical Education & Research, Nashik-422005, MS, India, Email: prashantlpingale@gmail.com
Received: 11-Dec-2022, Manuscript No. dpc-22-83077; Editor assigned: 13-Dec-2022, Pre QC No. dpc-22-83077; Reviewed: 27-Dec-2022, QC No. dpc-22-83077; Revised: 30-Dec-2022, Manuscript No. dpc-22-83077; Published: 06-Jan-2023, DOI: 10.4172/0975-413X.15.1.1-6
Abstract
Diabetic retinopathy (DR) is characterized by harm to the retinal blood vessels, which ultimately results in visual loss. Diseases that affect the posterior segment of the eye, such as cytomegalovirus retinitis, posterior uveitis, age-related macular degeneration, and diabetic retinopathy, necessitate the development of a novel delivery system that can increase the level of drug that reaches the posterior segment of the eye. The current therapies for DR are intrusive, costly, and time-consuming. The creation of innovative medication delivery systems has gained prominence in the area of research, with nanotechnology being the much heavily explored option. As a novel formulation for drug delivery, Nanoparticles, nanoliposomes, niosomes, nanomicelles, nanoemulsions, nanogels, cyclodextrins, dendrimers, and quantum dots are among the nanotechnology-based systems being developed. The logic behind nanoparticle systems is their capacity to build a painless, safe, non-invasive system with a prolonged, controlled release dosage form to succeed over primary limitations in the management of DR. This review study addresses newly developed sustained drug release as well as innovative non-invasive drug delivery systems that have had different degrees of success in local drug delivery to retinal circulation.
Keywords
Diabetes; Retina; Diabetic retinopathy; Nanoparticles; Proliferative Diabetic Retinopathy
INTRODUCTION
Diabetes Mellitus (DM) is a metabolic disorder caused by elevated blood sugar levels. DM can be broadly divided into 2 types, Type1 (T1DM) and Type2. (T2DM). More than 422 million population worldwide has DM and nearly 1.6 million have diabetes death related to DM per years. The worldwide frequency of diabetes Over 8.5% of the population over the age of 18. By 2045, the population of diabetics is predicted to reach 700 million [1,2]. DR is a very common chronic condition in patients with a long history of Diabetes Mellitus and harms the retina. Blood vessels and retinal nerves are believed to be the main caused of vision losses or blindness in diabetics around the world.
Diabetic Retinopathy can be divided into two classes Type: Proliferative Diabetic Retinopathy (PDR) and Non-Proliferative Diabetic Retinopathy (NPDR). In PDR, angiogenesis occurs in the blood vessels of the eye, causing sudden loss of vision. NPDR is, even more, and can be divided into three categories that are, light, medium, and heavy NPDR. Mild NPDR develops a microaneurysm that damages the tiny blood vessels (BVs) of the retina. Moderate NPDR clogs BVs into the retina and lacks the oxygen and nutrients needed for the retina to function properly. In severe NPDR, many retinal blood vessels are occluded, resulting in O2 and nutrient deficiency at the retinal levels. The prevalence of the anatomical and physiological retinal eye barrier poses challenges in the management of diabetic retinopathy. Successful treatment of diabetic retinopathy depends on directing the drugs to the posterior segment of the eye that is by passage through the eye barrier and reaches to the retina for the management of this disease [3].
In many, eye aliment is treated by two major methods: that is Topical drops and intravitreal injection. Topical drops have been the mainstay for decades, but they has become the main challenge for effective treatment. It has been reported that 33% of patients receiving approximately treatment discontinued treatment one year later [4,5]. The advent of intraocular injections imparts the first effectual treatment behind the eye. The acceptance of ranibizumab with aflibercept for the management of wet age-related macular degeneration represents a significant advancement in intraocular drug delivery. However, current research has reported that in most cases, the frequency of use and injection did not reproduce the study results due to inadequate dosing frequency [6]. The development of implantable intraocular devices like Vitrasert®, Retisert®, Ozurdex®, and Ilubein® may allow for long-term eye retention [7,8]. By, these intraocular devices were being investigated to provide small molecule therapeutics. Therefore, they provide an important range for delivering highly potent protein-based drugs through a novel and intelligent drug transfer system. In current years, many nanotechnology-based drug transfer system had investigated to control and decrease the adverse effect of diabetes. Nanoparticle provide many benefits in conquering challenges related to traditional delivery systems such as drug therapy alone or chemical and enzymatic deterioration. Drug-loaded nanoparticle can control these drawbacks. In addition, selected delivery to specific cell or tissue, continuous transfer of both water-insoluble drug [9,10]. This article examines various methods of longer-acting medication delivery devices for the treatment of DR.
ETIOLOGY
DR affects people who have been diagnosed with diabetes and those who have not. DR is proportional to the patient’s age and diabetes duration, as well as glucose management and blood pressure (BP) changes.
Currently available treatments for Diabetic Retinopathy
Treatment of hyperglycemia and hypertension has been advised to prevent or unusually develop DR in patients who have previously been treated with laser photocoagulation, anti-VEGF medicines, intravitreal corticosteroid injections, and vitreous retinal surgery. As a result, novel therapies require during the initial stage of this disease. Laser photocoagulation either directly treatments leaky blood vessels or removes newly generated retinal blood vessels. The eye's peripheral retina is concerned with the production of vascular endothelial growth factor, which is accountable for angiogenesis. This hypothesis of photoreceptor degeneration or chemical elimination has lately been validated by in vivo testing of decreased oxygen deficiency and increased retina functions in rats, and anti-angiogenic medications have been utilized to enhance patient visual acuity. DME and PDR should be increased.
Anti-VEGF therapy in DR
VEGF is a homodimeric polypeptide has a molar mass between 35 and 45 kDa that exists in many isoforms. Hypoxia promotes the production of vascular permeability factors as well as endothelial cell-specific angiogenic factors. In recent investigations, monoclonal antibodies have been used to treat ocular illnesses by intravitreal injection because they neutralize VEGF. Because of hypoxia, VEGFs are highly produced in DR, and their ability to increase the permeation of blood–tissue obstacles that take place in retinal cells is the primary stimulant for the formation of retinal neovascularization. Anti-VEGF medications include bevacizumab, ranibizumab, and pegaptanib.
Corticosteroids
These are the medications with the most anti-inflammatory responses. It reduced BV penetrability including deterioration of the blood-retinal barrier (BRB), as well as VEGF expression. Triamcinolone acetonide, dexamethasone, and fluocinolone acetonide are some of the corticosteroids used in the DR.
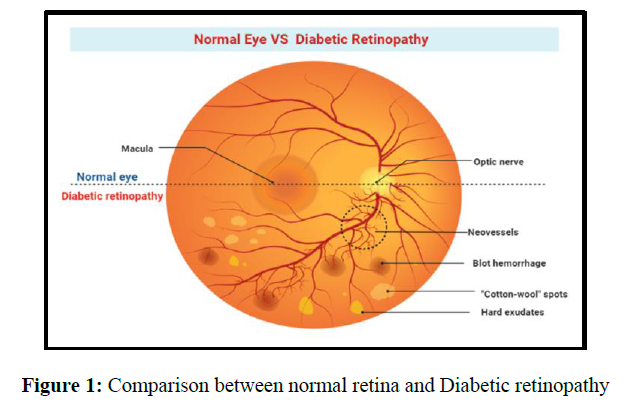
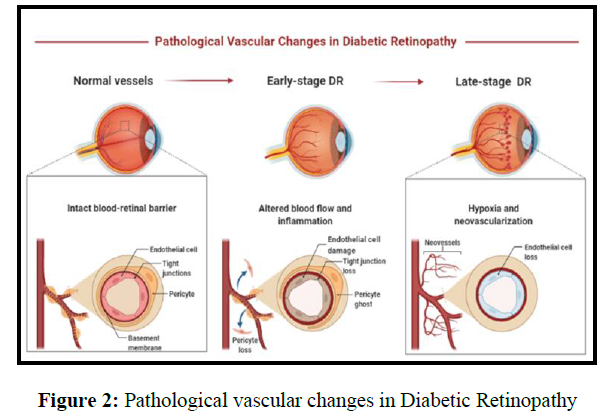
Unlike traditional liposomes, which are composed of natural (egg phosphatidylcholine) or synthetic (dimyristoyl phosphatidylcholine and dipalmitoyl phosphatidyl glycerol), transfersomes are composed of phosphatidylcholine and a single-chain surfactant.as shown in figure 2. Transferesomes are a new elastomeric or ultradeformable phospholipid, surfactant, and water-based vesicular drug carrier system to improve transdermal administration . It gets across the epidermal barrier by squeezing through the stratum corneum is intracellular gap. They can administer lower and higher molecular weight drugs into or through the skin based on the application because of their self-optimized and ultra-flexible membrane properties (Figures 1-3).
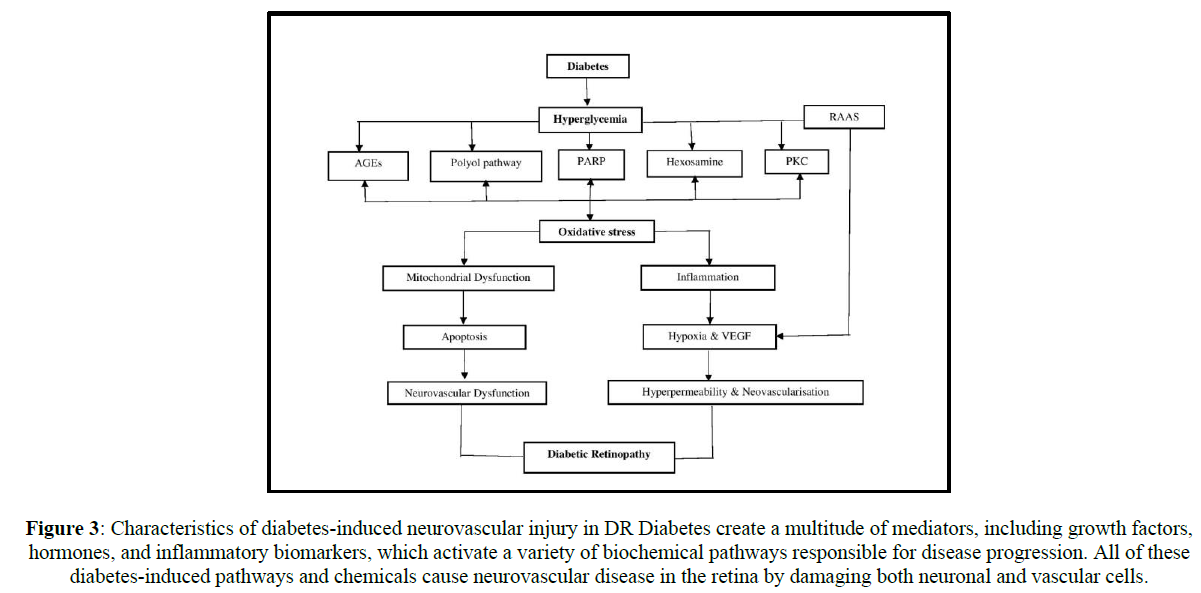
Figure 3: Characteristics of diabetes-induced neurovascular injury in DR Diabetes create a multitude of mediators, including growth factors, hormones, and inflammatory biomarkers, which activate a variety of biochemical pathways responsible for disease progression. All of these diabetes-induced pathways and chemicals cause neurovascular disease in the retina by damaging both neuronal and vascular cells.
Several disorders, including hypertension, diabetes, and ischemic cardiovascular disease, are caused by an inequality between the antioxidant system and reactive oxygen species (ROS). Diabetic patients experience as retinitis a result of increased oxidative stress. ROS influences inflammatory reactions by altering the expression of inflammatory genes. The level of polyunsaturated fatty acids in the retina is very high, as is the need for oxygen. In humans, the retina requires 300-600% more oxygen than the cerebral cortex and heart muscle. ROS products include oxidant, peroxyl, hydroxyl as well as non-radical H2O2.
NOVEL DRUG DELIVERY SYSTEMS (NDDS)
The limitations of present traditional dosage forms call for a reconsideration requirement of non-invasive, efficacious as well as cost-effective delivery technologies. In current years, this new delivery system is come out as an efficacious carrier capable of successfully delivering drugs to patients. The retina addresses the difficulties related to the traditional and surgical approach. Two basic techniques for increasing ocular bioavailability are as follows: improving corneal permeability and extending contact duration on the ocular surface. The primary goal of NDDS will minimize the costs and frequencies of injections, as well as enhance the effectiveness of injections. Therapeutic impact, reduce adverse effects, enhance patient compliance, and overcomes the limits of traditional dose forms. However, the key parameters that impact the efficacy of ocular drug administration include maximizing the polymer-drug system's lipophilic-hydrophilic characteristics, rate of degradation, safety, and compatibility of polymer utilized in the manufacture of nanomedicines. The careful choice of polymers is critical in the pharmacokinetics of drug release from nanomedicine. Bio-adhesion qualities of polymers are determined by their hydrating duration, characteristics of swell, molar mass, and the degrees of cross-linking are among the variables that govern retinal bioavailability from bioadhesive formulations. Furthermore, the production method has an impact on the nanoformulations' quality, particularly their ability to bind. Polymers use in the preparation of ocular medication are poly (acrylic acid), hyaluronic acid, poly (alkyl cyanoacrylates), poly (lactic acid), chitosan, Eudragit RL100, as well as RS100, modified polystyrene, albumin, and gelatin.
Nanoemulsion
Nanoemulsions are defined as o/w emulsions in which small droplets with an inner lipids core and an outer lipid membrane are formed with a surfactant. These drops can enter the ocular tissue and deliver the medicine to the desired location. A surface-modified nanoemulsion with the appropriate polymer can also give a prolonged releasing impact. This approach has the advantages of improved therapeutic effects, fewer toxic effects, and so more patient acceptance. According to current research, on the spot Rotepredonor etabonate, nanoemulsion gel preparation has been studied, which turns into a gel after being inserted into the rabbit eye. It is used to treat inflammations of the eye following cataract surgery.
Dendrimers
A dendrimer is a star-like, rather branched, large molecule that is a water-soluble structure having 3 parts: a valuable core, an indoor dendrimer structure, also an outdoor floor with a purposeful floor group. Such artificial polymeric macromolecule can offer a long-term release of the medication in the posterior segment of the eyes. Polyamidoamine dendrimers were synthesized and given in the rat eyes in one study to treat ocular inflammation. Broken DR retina confirmed structural and characteristic safety in PAMAM dendrimers via topical administration in animal’s model. Dendrimers as well as polyplexes containing anti-angiogenic oligonucleotide-1 and plasmid DNA as genes have been utilized to treat diabetic retinopathy by transfecting human retinal pigment epithelial cells after complex formation with the nucleotide. The degree of transfecting is circuitously determined with the aid of using the downregulation of the hVEGF polypeptide.
Nanomicelles
A nanomicelle is a self-assembled molecule with a polar head and a non-polar tail towards the center that comes into contact with the solvent. It is similar to lipid nanoparticles apart from the outer layers. Liposomes are an example of this, the outermost part is a bilayer of phospholipids, while in the case of nano cells, and the outermost part is a single layer. Those micelles are nano-sized and also have high drug permeability without irritating the eyes. They are composed of different surfactants as well as polymers.
Dexamethasone-loaded nanomicelle was tested for transscleral iontophoresis after topical administration in one of the trials. It has been discovered to attain the retina, in particular via the scleral/conjunctival route. Every other investigation has shown that the poly (ethylene glycol)-poly (-caprolactone) di-block copolymer used in the Dexamethasone-encapsulated polymeric nanomicelles technique was composed of poly (ethylene glycol)-poly (-caprolactone) di-blocks copolymers. The improved method accelerated dexamethasone permeation through 2 instances throughout the conjunctival molecular lines and through 2.5 instances throughout the excise rabbit sclera, compare to suspension of dexamethasone. The FDA has approved a cyclosporine-A (0.09%) loaded nanomicellar method for the management of dry ocular disorders. It has proven to step forward with the rapid commencement of effect, beginning as early as 4 weeks, and development in tear manufacturing in comparison to the emulsion of Cyclosporine-A throughout medical trials.
Solid Lipid Nanoparticles (SLNS)
It is sphere-shaped particles with mean diameters ranging from 10 to 1000 nm, have a large surface area, and also have a heavy drug load. These compositions are made up of solid lipids, surfactants, co-surfactants, as well as hydrophilic or lipophilic drugs. SLN is a controlled site due to its tiny size, presence of lipids, improved durability, superior biocompatibility, easy passage through the biomembrane like a retinal barrier, and improved biological availability all advantages achieve specific drug delivery goals.
Triamcinolone acetonide turned into investigated as a remedy for posterior eye illnesses such as conjunctivitis, swelling, diabetic macular edema, and retinopathy. TA-loaded stable lipid nanoparticles (TA-SLNs) and formulation of in situ gel (TA-SLN-IG) have been organized via way of means of warm homogenization and softened emulsification method. These have been introduced into the deep ocular tissues in the tropics. The natural section turned organized via way of means of melting glyceryl monostearate (GMS) and Compritol5 888ATO at 80º C as well as the drugs turned into introduced with non-stop stirring. The aqueous section turned organized via way of means of blending tween eighty and pluronic F-68 surfactant, glycerin, and boiled and condensed water. A preliminary emulsion turned into shape via means of including a water section to the natural section using a sonicator. SLNs have been received via way of means of homogenizing this emulsion. The results revealed that the lipid primarily depends completely on nanoparticulate systems, when used in conjunction with an in-situ gelling agent, demonstrated to the viable drug transport system for deep medication permeation into retinal tissue for the management of diabetic retinopathy.
The cold homogenizer was used to create myricitrin SLN, which was then tested in the STZ-induced T2DM animal model. The formulation of SLN demonstrated antioxidants, anti-apoptotic, also an anti-diabetic effect that protects the diabetic retina caused by DR. Myricitrin SLN enhanced antioxidant protection, increased glycogen abundance, also cell survival in hyperglycemic cell exposure to high glucose level conditions. The outcome in the SLN-treated group where much stronger than the metformin group.
Another study discovered that ciprofloxacin (CIP) SLNs were formulated using a sonication melt emulsifying technique for sustained delivery to cure ophthalmologic ailments, specifically DR. CIPSLNs was found to diameter is 165 to 320 nm in addition to great drug loading. In phosphate buffered saline, release tests were carried by dialysis bag with a molar mass of 12-14kDa. The preparations Information Classification: In comparison to CIP, General had a prolonged release pattern as well as stronger antimicrobial activities. According to this, it was observed CIP must be given to remaining in address with the retina for a long time period by bringing in SLNs. The SLN has resulted that have low transfected levels in an established photoreceptors line of the epithelium (ARPE19) also used as a non-viral vector for gene therapy to treat retinal disorders such as DR.
Nanosystem based on cyclodextrin (CD)
It is made up of cyclic oligosaccharides with 6, 7, or 8 glucopyranose units. The outside section of these units has a hydrophilic and hydrophobic matrix. This nanosystem can transport the medication to the back of the eyes. It was successfully studied for the transport of medication for the management of eye diseases to the eye. A study of CD fine particle eye drops loaded with dexamethasone to transport medication to the posterior segment for the management of eye disorders. In some other studies, loteprednol etabonate and hydroxypropyl βCD were also βCD produced in various types like ocular drops, as well as gel. These have been studied for their non-inflammatory effects on pinkeye and have been shown to be efficient in lowering inflammation markers within the rabbit's eyes. It was very crucial to remember that CD-related derivative medicines are a nowadays available treatment option for a variety of eye ailments.
Quantum dots
It is nanocrystals of semiconductors have diameters ranging from 2 to 6 nm. QDs made of zinc sulfide and cadmium selenide. It is mostly utilized agent for imaging to mark neurons, endothelial cell, and glia in the retina’s capillary. In a study, QDs based-silicon was produced and given intravitreally to provide retinal cells with electrical stimulation in a retinal photoreceptor model as a neuroprotective substance. This method was utilized to target drugs at the back of the eyes for the management of eye disorders.
Nanostructured lipid carriers (NLCs)
NLC is a drug transfer system commonly associated with two kinds of lipids. Solids and liquids are produced. The various benefits of NLC are described below.
1. Prolonged biological half-life of the drug.
2. Improves the permeability and bioavailability of various drugs.
3. Improved hydrophobic medication solubility across a range of dosage formulations.
4. Improving the durability of different dose types
5. Reduce the side effects of some medicines.
6. Specialised transfer of drugs is possible to reach different bodily tissues.
7. Extended drug released that has been coated with NLC
It is used topically to ease the pain. And tenderness associated with intravitreal injection. Due to the above benefits, NLC has been getting more and greater focus lately. In studies, scientists rearranged itraconazole (ITR) -NLC to control this disease with a highly effective undeveloped anti-angiogenic substance. ITR-NLC was prepared using the high-pressure homogenization method. ITR NLC was manufactured using tripartition, Capmul MCM (solid lipid), Transcutol HP (cosurfactant), and Tween 80 (surfactant). In addition, we performed optimized NLC surface modification using chitosan to alter the charge present on the surface as well as improved drug residence and permeation of the eye. Anti-angiogenic studies were performed with the HET CAM model (egg test-Chorioal into Membranes) and demonstrated the potential for excellent anti-angiogenesis of ITR-NLC. It showed anti-angiogenic effects on the rat cornea. Another study found that palmitoylethanolamide (PEA) has positive effects on many eye disorders like glaucoma and DR. PEA reduced the percentage of eye inflammation by maintaining the BRB in diabetics’ animals. NLC is loaded with myriocin (Myr) developed by Platania et al. (2019) for the management of retinitis pigmentosa (RP). The preparation uses a melt emulsifier and ultrasonic technology using Gelucire 44/14 (10% w / v) and Mygliol 812 (5% w / v) as lipids and Tween80 as a surfactant. Prepared Intraocular dispersion of MyrNLC preparation in mice C57BLJ and rabbits was performed also the effectiveness was contrasted with Myrwater suspension and Myr-loaded SLN. When compared with the suspension of myriocin or MyrSLN preparations, the NLC1 formulation given markedly (p 0001) higher abundance of retinal myriocin after topical ocular administration.
Nanoparticles
It has a size of nanometers and can overcome diverse physical limitations. Lipid NPs and polymeric NPs are the two types of nanoparticles. Polymeric NP is built of polymer, whereas lipids NPs are made up of lipid, surfactant, and co-surfactant distributed across 3 layers: surface layer, shell layer, and core layer. Lipids improve the penetration of the NPs since they are present, resulting in increased medication retention in the eye for a longer period of time SLNs as well as nanostructured lipid carriers are the two basic categories of lipid NPs (NLCs).
Recent research used thin-film hydration to create NPs that were then delivered IV to targeted choroids for the treatment of neovascularization of the choroids in the therapy of this disease. Irradiation in the eye changed it to a tissue-targeting condition after NPs injection. NPs that target photos were produced by chemically altered poly (ethylene oxide)-poly (D, L-lactic acid) (PEG-PLA) blocks copolymers self-assembly. The medicine was accumulated precisely in the damaged parts of the eye by the generated NPs, which decreased the extent of the neovascular lesion. Using Transmission Electron Microscopy (TEM) produced NPs. The normal size of the NPs is 50 nm. Fluorescence emission spectra for irradiated NPs exhibited a change in wavelength to 465 nm when compared to 481 nm for normal NPs. The spectral structure peaks of free CPP and various NPs in D2O were shown in the 1H NMR spectra, with trademark phenylalanine protons peaks emphasized in the blue square. The nanoparticle NP-CPP is made up of CPP-PEG-PLA and mPEG-PLA. HPLC at 390 nanometer absorbances after 1 minute of continuously irradiate (50 mWcm2, 400 nm) with a 400 NP-[CPP] photocleavage in Phosphate Buffered Saline (0.5 mg mL1) was determined after continuous irradiation (50 mWcm2, 400 nm) [64]. Kowluru et al. investigated the neuroprotection function of NPs in DR also medication delivery to the retinal site in 2001. This work, NPs function with neuroprotective chemicals like coenzyme Q10, glutamate, and somatostatin, among others, were employed to protect diabetic retinas for this disease control. These organic polymer materials nanoparticles are investigated as carriers for targeting particular locations into the eyes. In nature, the observed polymer was biologically compatible, and non-antigenic.
Two nanosystems for the treatment of DR in rats were developed by Amadio et al. (2016): siRNA-loaded SLNs and liposomes (SUV) that inhibit HuR expression. Lipoplexes were injected into the eyes of rats with diabetes produced by streptozotocin (Streptozotocin) in this work. Western blot and ELISA were used to assess retinal HuR and VEGF. The findings demonstrated that HuR siRNA treatment significantly decreased retinal HuR and VEGF levels, which were markedly elevated in STZ-rats. It was discovered that lipoplexes with a 4:1 N/P ratio and a modest positive surface charge were more successful in transfecting cells, considerably reducing retinal HuR and VEGF levels.
Niosomes
Niosomes are lamellar (bilayer) structures made of non-ionic surfactant molecules enclosing an aqueous compartment, comparable to liposomes. In ocular administration, lamellar structure is preferred over vesicular structures. Systems due to the fact that chemically stable; raw ingredients are readily accessible and inexpensive; they are biocompatible, biodegradable, and non-immunogenic, unlike phospholipids, and they do not need any conditions or cautions for using surfactants, making them more suitable for industrial production.
Nanogel
Nanogels, also called hydrogels, are made from nanoscale systems containing oleophobic polymers directly loaded with macromers (such as N-isopropyl acrylamide and 2-hydroxy methacrylate dextran macromers).
Both hydrophobic and hydrophilic medicines. The rate of crosslink degradation and peripheral cues such as pH and temperature can influence drug release kinetics from the nanogel. It has been found that previously described nanogels can bypass the biological barriers of the eye and are therefore used as a vehicle for intraocular delivery of drugs capable of delivering drugs to the retina. Nanogels have now demonstrated promising capabilities for ocular medication delivery and constitute a feasible alternative to traditional eye drops for the management of eye problems.
Nanoliposomes
Nanoliposomes are tiny spherical vesicles composed of a single or several lipid bilayers generated from organic or manufactured phospholipid with a watery core in the middle. These are used as delivery systems for drugs that are hydrophilic (laden in the core) and lipophilic (loaded in the bilayer). due to their little stature as well as extended residency period, it aids medications in reaching their target spot. By bypassing the retinal barriers, they improve corneal permeability and prolong drug contact duration on the ocular surface.
In experiments, rapamycin-loaded nanoliposome showed improved solubility compared to rapamycin alone. The medication was administered topically and demonstrated transscleral penetration with significant drug retention. This proposed topical administration in order to treat ocular diseases such as diabetic retinopathy.
By combining ammonium sulfate gradients with a pH gradients approach, timolol nanoparticles and timolol maleate chitosan-coated liposomes (TM-CHL) were produced. Compared to TM eye drops that are sold in stores, these liposomes improved contact duration of precorneal, eye permeability, bioavailability, and extended corneal contact duration without causing discomfort. The formulation demonstrated an outstanding intraocular force-reducing impact, which is to treat diabetic retinopathy, as well as efficient strategies for improving the retinal absorption of aqueous soluble TM to treat eye disorders such as DR, glaucoma, and so on.
In a different research, ranibizumab-encapsulated liposomes made using the dehydration-rehydration process were superior to invasive intravitreal injections in terms of prolonged release, better penetrating, and a greater standard of en-capsulation, penetration through into cornea, and depots impact.
CONCLUSION
Diabetic retinopathy (DR) is a common condition that causes retinal damage in persons with a long history of diabetes. The successful treatment of DR is based on the drug reaching the retina via the posterior region of the eye, i.e. crossing the ocular barrier. Novel drug delivery, i.e. longer-acting drug delivery technology, is the best option for delivering medication to the posterior region of the eye by overcoming the eye retinal barrier. Nanotechnology appears to be a viable choice since it can penetrate the blood-retinal barrier, reducing treatment administration and remaining in the eye for a longer period of time. Nanotechnology has been described as an extremely effective treatment for posterior eye segment problems like DR. As a result, these advancements have the potential to improve therapy efficacy and patient compliance, resulting in better outcomes than current therapeutic options. This paper explains the current alternatives for treating DR utilizing a nanotechnology-based system, such as nanoparticles, nanoemulsions, nanogels, CD, QDs, dendrimers, and nanomicelles, nanoliposomes, and niosomes.
REFERENCES
- Sharma DS, Wadhwa S, Gulati M, et al., Expert Opinion on Drug Delivery. 2021, 5: p. 553-576.
- Susilawati DS, Riana D. IAIC Transactions on Sustainable Digital Innovation (ITSDI). 2019, 1: p. 78-86.
- Kim JH, Kim JH, Kim KW, et al., Nanotechnology. 2009, 20(50): p. 505101.
- Cao Y, Samy KE, Bernards DA, et al., Drug discovery today. 2019, 24: p. 1694-700.
- Robin AL, Muir KW. Expert Review of Ophthalmology. 2019, 14: p. 199-210.
- Rofagha S, Bhisitkul RB, Boyer DS, et al., Ophthalmology. 2013, 120: p. 2-9.
- Haller JA, Bandello F, Belfort Jr R, et al., Ophthalmology. 2010, 117: p. 1134-1146.
- Callanan DG, Jaffe GJ, Martin DF, et al., Arch ophthalmol. 2008, 126(9): p. 1191-201.
- Fangueiro JF, Calpena AC, Clares B, et al., International journal of pharmaceutics. 2016, 502: p. 161-169.
- Souto EB, Souto SB, Campos JR, et al. Molecules. 2019, 24: p. 4209.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref